Combining Proteomics and Organoid Research to Unravel the Multifunctional Complexity of Kidney Physiology Enhances the Need for Controlled Organoid Maturation
Abstract
1. Methods
2. Using Proteomics to Study Organ-Specific Spatial and Dynamic Proteome Patterns in Healthy and Pathological Conditions in Human Kidneys
3. Techniques to Generate Complex Renal Organoids
| Ref | Cell Source | Method | Advantage | Limitations | Clinical Model? |
|---|---|---|---|---|---|
| [21] | hPSC | suspension cell cultue | cost effective, high throughput possible | no flow or vascularization, no CDs, limited organoid size | |
| [30] | hPSC | polyester membrane | enhanced sprouting and interconnectivity | batch to batch variability | |
| physiological hypoxia | increased vessel length | ambient oxygen conc during handling | |||
| [27] | hPSC | permeable 3D nanofibre membrane (UniMat) | enhanced uniformity, enhanced maturation | not commercially available, permeability and scattering limits experimental design | PKD, AKI |
| [28] | hPSC | semi-synthetic hydrogel | improved maturation, enhanced reproducibility, controlled environment | DKD | |
| [29] | hPSC | alginate hydrogel | improved extracellular matrix composition | no structural improvement | |
| [22] | hUB, hUB tip | soft hydrogel, co-culture | epithelial polarity, tubular lumen, branching, CD progenitors | immature CDs | MCDK |
| [24,25] | hPSC derived UB and NPCs | co-culture | structurally integrated CDs | efficiency of nephron fusion to CDs and organization needs to be increased | |
| [33] | hPSC | co-culture, genetically induced endothelial niche | improved endotheliazation, improved maturation, drug-responsive renin expressing cells | ||
| [34] | hPSC, HUVECs | microfluidic organ-on-chip, co-culture | increased endothelial maturation, vascularization | no high throughput, transfer of organoids from transwell to chip necessary | |
| [31] | hPSC | microbioreactor array | constant perfusion | no strong maturation, high throughput possible | |
| [23] | mRPCs, hPSC derived NPCs | human-mouse chimera, transplantation, magnetic sorting of NPCs | increased NPC purity and increased hNPC:mRPC ratio, increased chimera formation | mouse model, animals needed, elaborate protocols | transplantation research |
| [26] | mPSC | in vitro induction protocol for SPs | complex structures | mouse model, animals needed, elaborate protocols | |
| co-culture with NP and UB | |||||
