Influence of Infection Origin, Type of Sampling, and Weather Factors on the Periodicity of Some Infectious Pathogens in Marseille University Hospitals, France
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Bacterial Identifications and Related Data
2.1.2. Weather Data
2.1.3. Legal Statement
2.2. Methods
- -
- A Kwiatkowski–Phillips–Schmidt–Shin (KPSS) test for testing trend stationarity, with stationarity rejection if p > 0.05 [28]. The KPSS test is a type of unit root test that tests for the stationarity of a given series around a deterministic trend. The p-value reported by the test is the probability score, based on which you can decide whether to reject the null hypothesis or not.
- -
- A ‘Seasonal and Trend decomposition using LOESS’ (STL), which is a versatile and robust method for decomposing time series. The LOESS method used for this decomposition is a method for estimating nonlinear relationships [29]. STL uses LOESS (locally estimated scatterplot smoothing) to extract smooth estimates of the three components. The advantage of this method is that the seasonal component is allowed to change over time, and it is robust to outliers. For the purpose of this study, we have forced the seasonal component to be identical across years.
- -
- A ‘Trigonometric seasonality, Box-Cox transformation, ARMA errors, Trend and Seasonal components’ (TBATS) analysis for allowing a search for multiple seasonalities [30].
- -
- An extraction of the detrended time series using the STL results.
3. Results
3.1. Seasonality Study
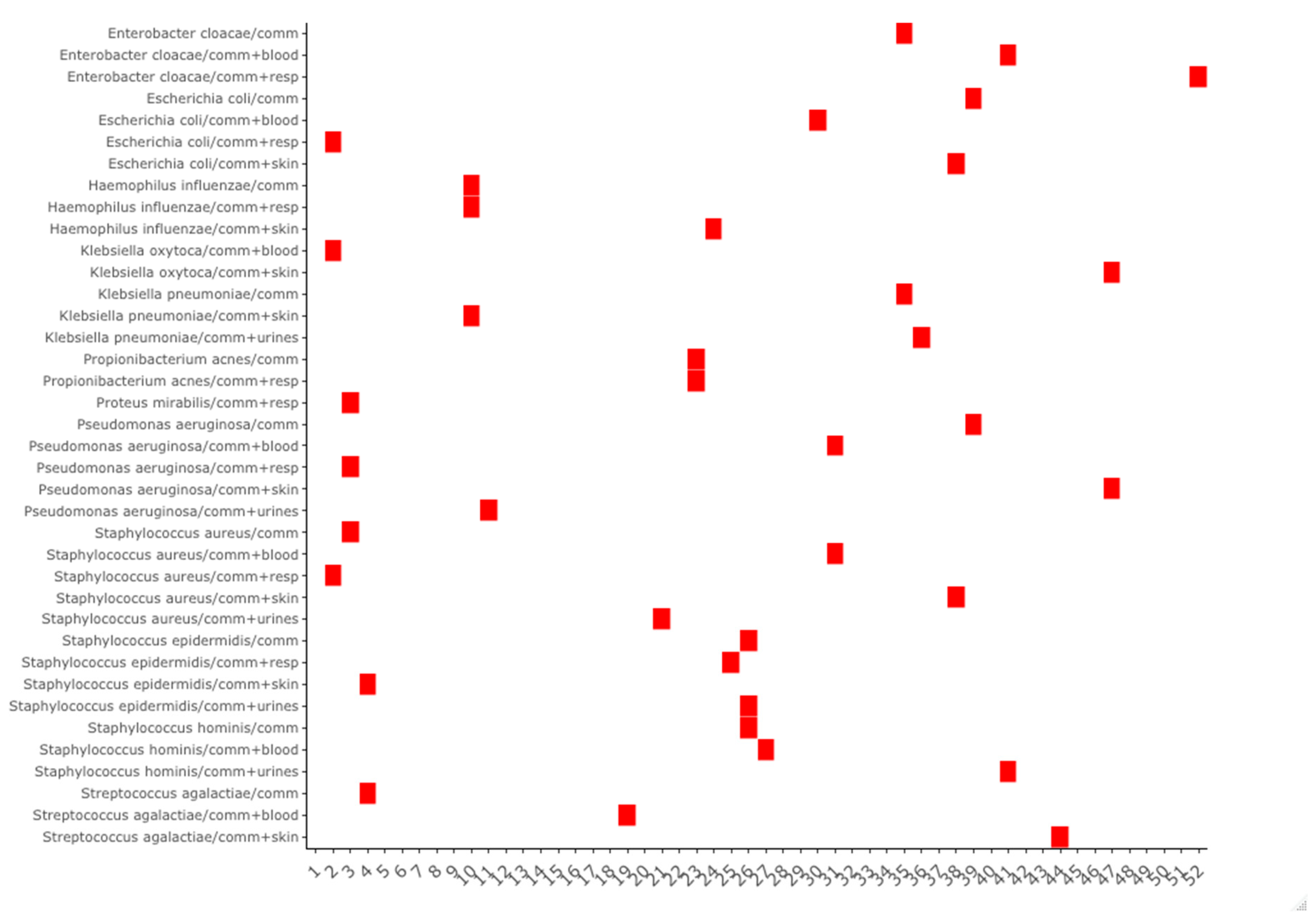
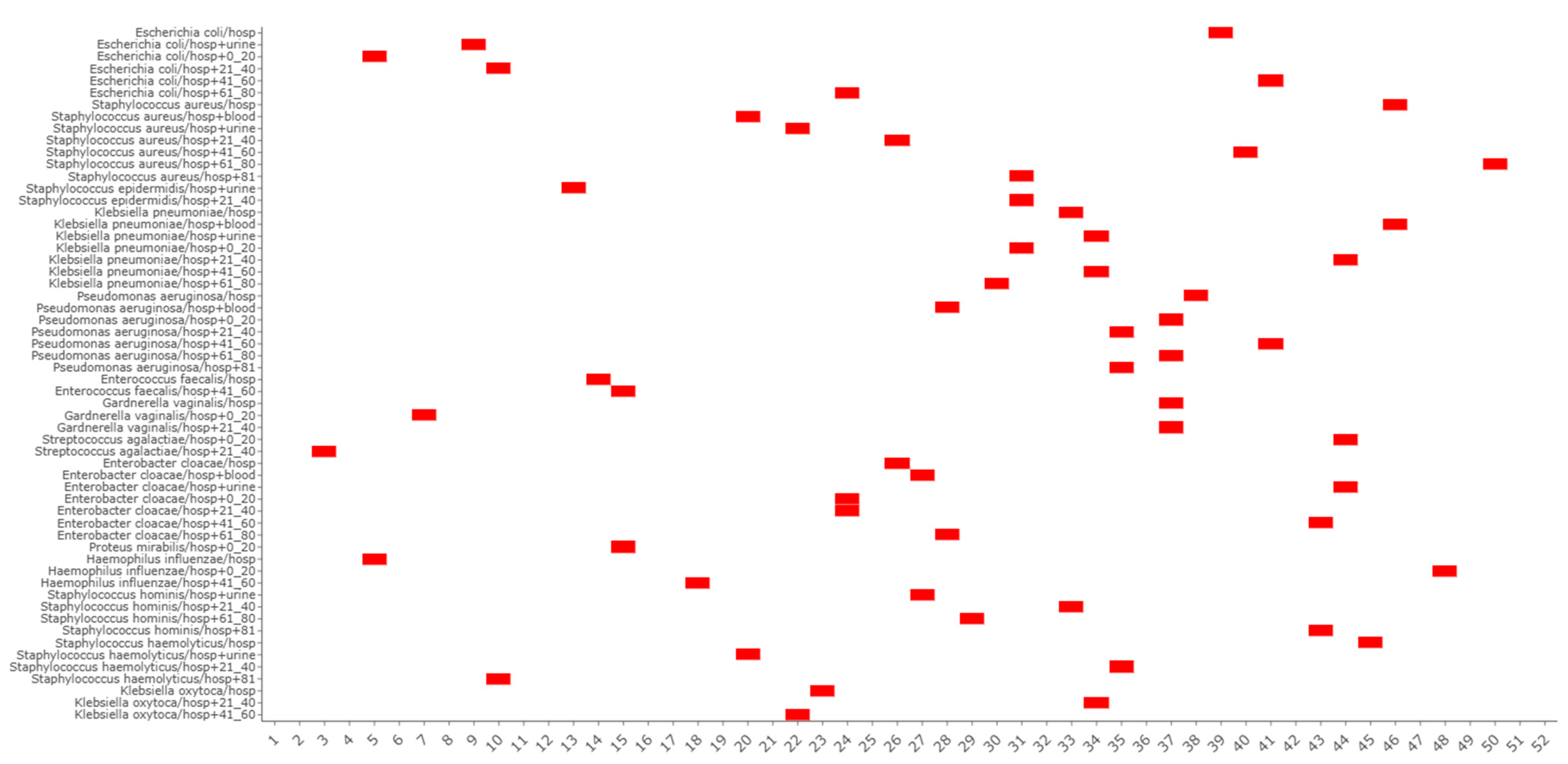
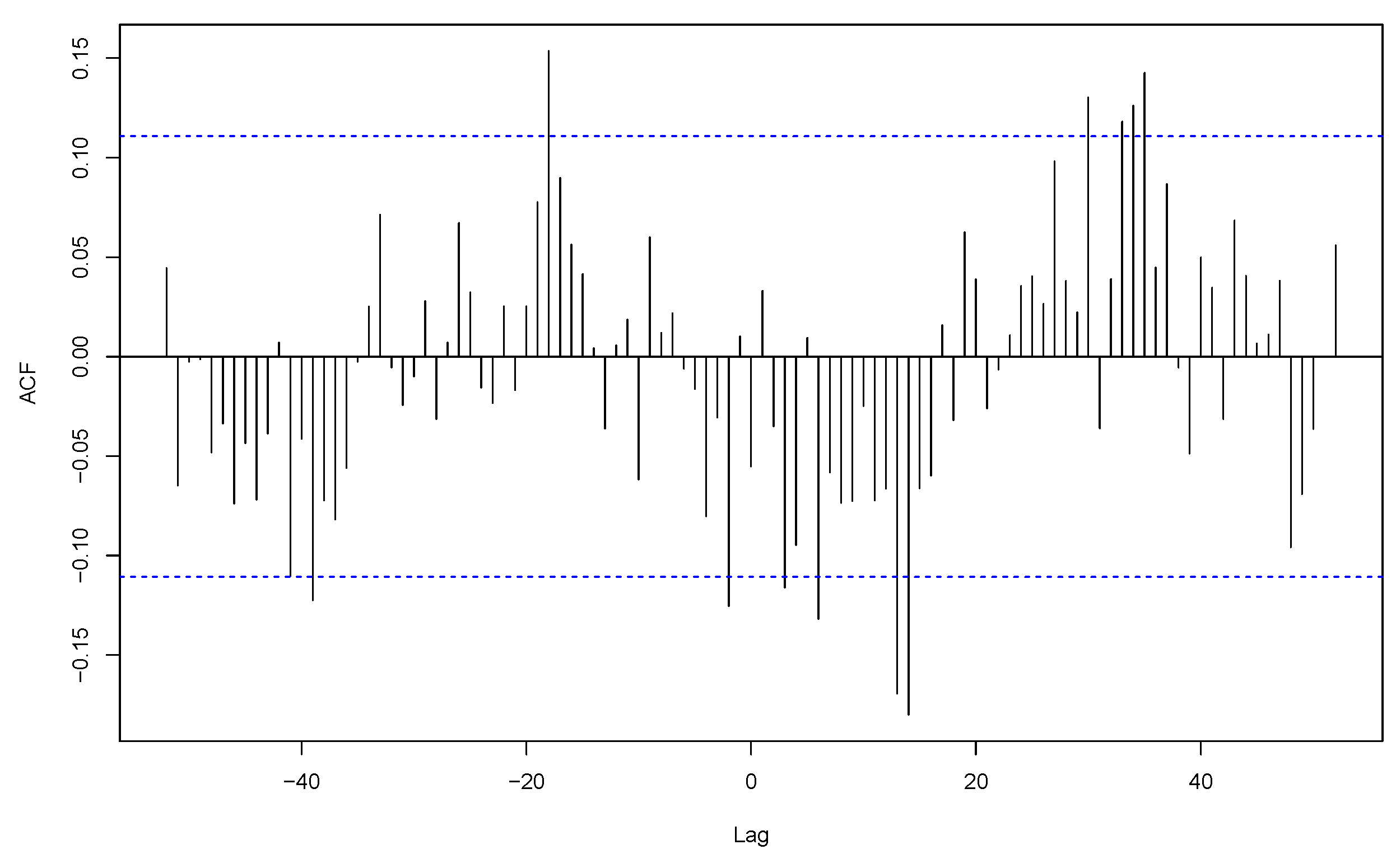
3.2. Co-Seasonality and Cross-Correlation Analysis
