Case Report: Inflammation-Driven Species-Level Shifts in the Oral Microbiome of Refractory Feline Chronic Gingivostomatitis
Abstract
1. Introduction
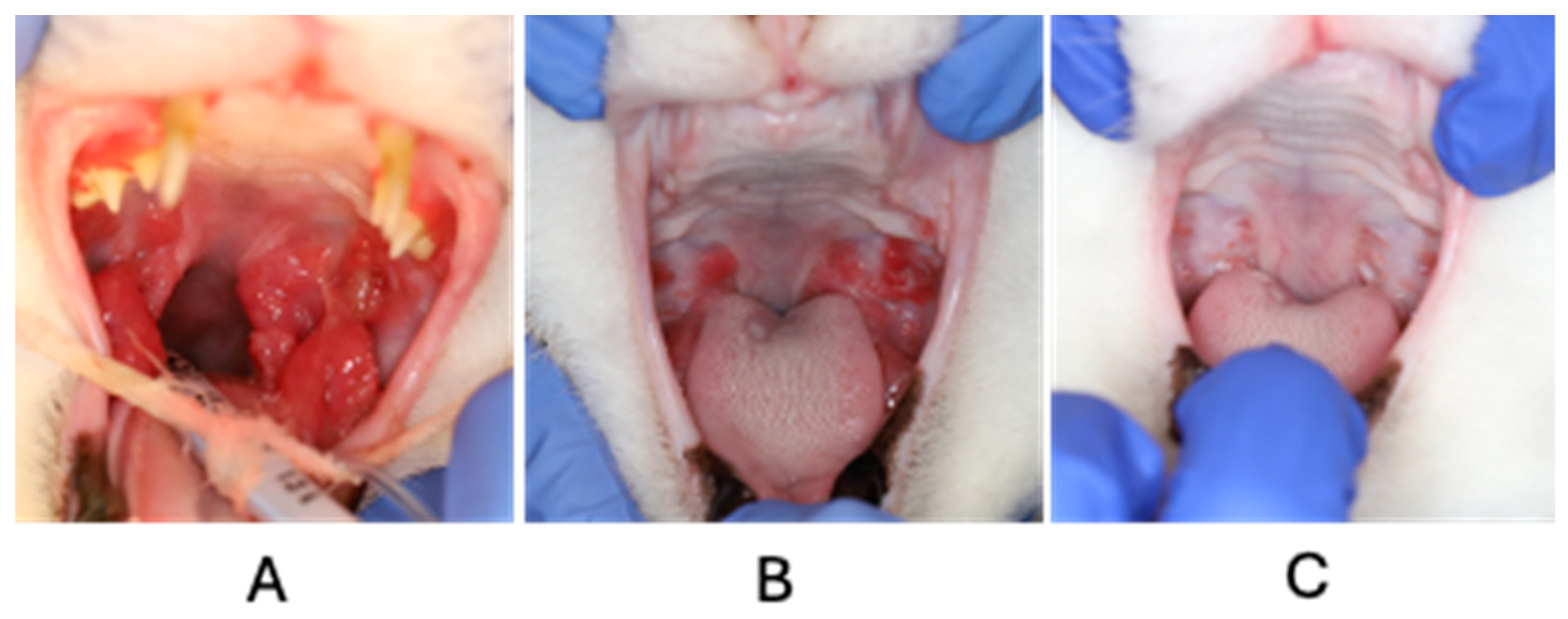
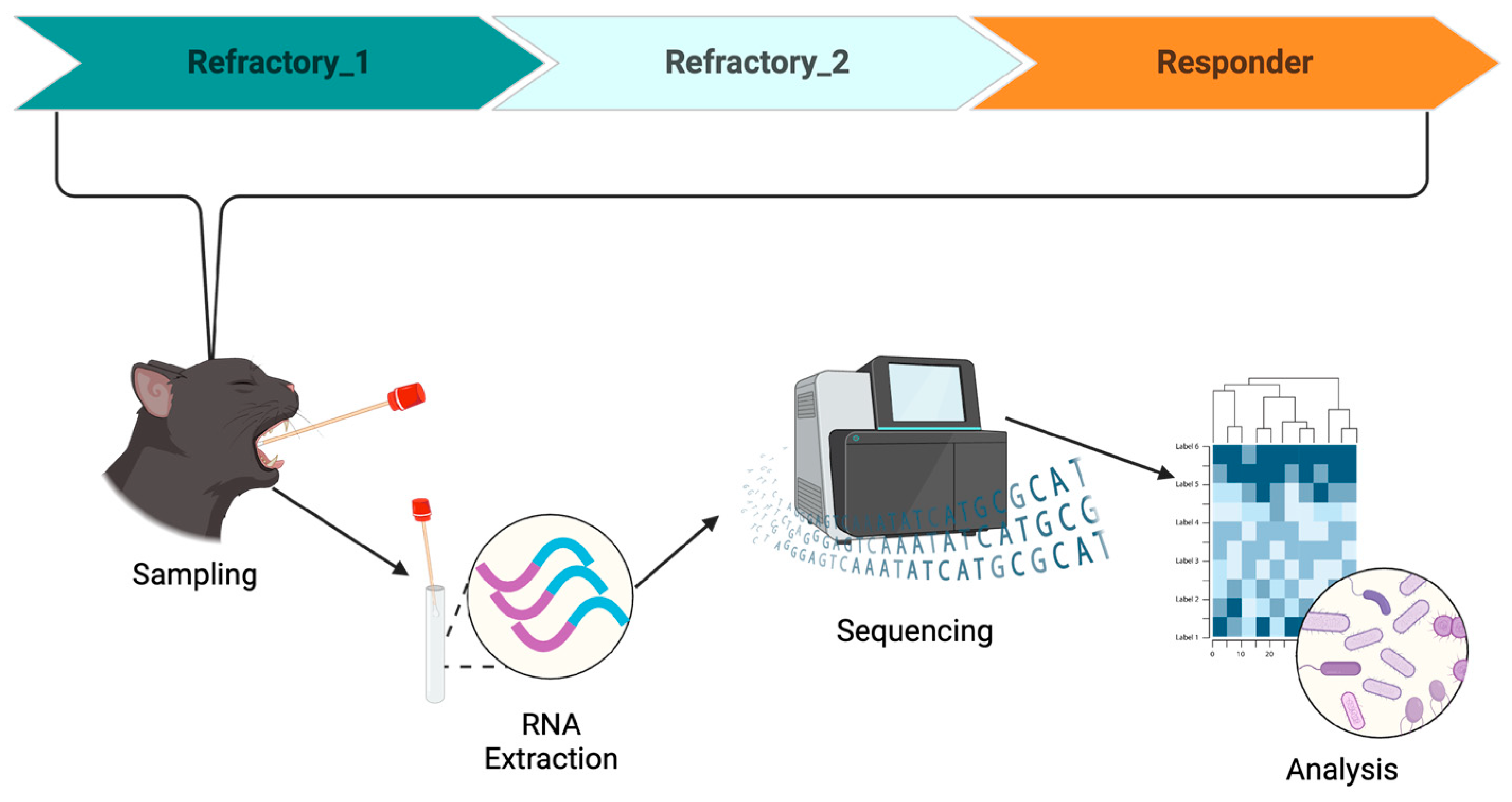
2. Case Description
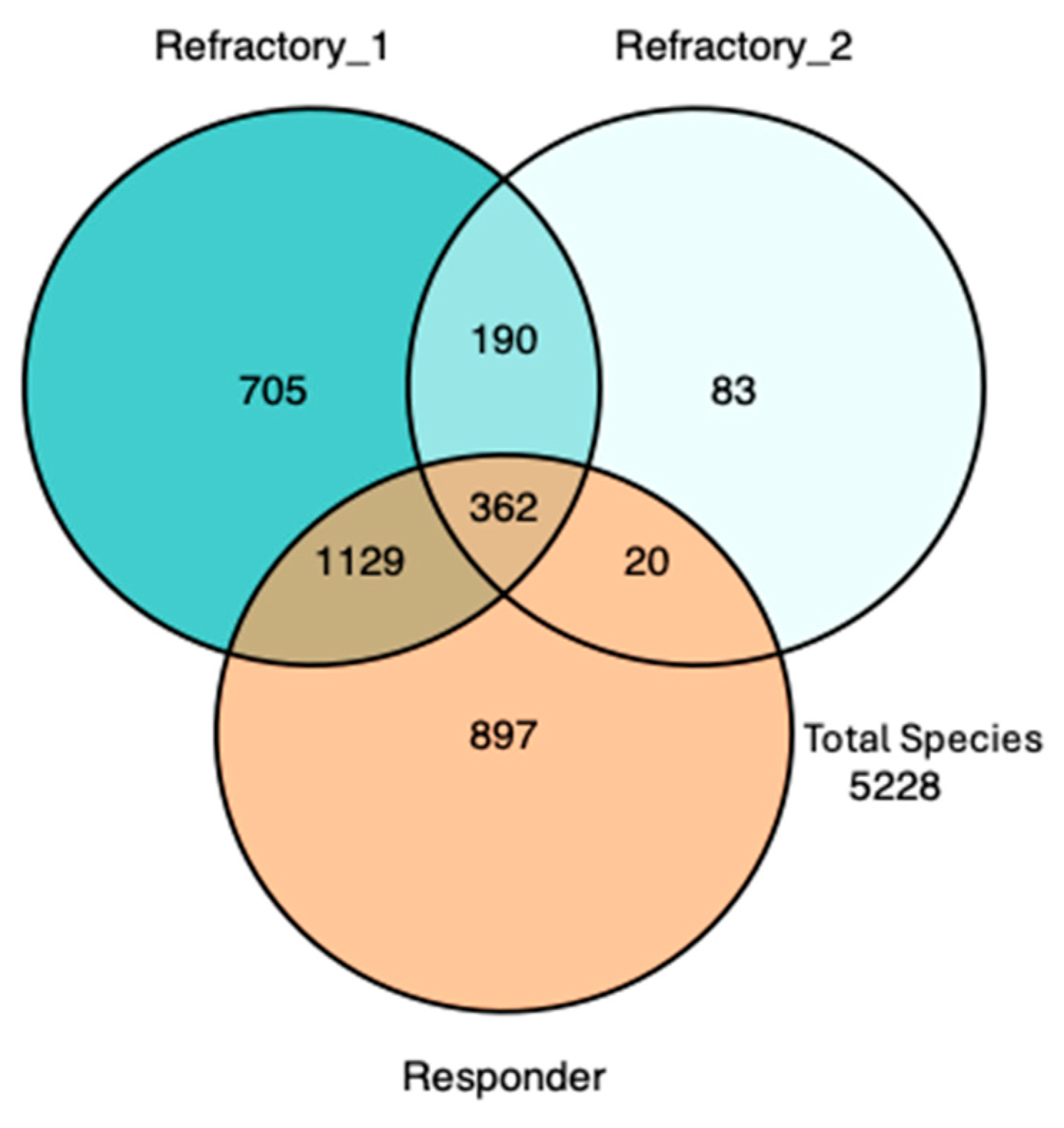
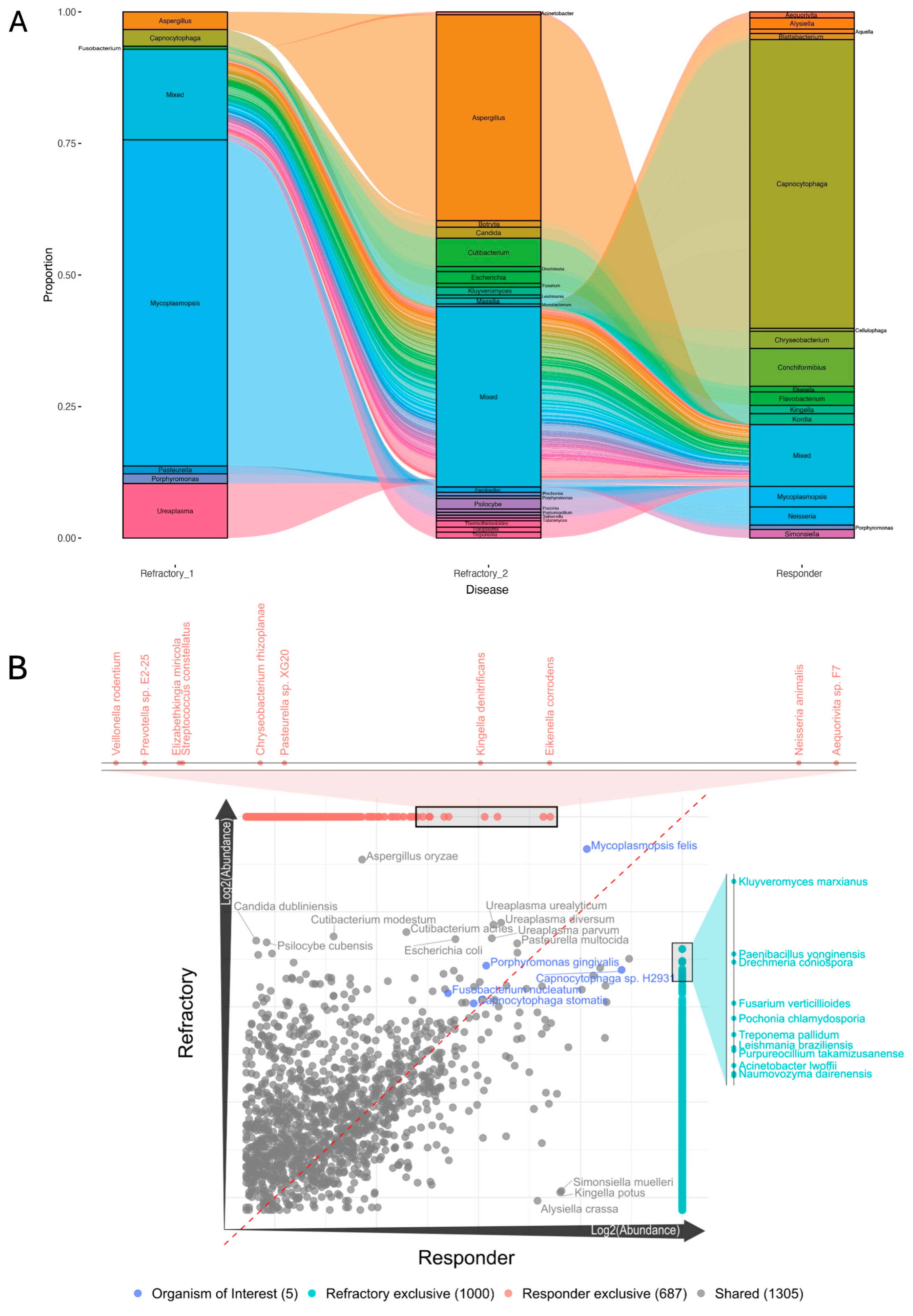
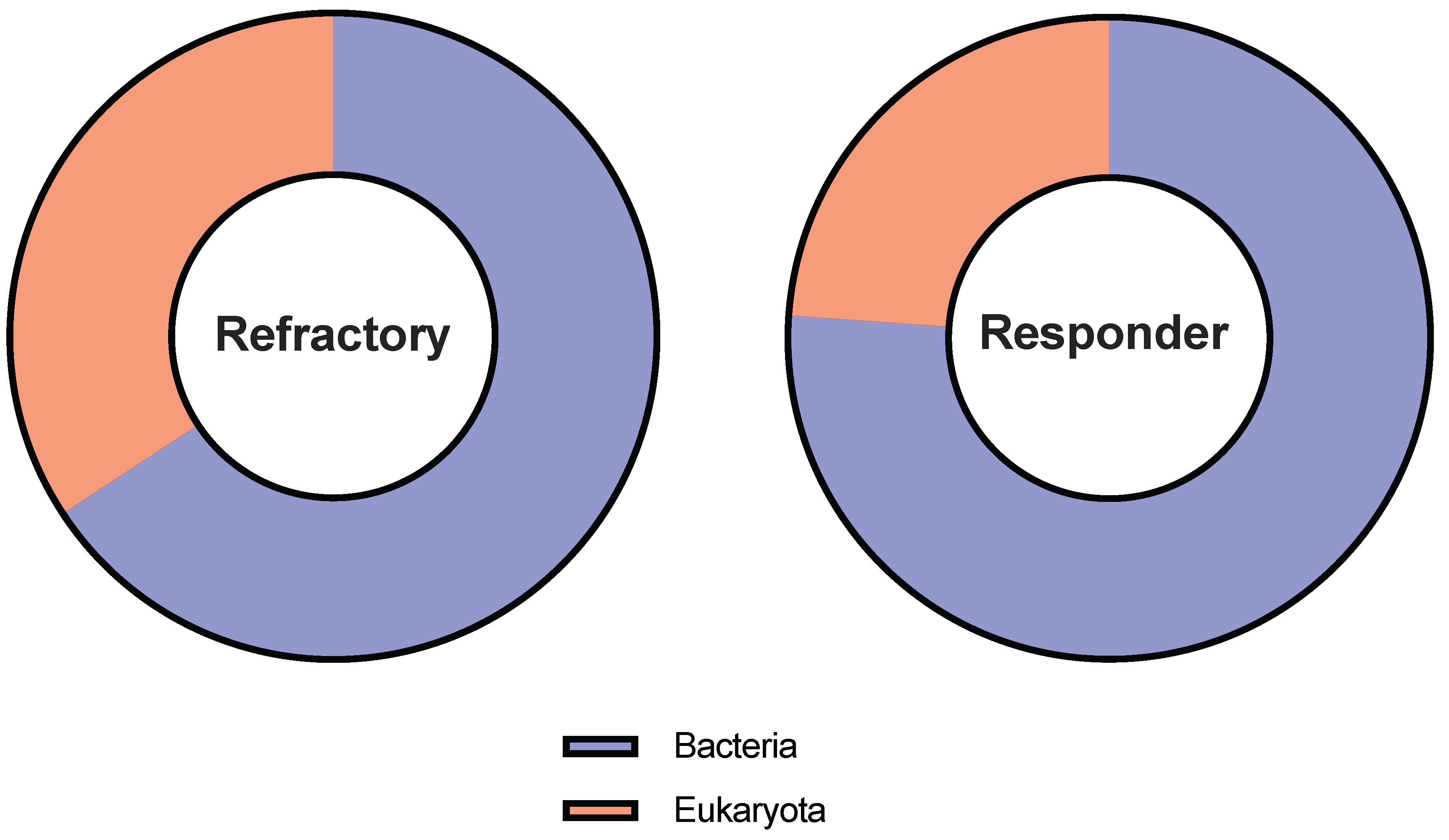
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, E.M.; Weese, J.S. Oral Microbiome in Dogs and Cats: Dysbiosis and the Utility of Antimicrobial Therapy in the Treatment of Periodontal Disease. Veter. Clin. N. Am. Small Anim. Pract. 2021, 52, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Reiter, A.M.; Pohl, J.C.; Tang, S.; Kim, Y.J.; Linde, A.; Prem, A.; Melgarejo, T. Characterization of Oral Microbiota in Cats: Novel Insights on the Potential Role of Fungi in Feline Chronic Gingivostomatitis. Pathogens 2021, 10, 904. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Davis, E.M. Gene Sequence Analyses of the Healthy Oral Microbiome in Humans and Companion Animals. J. Veter. Dent. 2016, 33, 97–107. [Google Scholar] [CrossRef]
- Rodrigues, M.X.; Bicalho, R.C.; Fiani, N.; Lima, S.F.; Peralta, S. The subgingival microbial community of feline periodontitis and gingivostomatitis: Characterization and comparison between diseased and healthy cats. Sci. Rep. 2019, 9, 12340. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.; Croft, J.; O’flynn, C.; Deusch, O.; Colyer, A.; Allsopp, J.; Milella, L.; Davis, I.J. A Pyrosequencing Investigation of Differences in the Feline Subgingival Microbiota in Health, Gingivitis and Mild Periodontitis. PLoS ONE 2015, 10, e0136986. [Google Scholar] [CrossRef]