| transplantation | |||||
| [32] | hPSC | transplantation | improved vascularization, improved maturation and morphogenesis | animals needed (chicken embryos), elaborate protocols | |
| major protocol categories coded by color: | |||||
| chip | |||||
| matrix | |||||
| co-culture | |||||
| transplantation | |||||
| combining co-culture and transplantation | |||||
| combining co-culture and chip | |||||
| combining co-culture and matrix | |||||
4. Proteomics to Ensure the Complex and Differentiated Maturation of Renal Organoids That Mirrors the Kidney
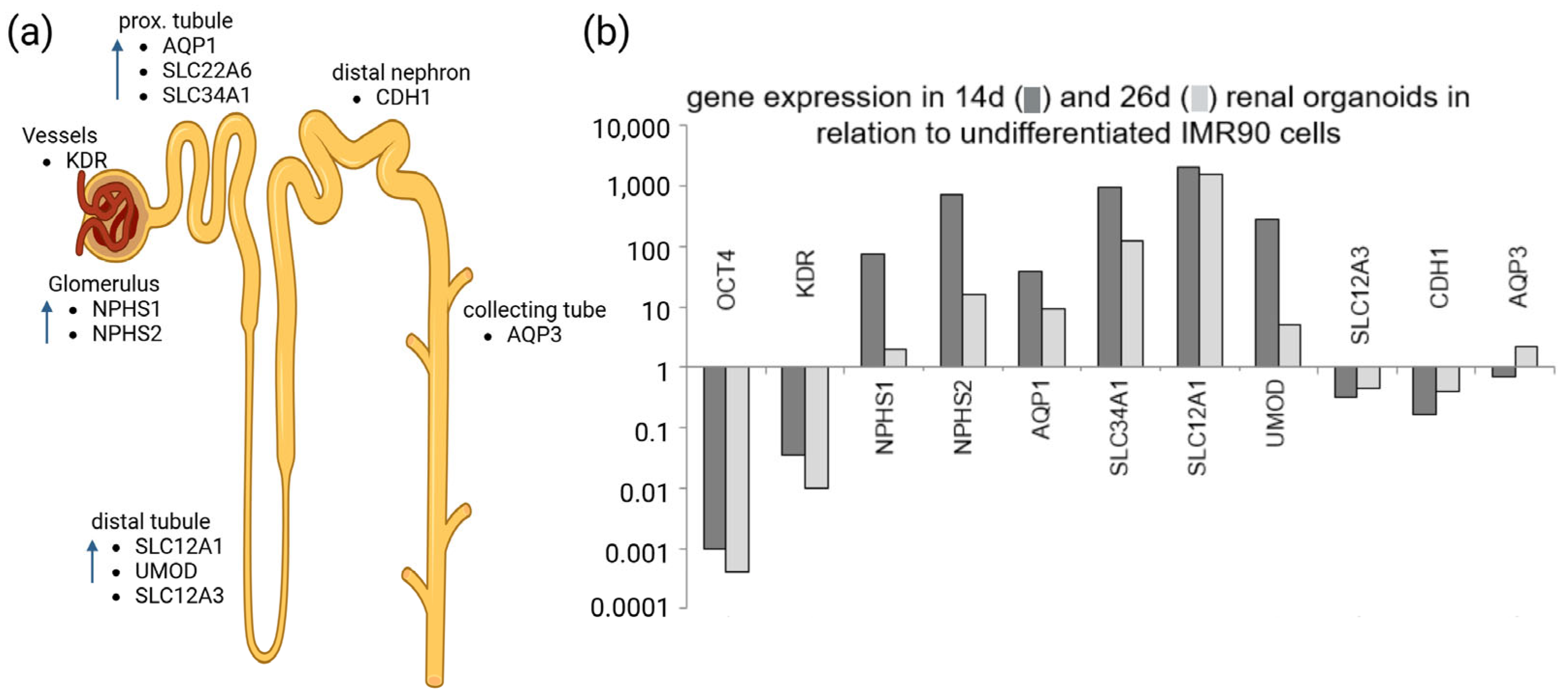
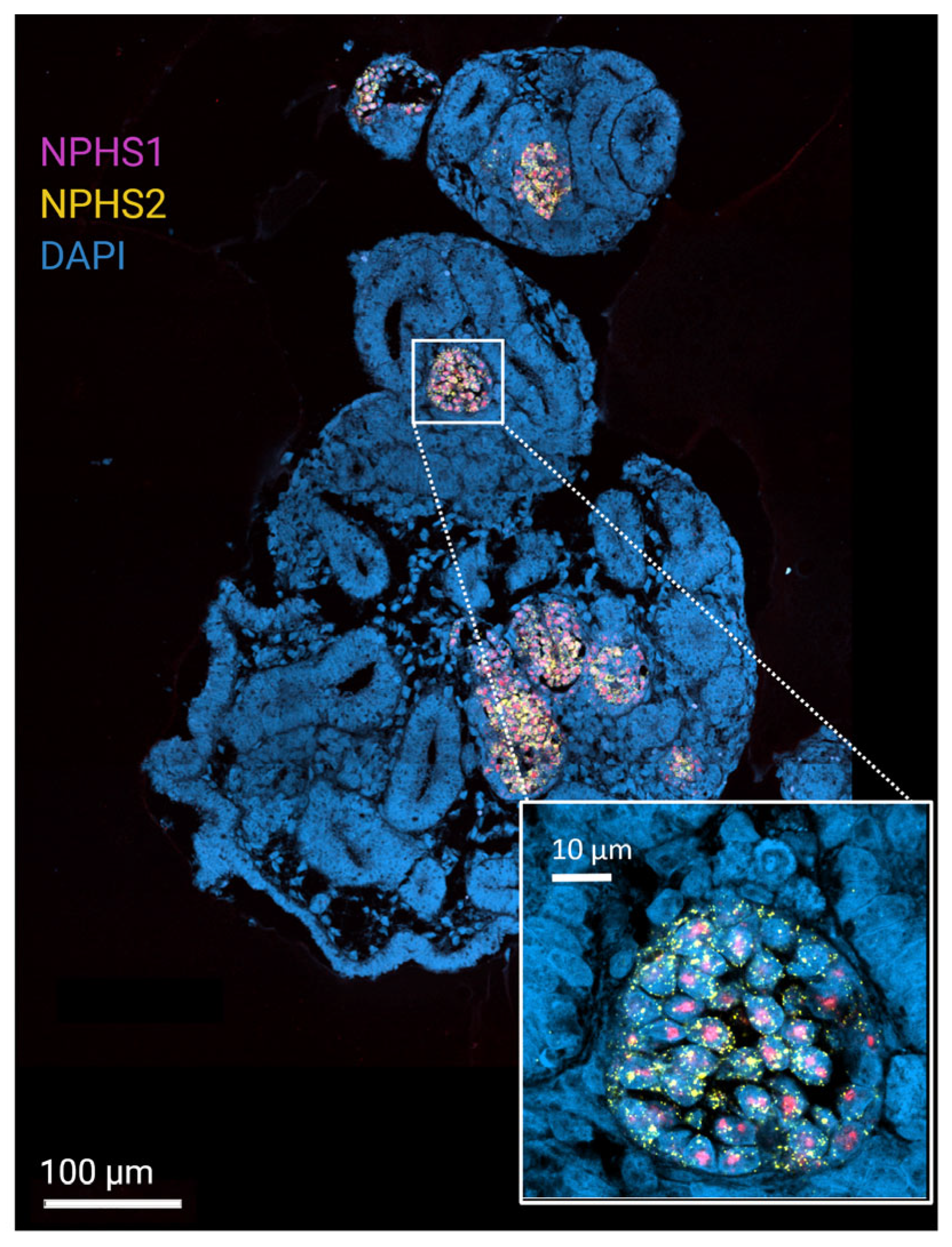
5. Proteomics to Study Healthy and Pathological Patterns in Matured Renal Organoids
6. Proteomics Methods That Are Employed for Kidney and Renal Organoid Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Consensus: AI-Powered Academic Search Engine. 2025. Available online: https://consensus.app/ (accessed on 23 June 2025).
- Ilmenau, T.U. Ai-Chatbot.tu-Ilmenau.de; Technische Universität Ilmenau: Ilmenau, Germany, 2018. [Google Scholar]
- USA. National Library of Medicine, National Center for Biotechnology Information. 2025. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 23 June 2025).