3.3. Species Periodicities and Meteorological Drivers
3.4. Seasonal Weekly Indexes per Pathogen
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Paul, M. Seasonality in Infectious Diseases: Does It Exist for All Pathogens? Clin. Microbiol. Infect. 2012, 18, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, J.; Fletcher, W.M. VI. An Investigation into the Periodicity of Measles Epidemics in London from 1703 to the Present Day by the Method of the Periodogram. Philos. Trans. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character 1918, 208, 225–250. [Google Scholar] [CrossRef]
- Fisman, D. Seasonality of Viral Infections: Mechanisms and Unknowns. Clin. Microbiol. Infect. 2012, 18, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Dowell, S.F. Seasonal Variation in Host Susceptibility and Cycles of Certain Infectious Diseases. Emerg. Infect. Dis. J. CDC 2001, 7, 369–374. [Google Scholar] [CrossRef]
- Martinez, M.E. The Calendar of Epidemics: Seasonal Cycles of Infectious Diseases. PLoS Pathog. 2018, 14, e1007327. [Google Scholar] [CrossRef]
- Greer, A.L.; Drews, S.J.; Fisman, D.N. Why “Winter” Vomiting Disease? Seasonality, Hydrology, and Norovirus Epidemiology in Toronto, Canada. EcoHealth 2009, 6, 192–199. [Google Scholar] [CrossRef]
- Linthicum, K.J.; Britch, S.C.; Anyamba, A. Rift Valley Fever: An Emerging Mosquito-Borne Disease. Annu. Rev. Entomol. 2016, 61, 395–415. [Google Scholar] [CrossRef]
- Schuster, G.; Ebert, E.E.; Stevenson, M.A.; Corner, R.J.; Johansen, C.A. Application of Satellite Precipitation Data to Analyse and Model Arbovirus Activity in the Tropics. Int. J. Health Geogr. 2011, 10, 8. [Google Scholar] [CrossRef]
- Coletti, P.; Poletto, C.; Turbelin, C.; Blanchon, T.; Colizza, V. Shifting Patterns of Seasonal Influenza Epidemics. Sci. Rep. 2018, 8, 12786. [Google Scholar] [CrossRef]