- Lin, D.; Yang, L.; Wen, L.; Lu, H.; Chen, Q.; Wang, Z. Crosstalk between the oral microbiota, mucosal immunity, and the epithelial barrier regulates oral mucosal disease pathogenesis. Mucosal Immunol. 2021, 14, 1247–1258. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Yerezhepov, A.Y.; Shardarbek, R.S.; Wu, X.; Zha, J. Nutritional factors influencing microbiota-mediated colonization resistance of the oral cavity: A literature review. Front. Nutr. 2022, 9, 1029324. [Google Scholar] [CrossRef]
- Soltero-Rivera, M.; Goldschmidt, S.; Arzi, B. Feline chronic gingivostomatitis: Current concepts in clinical management. J. Feline Med. Surg. 2023, 25. [Google Scholar] [CrossRef]
- Soltero-Rivera, M.; Shaw, C.; Arzi, B.; Lommer, M.; Weimer, B.C. Feline Chronic Gingivostomatitis Diagnosis and Treatment through Transcriptomic Insights. Pathogens 2024, 13, 192. [Google Scholar] [CrossRef]
- Soltero-Rivera, M.; Vapniarsky, N.; Rivas, I.L.; Arzi, B. Clinical, radiographic and histopathologic features of early-onset gingivitis and periodontitis in cats (1997–2022). J. Feline Med. Surg. 2023, 25. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.N.; Arzi, B.; Verstraete, F.J.M. Therapeutic Management of Feline Chronic Gingivostomatitis: A Systematic Review of the Literature. Front. Veter. Sci. 