- Al-Majdoub, Z.M.; Scotcher, D.; Achour, B.; Barber, J.; Galetin, A.; Rostami-Hodjegan, A. Quantitative Proteomic Map of Enzymes and Transporters in the Human Kidney: Stepping Closer to Mechanistic Kidney Models to Define Local Kinetics. Clin. Pharmacol. Ther. 2021, 110, 1389–1400. [Google Scholar] [CrossRef]
- Hansen, J.; Sealfon, R.; Menon, R.; Eadon, M.T.; Lake, B.B.; Steck, B.; Anjani, K.; Parikh, S.; Sigdel, T.K.; Zhang, G.; et al. A reference tissue atlas for the human kidney. Sci. Adv. 2022, 8, eabn4965. [Google Scholar] [CrossRef]
- Lewis, S.; Chen, L.; Raghuram, V.; Khundmiri, S.J.; Chou, C.L.; Yang, C.R.; Knepper, M.A. “SLC-omics” of the kidney: Solute transporters along the nephron. Am. J. Physiol. Cell Physiol. 2021, 321, C507–C518. [Google Scholar] [CrossRef]
- Chen, L.; Chou, C.L.; Knepper, M.A. A Comprehensive Map of mRNAs and Their Isoforms across All 14 Renal Tubule Segments of Mouse. J. Am. Soc. Nephrol. 2021, 32, 897–912. [Google Scholar] [CrossRef]
- Limbutara, K.; Chou, C.L.; Knepper, M.A. Quantitative Proteomics of All 14 Renal Tubule Segments in Rat. J. Am. Soc. Nephrol. 2020, 31, 1255–1266. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Wang, T.; Wang, K.; Qiu, F. Integrative analysis of metabolomics and proteomics reveals mechanism of berberrubine-induced nephrotoxicity. Toxicol. Appl. Pharmacol. 2024, 488, 116992. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Heijs, B.; Kostidis, S.; Rietjens, R.G.J.; Koning, M.; Yuan, L.; Tiemeier, G.L.; Mahfouz, A.; Dumas, S.J.; Giera, M.; et al. Spatial dynamic metabolomics identifies metabolic cell fate trajectories in human kidney differentiation. Cell Stem Cell 2022, 29, 1580–1593.e1587. [Google Scholar] [CrossRef] [PubMed]
- Fédou, C.; Camus, M.; Lescat, O.; Feuillet, G.; Mueller, I.; Ross, B.; Buléon, M.; Neau, E.; Alves, M.; Goudounéche, D.; et al. Mapping of the amniotic fluid proteome of fetuses with congenital anomalies of the kidney and urinary tract identifies plastin 3 as a protein involved in glomerular integrity. J. Pathol. 2021, 254, 575–588. [Google Scholar] [CrossRef]
- Yang, H.; Liu, F.; Huang, J.; Zheng, F.; Qiu, J.; Zhu, H.; Tang, D.; Yan, Q.; Li, S.-S.; Luo, Z.; et al. Proteomic Insights into IgA Nephropathy: A Comprehensive Analysis of Differentially Expressed Proteins in the Kidney. ACS Omega 2025, 10, 17208–17220. [Google Scholar] [CrossRef]
- Dubin, R.; Rhee, E. Proteomics and Metabolomics in Kidney Disease, including Insights into Etiology, Treatment, and Prevention. Clin. J. Am. Soc. Nephrol. CJASN 2019, 15, 404–411. [Google Scholar] [CrossRef]
- Davies, E.; McDowell, G.; Oni, L.; Rao, A.; Chetwynd, A. The current use of proteomics and metabolomics in glomerulonephritis: A systematic literature review. J. Nephrol. 2024, 37, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, N.; Yun, D.; Han, D.; Ginley, B.; Moon, K.C.; Rosenberg, A.Z.; Tomaszewski, J.E.; Zee, J.; Jen, K.Y.; Han, S.S.; et al. Discovery of Novel Digital Biomarkers for Type 2 Diabetic Nephropathy Classification via Integration of Urinary Proteomics and Pathology. medRxiv 2023. [Google Scholar] [CrossRef]
- Zürbig, P.; Jerums, G.; Hovind, P.; MacIsaac, R.J.; Mischak, H.; Nielsen, S.E.; Panagiotopoulos, S.; Persson, F.; Rossing, P. Urinary Proteomics for Early Diagnosis in Diabetic Nephropathy. Diabetes 2012, 61, 3304–3313. [Google Scholar] [CrossRef] [PubMed]