- Viboud, C.; Bjørnstad, O.N.; Smith, D.L.; Simonsen, L.; Miller, M.A.; Grenfell, B.T. Synchrony, Waves, and Spatial Hierarchies in the Spread of Influenza. Science 2006, 312, 447–451. [Google Scholar] [CrossRef]
- Salmon-Rousseau, A.; Piednoir, E.; Cattoir, V.; de La Blanchardière, A. Hajj-Associated Infections. Med. Mal. Infect. 2016, 46, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Park, K.H.; Bahk, G.J. Combined Influence of Multiple Climatic Factors on the Incidence of Bacterial Foodborne Diseases. Sci. Total Environ. 2018, 610–611, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Ikeda, T.; French, N.; Baker, M.G.; Hales, S. Climate Variability, Weather and Enteric Disease Incidence in New Zealand: Time Series Analysis. PLoS ONE 2013, 8, e83484. [Google Scholar] [CrossRef] [PubMed]
- Kovats, R.S.; Edwards, S.J.; Charron, D.; Cowden, J.; D’Souza, R.M.; Ebi, K.L.; Gauci, C.; Gerner-Smidt, P.; Hajat, S.; Hales, S.; et al. Climate Variability and Campylobacter Infection: An International Study. Int. J. Biometeorol. 2005, 49, 207–214. [Google Scholar] [CrossRef]
- Stashevsky, P.S.; Yakovina, I.N.; Alarcon Falconi, T.M.; Naumova, E.N. Agglomerative Clustering of Enteric Infections and Weather Parameters to Identify Seasonal Outbreaks in Cold Climates. Int. J. Environ. Res. Public Health 2019, 16, 2083. [Google Scholar] [CrossRef]
- Nichols, G.L.; Richardson, J.F.; Sheppard, S.K.; Lane, C.; Sarran, C. Campylobacter Epidemiology: A Descriptive Study Reviewing 1 Million Cases in England and Wales Between 1989 and 2011. BMJ Open 2012, 2, e001179. [Google Scholar] [CrossRef]