2016, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Vulturar, R.; Buduru, S.; Cozma, A.; Fodor, A.; Chiș, A.; Lucaciu, O.; Damian, L.; Moldovan, M.L. Oral Microbiome: Getting to Know and Befriend Neighbors, a Biological Approach. Biomedicines 2022, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, T.; Shiba, T.; Komatsu, K.; Watanabe, T.; Shimogishi, M.; Shibasaki, M.; Koyanagi, T.; Nagai, T.; Katagiri, S.; Takeuchi, Y.; et al. Discrimination of Bacterial Community Structures among Healthy, Gingivitis, and Periodontitis Statuses through Integrated Metatranscriptomic and Network Analyses. mSystems 2021, 6, e0088621. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.G.; Rojas, C.A.; Scarsella, E.; Entrolezo, Z.; Jospin, G.; Hoffman, S.L.; Force, J.; MacLellan, R.H.; Peak, M.; Shope, B.H.; et al. The Oral Microbiome across Oral Sites in Cats with Chronic Gingivostomatitis, Periodontal Disease, and Tooth Resorption Compared with Healthy Cats. Animals 2023, 13, 3544. [Google Scholar] [CrossRef]
- Fried, W.A.; Soltero-Rivera, M.; Ramesh, A.; Lommer, M.J.; Arzi, B.; DeRisi, J.L.; Horst, J.A. Use of unbiased metagenomic and transcriptomic analyses to investigate the association between feline calicivirus and feline chronic gingivostomatitis in domestic cats. Am. J. Veter. Res. 2021, 82, 381–394. [Google Scholar] [CrossRef]
- Peralta, S.; Grenier, J.K.; Webb, S.M.; Miller, A.D.; Miranda, I.C.; Parker, J.S.L. Transcriptomic signatures of feline chronic gingivostomatitis are influenced by upregulated IL6. Sci. Rep. 2023, 13, 13437. [Google Scholar] [CrossRef]