- Siwy, J.; Zürbig, P.; Argiles, A.; Beige, J.; Haubitz, M.; Jankowski, J.; Julian, B.A.; Linde, P.G.; Marx, D.; Mischak, H.; et al. Noninvasive diagnosis of chronic kidney diseases using urinary proteome analysis. Nephrol. Dial. Transplant. 2016, 32, 2079–2089. [Google Scholar] [CrossRef]
- Yoodee, S.; Malaitad, T.; Plumworasawat, S.; Thongboonkerd, V. E53, E96, D162, E247 and D322 in Ca2+-binding domains of annexin A2 are essential for regulating intracellular [Ca2+] and crystal adhesion to renal cells via ERK1/2 and JNK signaling pathways. Arch. Biochem. Biophys. 2025, 769, 110410. [Google Scholar] [CrossRef]
- Phipson, B.; Er, P.X.; Combes, A.; Forbes, T.; Howden, S.; Zappia, L.; Yen, H.-J.; Lawlor, K.; Hale, L.; Sun, J.; et al. Evaluation of variability in human kidney organoids. Nat. Methods 2018, 16, 79–87. [Google Scholar] [CrossRef]
- Przepiorski, A.; Crunk, A.E.; Holm, T.M.; Sander, V.; Davidson, A.J.; Hukriede, N.A. A Simplified Method for Generating Kidney Organoids from Human Pluripotent Stem Cells. J. Vis. Exp. 2021, 170, e62452. [Google Scholar] [CrossRef]
- Mae, S.I.; Ryosaka, M.; Sakamoto, S.; Matsuse, K.; Nozaki, A.; Igami, M.; Kabai, R.; Watanabe, A.; Osafune, K. Expansion of Human iPSC-Derived Ureteric Bud Organoids with Repeated Branching Potential. Cell Rep. 2020, 32, 107963. [Google Scholar] [CrossRef]
- Matsumoto, N.; Yamanaka, S.; Morimoto, K.; Matsui, K.; Nishimura, S.; Kinoshita, Y.; Inage, Y.; Fujimori, K.; Kuroda, T.; Saito, Y.; et al. Evaluation of the ability of human induced nephron progenitor cells to form chimeric renal organoids using mouse embryonic renal progenitor cells. Biochem. Biophys. Res. Commun. 2023, 662, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Crouse, B.; Sundaram, N.; Pode Shakked, N.; Thorner, K.; King, N.M.; Dutta, P.; Ester, L.; Zhang, W.; Govindarajah, V.; et al. Integrating collecting systems in human kidney organoids through fusion of distal nephron to ureteric bud. Cell Stem Cell 2025, 32, 1055–1070.e8. [Google Scholar] [CrossRef]
- Shi, M.; McCracken, K.W.; Patel, A.B.; Zhang, W.; Ester, L.; Valerius, M.T.; Bonventre, J.V. Human ureteric bud organoids recapitulate branching morphogenesis and differentiate into functional collecting duct cell types. Nat. Biotechnol. 2023, 41, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, S.; Tanaka, E.; Miike, K.; Ohmori, T.; Inoue, D.; Cai, C.L.; Taguchi, A.; Kobayashi, A.; Nishinakamura, R. Generation of the organotypic kidney structure by integrating pluripotent stem cell-derived renal stroma. Nat. Commun. 2022, 13, 611. [Google Scholar] [CrossRef]
- Kim, D.; Lim, H.; Youn, J.; Park, T.-E.; Kim, D.S. Scalable production of uniform and mature organoids in a 3D geometrically-engineered permeable membrane. Nat. Commun. 2024, 15, 9420. [Google Scholar] [CrossRef]
- Clerkin, S.; Singh, K.; Davis, J.L.; Treacy, N.J.; Krupa, I.; Reynaud, E.G.; Lees, R.M.; Needham, S.R.; MacWhite-Begg, D.; Wychowaniec, J.K.; et al. Tuneable gelatin methacryloyl (GelMA) hydrogels for the directed specification of renal cell types for hiPSC-derived kidney organoid maturation. Biomaterials 2025, 322, 123349. [Google Scholar] [CrossRef]