- Djennad, A.; Lo Iacono, G.; Sarran, C.; Fleming, L.E.; Kessel, A.; Haines, A.; Nichols, G.L. A Comparison of Weather Variables Linked to Infectious Disease Patterns Using Laboratory Addresses and Patient Residence Addresses. BMC Infect. Dis. 2018, 18, 198. [Google Scholar] [CrossRef]
- Naumova, E.N.; Christodouleas, J.; Hunter, P.R.; Syed, Q. Effect of Precipitation on Seasonal Variability in Cryptosporidiosis Recorded by the North West England Surveillance System in 1990-1999. J. Water Health 2005, 3, 185–196. [Google Scholar] [CrossRef]
- Al-Hasan, M.N.; Lahr, B.D.; Eckel-Passow, J.E.; Baddour, L.M. Seasonal Variation in Escherichia Coli Bloodstream Infection: A Population-Based Study. Clin. Microbiol. Infect. 2009, 15, 947–950. [Google Scholar] [CrossRef]
- Eber, M.R.; Shardell, M.; Schweizer, M.L.; Laxminarayan, R.; Perencevich, E.N. Seasonal and Temperature-Associated Increases in Gram-Negative Bacterial Bloodstream Infections among Hospitalized Patients. PLoS ONE 2011, 6, e25298. [Google Scholar] [CrossRef]
- Freeman, J.T.; Anderson, D.J.; Sexton, D.J. Seasonal Peaks in Escherichia Coli Infections: Possible Explanations and Implications. Clin. Microbiol. Infect. 2009, 15, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Talbot, T.R.; Poehling, K.A.; Hartert, T.V.; Arbogast, P.G.; Halasa, N.B.; Edwards, K.M.; Schaffner, W.; Craig, A.S.; Griffin, M.R. Seasonality of Invasive Pneumococcal Disease: Temporal Relation to Documented Influenza and Respiratory Syncytial Viral Circulation. Am. J. Med. 2005, 118, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.H.; Armstrong, C.W.; Jenkins, S.R.; Harmon, M.W.; Ajello, G.W.; Miller, G.B., Jr.; Broome, C.V. A Cluster of Meningococcal Disease on a School Bus Following Epidemic Influenza. Arch. Intern. Med. 1991, 151, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Fisman, D.N. Seasonality of Infectious Diseases. Annu. Rev. Public Health 2007, 28, 127–143. [Google Scholar] [CrossRef]