- Hong, B.-Y.; Sobue, T.; Choquette, L.; Dupuy, A.K.; Thompson, A.; Burleson, J.A.; Salner, A.L.; Schauer, P.K.; Joshi, P.; Fox, E.; et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 2019, 7, 66. [Google Scholar] [CrossRef]
- Negrini, T.d.C.; Carlos, I.Z.; Duque, C.; Caiaffa, K.S.; Arthur, R.A. Interplay Among the Oral Microbiome, Oral Cavity Conditions, the Host Immune Response, Diabetes Mellitus, and Its Associated-Risk Factors—An Overview. Front. Oral Health 2021, 2, 697428. [Google Scholar] [CrossRef]
- Moreno, C.M.; Boeree, E.; Freitas, C.M.T.; Weber, K.S. Immunomodulatory role of oral microbiota in inflammatory diseases and allergic conditions. Front. Allergy 2023, 4, 1067483. [Google Scholar] [CrossRef]
- Dolieslager, S.M.J.; Lappin, D.F.; Bennett, D.; Graham, L.; Johnston, N.; Riggio, M.P. The influence of oral bacteria on tissue levels of Toll-like receptor and cytokine mRNAs in feline chronic gingivostomatitis and oral health. Veter Immunol. Immunopathol. 2013, 151, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Yama, K.; Nishimoto, Y.; Kumagai, K.; Jo, R.; Harada, M.; Maruyama, Y.; Aita, Y.; Fujii, N.; Inokuchi, T.; Kawamata, R.; et al. Dysbiosis of oral microbiome persists after dental treatment-induced remission of periodontal disease and dental caries. mSystems 2023, 8, e0068323. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Ng, W.; Lee, V.; Kelly, L.; Weimer, B. Production and analysis of high molecular weight genomic DNA for NGS pipelines using Agilent DNA extraction kit (p/n 200600). In Agilent Technologies Application Note; Agilent Technologies: Santa Clara, CA, USA, 2013. [Google Scholar]