- Geuens, T.; Ruiter, F.A.A.; Schumacher, A.; Morgan, F.L.C.; Rademakers, T.; Wiersma, L.E.; van den Berg, C.W.; Rabelink, T.J.; Baker, M.B.; LaPointe, V.L.S. Thiol-ene cross-linked alginate hydrogel encapsulation modulates the extracellular matrix of kidney organoids by reducing abnormal type 1a1 collagen deposition. Biomaterials 2021, 275, 120976. [Google Scholar] [CrossRef]
- Schumacher, A.; Roumans, N.; Rademakers, T.; Joris, V.; Eischen-Loges, M.J.; van Griensven, M.; LaPointe, V.L.S. Enhanced Microvasculature Formation and Patterning in iPSC-Derived Kidney Organoids Cultured in Physiological Hypoxia. Front. Bioeng. Biotechnol. 2022, 10, 860138. [Google Scholar] [CrossRef]
- Glass, N.R.; Takasako, M.; Er, P.X.; Titmarsh, D.M.; Hidalgo, A.; Wolvetang, E.J.; Little, M.H.; Cooper-White, J.J. Multivariate patterning of human pluripotent cells under perfusion reveals critical roles of induced paracrine factors in kidney organoid development. Sci. Adv. 2020, 6, eaaw2746. [Google Scholar] [CrossRef] [PubMed]
- Koning, M.; Dumas, S.; Avramut, M.; Koning, R.; Meta, E.; Lievers, E.; Wiersma, L.; Borri, M.; Liang, X.; Xie, L.; et al. Vasculogenesis in kidney organoids upon transplantation. NPJ Regen. Med. 2022, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Maggiore, J.C.; LeGraw, R.; Przepiorski, A.; Velazquez, J.; Chaney, C.; Vanichapol, T.; Streeter, E.; Almuallim, Z.; Oda, A.; Chiba, T.; et al. A genetically inducible endothelial niche enables vascularization of human kidney organoids with multilineage maturation and emergence of renin expressing cells. Kidney Int. 2024, 106, 1086–1100. [Google Scholar] [CrossRef]
- Bas-Cristóbal Menéndez, A.; Du, Z.; van den Bosch, T.P.P.; Othman, A.; Gaio, N.; Silvestri, C.; Quirós, W.; Lin, H.; Korevaar, S.; Merino, A.; et al. Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system. Sci. Rep. 2022, 12, 20699. [Google Scholar] [CrossRef]
- Carrisoza-Gaytan, R.; Kroll, K.T.; Hiratsuka, K.; Gupta, N.R.; Morizane, R.; Lewis, J.A.; Satlin, L.M. Functional maturation of kidney organoid tubules: PIEZO1-mediated Ca2+ signaling. Am. J. Physiol. Cell Physiol. 2023, 324, C757–C768. [Google Scholar] [CrossRef]
- Goux Corredera, I.; Amato, G.; Moya-Rull, D.; Garreta, E.; Montserrat, N. Unlocking the full potential of human pluripotent stem cell-derived kidney organoids through bioengineering. Kidney Int. 2025, 108, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y. Revolutionizing renal research: The future of kidney-on-a-chip in biotechnology. Regen. Ther. 2024, 26, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, N.; Morizane, R. Advancements in therapeutic development: Kidney organoids and organs on a chip. Kidney Int. 2024, 105, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.V.T.; Gkouzioti, V.; Maass, C.; Verhaar, M.C.; Vernooij, R.W.M.; van Balkom, B.W.M. A systematic review of kidney-on-a-chip-based models to study human renal (patho-)physiology. Dis. Model. Mech. 2023, 16, dmm050113. [Google Scholar] [CrossRef]
- Lassé, M.; El Saghir, J.; Berthier, C.C.; Eddy, S.; Fischer, M.; Laufer, S.D.; Kylies, D.; Hutzfeldt, A.; Bonin, L.L.; Dumoulin, B.; et al. An integrated organoid omics map extends modeling potential of kidney disease. Nat. Commun. 2023, 14, 4903. [Google Scholar] [CrossRef]