- Cherrie, M.P.C.; Nichols, G.; Iacono, G.L.; Sarran, C.; Hajat, S.; Fleming, L.E. Pathogen Seasonality and Links with Weather in England and Wales: A Big Data Time Series Analysis. BMC Public Health 2018, 18, 1067. [Google Scholar] [CrossRef]
- Abat, C.; Chaudet, H.; Rolain, J.-M.; Colson, P.; Raoult, D. Traditional and Syndromic Surveillance of Infectious Diseases and Pathogens. Int. J. Infect. Dis. 2016, 48, 22–28. [Google Scholar] [CrossRef]
- Alarcon Falconi, T.M.; Estrella, B.; Sempértegui, F.; Naumova, E.N. Effects of Data Aggregation on Time Series Analysis of Seasonal Infections. Int. J. Environ. Res. Public Health 2020, 17, 5887. [Google Scholar] [CrossRef]
- Kwiatkowski, D.; Phillips, P.C.B.; Schmidt, P.; Shin, Y. Testing the Null Hypothesis of Stationarity against the Alternative of a Unit Root. J. Econom. 1992, 54, 159–178. [Google Scholar] [CrossRef]
- Cleveland, R.B.; Cleveland, W.S.; McRae, J.E.; Terpenning, I.J. A Seasonal-Trend Decomposition Procedure Based on Loess. J. Off. Stat. 1990, 6, 3–33. [Google Scholar]
- De Livera, A.M.; Hyndman, R.J.; Snyder, R.D. Forecasting Time Series with Complex Seasonal Patterns Using Exponential Smoothing. J. Am. Stat. Assoc. 2011, 106, 1513–1527. [Google Scholar] [CrossRef]
- Probst, W.N.; Stelzenmüller, V.; Fock, H.O. Using Cross-Correlations to Assess the Relationship Between Time-Lagged Pressure and State Indicators: An Exemplary Analysis of North Sea Fish Population Indicators. ICES J. Mar. Sci. 2012, 69, 670–681. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 10 March 2021).