- Basbas, C.; Garzon, A.; Schlesener, C.; van Heule, M.; Profeta, R.; Weimer, B.C.; Silva-Del-Rio, N.; Byrne, B.A.; Karle, B.; Aly, S.S.; et al. Unveiling the microbiome during post-partum uterine infection: A deep shotgun sequencing approach to characterize the dairy cow uterine microbiome. Anim. Microbiome 2023, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Garzon, A.; Basbas, C.; Schlesener, C.; Silva-Del-Rio, N.; Karle, B.M.; Lima, F.S.; Weimer, B.C.; Pereira, R.V. WGS of intrauterine E. coli from cows with early postpartum uterine infection reveals a non-uterine specific genotype and virulence factors. mBio 2024, 15, e0102724. [Google Scholar] [CrossRef]
- Beck, K.L.; Haiminen, N.; Chambliss, D.; Edlund, S.; Kunitomi, M.; Huang, B.C.; Kong, N.; Ganesan, B.; Baker, R.; Markwell, P.; et al. Monitoring the microbiome for food safety and quality using deep shotgun sequencing. npj Sci. Food 2021, 5, 3. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- Lu, J.; Breitwieser, F.P.; Thielen, P.; Salzberg, S.L. Bracken: Estimating species abundance in metagenomics data. PeerJ Comput. Sci. 2017, 3, e104. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; A Wlodarski, M.; Edalatmand, A.; Petkau, A.; A Syed, S.; Tsang, K.K.; et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2022, 51, D690–D699. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.; Sun, L.; Yu, J.; Jin, Q. VFDB 2008 release: An enhanced web-based resource for comparative pathogenomics. Nucleic Acids Res. 2007, 36, D539–D542. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, A.; Pinder, S.L.; Costa, M.C.; Weese, J.S. Characterization of the oral microbiota of healthy cats using next-generation sequencing. Vet. J. 2014, 201, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, A.; Kuboniwa, M.; Shimma, S.; Alghamdi, S.A.; Mayumi, S.; Lamont, R.J.; Fukusaki, E.; Amano, A. Fusobacterium nucleatum Metabolically Integrates Commensals and Pathogens in Oral Biofilms. mSystems 2022, 7, e0017022. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhou, F.; Wang, H.; Xu, X.; Xu, S.; Zhang, C.; Zhang, Y.; Lu, M.; Zhang, Y.; Zhou, M.; et al. Systemic antibiotics increase microbiota pathogenicity and oral bone loss. Int. J. Oral Sci. 2023, 15, 4. [Google Scholar] [CrossRef]
- Bartold, P.M.; Van Dyke, T.E. An appraisal of the role of specific bacteria in the initial pathogenesis of periodontitis. J. Clin. Periodontol. 2018, 46, 6–11. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The Nexus Between Periodontal Inflammation and Dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef]
- Blackmore, D.K.; Hill, A.; Jackson, O.F. The incidence of mycoplasma in pet and colony maintained cats. J. Small Anim. Pract. 1971, 12, 207–217. [Google Scholar] [CrossRef]
- Foster, S.; Martin, P.; Braddock, J.; Malik, R. A retrospective analysis of feline bronchoalveolar lavage cytology and microbiology (1995–2000). J. Feline Med. Surg. 2004, 6, 189–198. [Google Scholar] [CrossRef]