- Hutzfeldt, A.; Kretzler, M.; Lindenmeyer, M.; Lassé, M.; Demir, F.; Saghir, E.; Schlüter, H.; Bonin, L.; Harder, J.; Beck, B.; et al. MO059: Trajectory Analysis of the Kidney Organoid Proteome Extends its Modelling Potential of Disease. Nephrol. Dial. Transplant. 2022, 37, gfac063.011. [Google Scholar] [CrossRef]
- Tian, P.; Lennon, R. The myriad possibility of kidney organoids. Curr. Opin. Nephrol. Hypertens. 2019, 28, 211–218. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Harder, J.L.; Carter-Timofte, M.E.; Zanon Rodriguez, L.; Mirabelli, C.; Demir, F.; Kurmasheva, N.; Ramakrishnan, S.K.; Kunke, M.; Tan, Y.; et al. VPS34-dependent control of apical membrane function of proximal tubule cells and nutrient recovery by the kidney. Sci. Signal. 2022, 15, eabo7940. [Google Scholar] [CrossRef]
- Nakazono, Y.; Takahashi, E.; Mae, S.-I.; Kitagawa, F.; Tamai, I.; Morinaga, G.; Kadoguchi, M.; Arakawa, H.; Kudo, T.; Higuchi, D.; et al. Improvement of Protein Expression Profile in Three-Dimensional Renal Proximal Tubular Epithelial Cell Spheroids Selected Based on OAT1 Gene Expression: A Potential In Vitro Tool for Evaluating Human Renal Proximal Tubular Toxicity and Drug Disposition. Drug Metab. Dispos. 2023, 51, 1177–1187. [Google Scholar] [CrossRef]
- Lindgren, D.; Eriksson, P.; Krawczyk, K.; Nilsson, H.; Hansson, J.; Veerla, S.; Sjölund, J.; Höglund, M.; Johansson, M.E.; Axelson, H. Cell-Type-Specific Gene Programs of the Normal Human Nephron Define Kidney Cancer Subtypes. Cell Rep. 2017, 20, 1476–1489. [Google Scholar] [CrossRef]
- Duan, A.; Wang, H.; Zhu, Y.; Wang, Q.; Zhang, J.; Hou, Q.; Xing, Y.; Shi, J.; Hou, J.; Qin, Z.; et al. Chromatin architecture reveals cell type-specific target genes for kidney disease risk variants. BMC Biol. 2021, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shrestha, R.; Qiu, C.; Kondo, A.; Huang, S.; Werth, M.; Li, M.; Barasch, J.; Suszták, K. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 2018, 360, 758–763. [Google Scholar] [CrossRef]
- Wuttke, M.; Li, Y.; Li, M.; Sieber, K.B.; Feitosa, M.F.; Gorski, M.; Tin, A.; Wang, L.; Chu, A.Y.; Hoppmann, A.; et al. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat. Genet. 2019, 51, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Huang, S.; Park, J.; Park, Y.; Ko, Y.A.; Seasock, M.J.; Bryer, J.S.; Xu, X.X.; Song, W.C.; Palmer, M.; et al. Renal compartment-specific genetic variation analyses identify new pathways in chronic kidney disease. Nat. Med. 2018, 24, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Dilz, J.; Auge, I.; Groeneveld, K.; Reuter, S.; Mrowka, R. A proof-of-concept assay for quantitative and optical assessment of drug-induced toxicity in renal organoids. Sci. Rep. 2023, 13, 6167. [Google Scholar] [CrossRef]
- Rutten, L.L.M. Morphologische Charakterisierung renaler Organoide aus humanen induzierten pluripotenten Stammzellen. In Medizinischen Fakultät; Friedrich-Schiller-Universität Jena: Jena, Germany, 2023. [Google Scholar]
- Reuter, S. qPCR Targeting Genes of Renal Site-Specific Marker Proteins. BioRender. 2025. Available online: https://BioRender.com/h36juj7 (accessed on 11 May 2025).
- Reuter, S. RNAScope Staining Targeting Renal Site-Specific Marker Probes for RNA. BioRender. 2025. Available online: https://BioRender.com/24x9svc (accessed on 24 June 2025).