- Zhang, X.; Zhang, T.; Young, A.A.; Li, X. Applications and Comparisons of Four Time Series Models in Epidemiological Surveillance Data. PLoS ONE 2014, 9, e88075. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhou, S.; Zhang, S.; Wang, H.; Tang, L. Temporal Correlation Analysis between Malaria and Meteorological Factors in Motuo County, Tibet. Malar. J. 2011, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Warren-Gash, C.; Bhaskaran, K.; Hayward, A.; Leung, G.M.; Lo, S.-V.; Wong, C.-M.; Ellis, J.; Pebody, R.; Smeeth, L.; Cowling, B.J. Circulating Influenza Virus, Climatic Factors, and Acute Myocardial Infarction: A Time Series Study in England and Wales and Hong Kong. J. Infect. Dis. 2011, 203, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Banerjee, S.N.; Jarvis, W.R. National Nosocomial Infections Surveillance System Seasonal Variation of Acinetobacter Infections: 1987–1996. Clin. Infect. Dis. 1999, 29, 1133–1137. [Google Scholar] [CrossRef]
- Richet, H. Seasonality in Gram-Negative and Healthcare-Associated Infections. Clin. Microbiol. Infect. 2012, 18, 934–940. [Google Scholar] [CrossRef]
- Dailiana, Z.H.; Rigopoulos, N.; Varitimidis, S.E.; Poultsides, L.; Petinaki, E.; Malizos, K.N. Clinical and Epidemiological Features of Upper-Extremity Infections Caused by Staphylococcus aureus Carrying the PVL Gene: A Four-Year Study in Greece. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2008, 14, CR511-4. [Google Scholar]
- Tveten, Y.; Jenkins, A.; Kristiansen, B.-E. A Fusidic Acid-Resistant Clone of Staphylococcus aureus Associated with Impetigo Bullosa Is Spreading in Norway. J. Antimicrob. Chemother. 2002, 50, 873–876. [Google Scholar] [CrossRef]
- Leekha, S.; Diekema, D.J.; Perencevich, E.N. Seasonality of Staphylococcal Infections. Clin. Microbiol. Infect. 2012, 18, 927–933. [Google Scholar] [CrossRef]
- Shaman, J.; Kohn, M. Absolute Humidity Modulates Influenza Survival, Transmission, and Seasonality. Proc. Natl. Acad. Sci. USA 2009, 106, 3243–3248. [Google Scholar] [CrossRef]





| N° | Pathogens | Min | 1st Qu | Median | Mean | 3rd Qu | Max |
|---|---|---|---|---|---|---|---|
| 1 | Enterobacter cloacae | 3 | 13 | 16 | 16.60 | 20 | 34 |
| 2 | Enterococcus faecalis | 9 | 23 | 27 | 27.75 | 32 | 48 |
| 3 | Escherichia coli | 109 | 135 | 145 | 145.4 | 155 | 203 |
| 4 | Gardnerella vaginalis | 3 | 16 | 22 | 23.46 | 30 | 51 |
| 5 | Haemophilus influenzae | 1 | 7 | 9 | 10.03 | 13 | 28 |
| 6 | Klebsiella oxytoca | 0 | 4 | 6 | 6.02 | 8 | 16 |
| 7 | Klebsiella pneumoniae | 18 | 31 | 35 | 36.06 | 41 | 66 |
| 8 | Propionibacterium acnes | 0 | 4 | 6 | 6.35 | 9 | 22 |
| 9 | Proteus mirabilis | 5 | 11 | 14 | 14.03 | 16 | 33 |
| 10 | Pseudomonas aeruginosa | 18 | 29 | 34 | 33.96 | 39 | 55 |
| 11 | Staphylococcus aureus | 45 | 67 | 74 | 74.04 | 80 | 111 |
| 12 | Staphylococcus epidermidis | 22 | 35 | 39 | 39.38 | 44 | 66 |
| 13 | Staphylococcus haemolyticus | 1 | 4 | 6 | 6.54 | 8 | 19 |
| 14 | Staphylococcus hominis | 0 | 6 | 8 | 8.04 | 10 | 23 |