- Bemis, D.A. Bordetella and Mycoplasma Respiratory Infections in Dogs and Cats. Veter. Clin. N. Am. Small Anim. Pract. 1992, 22, 1173–1186. [Google Scholar] [CrossRef]
- Hooper, P.T.; Ireland, L.A.; Carter, A. Mycoplasma polyarthritis in a cat with probable severe immune deficiency. Aust. Veter J. 1985, 62, 352. [Google Scholar] [CrossRef]
- Campbell, L.; Fox, J.; Snyder, S. Ocular bacteria and mycoplasma of the clinically normal cat. Feline Pract. 1973, 3, 10–12. [Google Scholar]
- Jin, F.-J.; Hu, S.; Wang, B.-T.; Jin, L. Advances in Genetic Engineering Technology and Its Application in the Industrial Fungus Aspergillus oryzae. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Ledder, R.G.; Madhwani, T.; Sreenivasan, P.K.; De Vizio, W.; McBain, A.J. An in vitro evaluation of hydrolytic enzymes as dental plaque control agents. J. Med. Microbiol. 2009, 58, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.M.L.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Barachetti, L.; Mortellaro, C.M.; Di Giancamillo, M.; Giudice, C.; Martino, P.; Travetti, O.; Miller, P.E. Bilateral orbital and nasal aspergillosis in a cat. Veter. Ophthalmol. 2009, 12, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Renner, K.; Hill, S.; Grinberg, A.; Weeden, A. Pancreatic candidiasis in a cat. J. Feline Med. Surg. 2021, 7. [Google Scholar] [CrossRef]