| 15 | Streptococcus agalactiae | 7 | 18 | 22 | 21.52 | 26 | 39 |
| Pathogens | Sample Size | Hospital-Associated Infections | p-Value | Community-Acquired Infections | p-Value | ||
|---|---|---|---|---|---|---|---|
| Samples | Drivers | Samples | Drivers | ||||
| S. aureus | 23,173 | all samples | temperature | 0.009 | all samples | rain | 0.004 |
| humidity | 0.011 | urines | rain | 0.037 | |||
| pressure change | 0.013 | pressure change | 0.039 | ||||
| urines | rain | 0.021 | |||||
| S. epidermidis | 12,325 | resp | rain | 0.018 | |||
| humidity | 0.030 | ||||||
| K. pneumoniae | 11,287 | urines | humidity | 0.016 | |||
| P. aeruginosa | 10,631 | blood | rain | 0.020 | blood | humidity | 0.019 |
| skin | rain | 0.043 | |||||
| pressure change | 0.015 | ||||||
| G. vaginalis | 7344 | all samples | rain | 0.013 | |||
| urines | temperature | 0.011 | |||||
| S. agalactiae | 6735 | urines | temperature | 0.005 | resp | rain | 0.010 |
| rain | 0.013 | humidity | 0.029 | ||||
| E. cloacae | 5195 | all samples | wind | 0.045 | all samples | humidity | 0.002 |
| pressure change | 0.019 | ||||||
| H. influenzae | 3139 | all samples | humidity | 0.013 | all samples | humidity | 0.022 |
| blood | humidity | 0.008 | pressure change | 0.004 | |||
| blood | rain | 0.023 | |||||
| pressure change | 0.011 | ||||||
| urines | wind | 0.049 | |||||
| S. hominis | 2516 | urines | pressure change | 0.043 | |||
| S. haemolyticus | 2048 | all samples | humidity | 0.024 | |||
| urines | rain | 0.023 | |||||
| humidity | 0.033 | ||||||
| wind | 0.046 | ||||||
| P. acnes | 1986 | all samples | humidity | 0.016 | |||
| skin | rain | 0.001 | |||||
| K. oxytoca | 1884 | all samples | rain | 0.041 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaba, L.; Giraud-Gatineau, A.; Colson, P.; Fournier, P.-E.; Chaudet, H. Influence of Infection Origin, Type of Sampling, and Weather Factors on the Periodicity of Some Infectious Pathogens in Marseille University Hospitals, France. Bacteria 2025, 4, 4. https://doi.org/10.3390/bacteria4010004
Kaba L, Giraud-Gatineau A, Colson P, Fournier P-E, Chaudet H. Influence of Infection Origin, Type of Sampling, and Weather Factors on the Periodicity of Some Infectious Pathogens in Marseille University Hospitals, France. Bacteria. 2025; 4(1):4. https://doi.org/10.3390/bacteria4010004
Chicago/Turabian StyleKaba, Lanceï, Audrey Giraud-Gatineau, Philippe Colson, Pierre-Edouard Fournier, and Hervé Chaudet. 2025. "Influence of Infection Origin, Type of Sampling, and Weather Factors on the Periodicity of Some Infectious Pathogens in Marseille University Hospitals, France" Bacteria 4, no. 1: 4. https://doi.org/10.3390/bacteria4010004
APA StyleKaba, L., Giraud-Gatineau, A., Colson, P., Fournier, P.-E., & Chaudet, H. (2025). Influence of Infection Origin, Type of Sampling, and Weather Factors on the Periodicity of Some Infectious Pathogens in Marseille University Hospitals, France. Bacteria, 4(1), 4. https://doi.org/10.3390/bacteria4010004