- Reagan, K.L.; Dear, J.D.; Kass, P.H.; Sykes, J.E. Risk factors for Candida urinary tract infections in dogs and cats. J. Veter. Intern. Med. 2019, 33, 648–653. [Google Scholar] [CrossRef]
- Hennet, P.R.; Camy, G.A.; McGahie, D.M.; Albouy, M.V. Comparative efficacy of a recombinant feline interferon omega in refractory cases of calicivirus-positive cats with caudal stomatitis: A randomised, multi-centre, controlled, double-blind study in 39 cats. J. Feline Med. Surg. 2011, 13, 577–587. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaw, C.A.; Soltero-Rivera, M.; Profeta, R.; Weimer, B.C. Case Report: Inflammation-Driven Species-Level Shifts in the Oral Microbiome of Refractory Feline Chronic Gingivostomatitis. Bacteria 2025, 4, 1. https://doi.org/10.3390/bacteria4010001
Shaw CA, Soltero-Rivera M, Profeta R, Weimer BC. Case Report: Inflammation-Driven Species-Level Shifts in the Oral Microbiome of Refractory Feline Chronic Gingivostomatitis. Bacteria. 2025; 4(1):1. https://doi.org/10.3390/bacteria4010001
Chicago/Turabian StyleShaw, Claire A., Maria Soltero-Rivera, Rodrigo Profeta, and Bart C. Weimer. 2025. "Case Report: Inflammation-Driven Species-Level Shifts in the Oral Microbiome of Refractory Feline Chronic Gingivostomatitis" Bacteria 4, no. 1: 1. https://doi.org/10.3390/bacteria4010001
APA StyleShaw, C. A., Soltero-Rivera, M., Profeta, R., & Weimer, B. C. (2025). Case Report: Inflammation-Driven Species-Level Shifts in the Oral Microbiome of Refractory Feline Chronic Gingivostomatitis. Bacteria, 4(1), 1. https://doi.org/10.3390/bacteria4010001