- Krause, M.; Rak-Raszewska, A.; Naillat, F.; Saarela, U.; Schmidt, C.; Ronkainen, V.P.; Bart, G.; Ylä-Herttuala, S.; Vainio, S.J. Exosomes as secondary inductive signals involved in kidney organogenesis. J. Extracell. Vesicles 2018, 7, 1422675. [Google Scholar] [CrossRef]
- Juliar, B.A.; Stanaway, I.B.; Sano, F.; Fu, H.; Smith, K.D.; Akilesh, S.; Scales, S.J.; El Saghir, J.; Bhatraju, P.K.; Liu, E.; et al. Interferon-γ induces combined pyroptotic angiopathy and APOL1 expression in human kidney disease. Cell Rep. 2024, 43, 114310. [Google Scholar] [CrossRef]
- Li, L.; Galichon, P.; Xiao, X.; Figueroa-Ramirez, A.C.; Tamayo, D.; Lee, J.J.; Kalocsay, M.; Gonzalez-Sanchez, D.; Chancay, M.S.; McCracken, K.W.; et al. Orphan nuclear receptor COUP-TFII enhances myofibroblast glycolysis leading to kidney fibrosis. EMBO Rep. 2021, 22, e51169. [Google Scholar] [CrossRef]
- Su, J.; Morgani, S.M.; David, C.J.; Wang, Q.; Er, E.E.; Huang, Y.H.; Basnet, H.; Zou, Y.; Shu, W.; Soni, R.K.; et al. TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature 2020, 577, 566–571. [Google Scholar] [CrossRef]
- Digby, J.L.M.; Vanichapol, T.; Przepiorski, A.; Davidson, A.J.; Sander, V. Evaluation of cisplatin-induced injury in human kidney organoids. Am. J. Physiol. Renal Physiol. 2020, 318, F971–F978. [Google Scholar] [CrossRef]
- Sakolish, C.; Moyer, H.L.; Tsai, H.D.; Ford, L.C.; Dickey, A.N.; Wright, F.A.; Han, G.; Bajaj, P.; Baltazar, M.T.; Carmichael, P.L.; et al. Analysis of reproducibility and robustness of a renal proximal tubule microphysiological system OrganoPlate 3-lane 40 for in vitro studies of drug transport and toxicity. Toxicol. Sci. 2023, 196, 52–70. [Google Scholar] [CrossRef]
- Zhang, T.; Widdop, R.E.; Ricardo, S.D. Transition from acute kidney injury to chronic kidney disease: Mechanisms, models, and biomarkers. Am. J. Physiol. Renal Physiol. 2024, 327, F788–F805. [Google Scholar] [CrossRef]
- Hirayama, R.; Toyohara, K.; Watanabe, K.; Otsuki, T.; Araoka, T.; Mae, S.-I.; Horinouchi, T.; Yamamura, T.; Okita, K.; Hotta, A.; et al. iPSC-derived type IV collagen α5-expressing kidney organoids model Alport syndrome. Commun. Biol. 2023, 6, 854. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.S.; Brooks, C.R.; Lam, A.Q.; Fu, H.; Morizane, R.; Agrawal, V.; Saad, A.F.; Li, M.K.; Hughes, M.R.; Werff, R.V.; et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat. Commun. 2015, 6, 8715. [Google Scholar] [CrossRef]
- Scarlat, A.; Trionfini, P.; Rizzo, P.; Conti, S.; Longaretti, L.; Breno, M.; Longhi, L.; Xinaris, C.; Remuzzi, G.; Benigni, A.; et al. PKD1 mutation perturbs morphogenesis in tubular epithelial organoids derived from human pluripotent stem cells. Sci. Rep. 2025, 15, 10375. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, S.; Tanigawa, S.; Taguchi, A.; Hotta, A.; Nakazato, H.; Osafune, K.; Kobayashi, A.; Nishinakamura, R. PKD1-Dependent Renal Cystogenesis in Human Induced Pluripotent Stem Cell-Derived Ureteric Bud/Collecting Duct Organoids. J. Am. Soc. Nephrol. 2020, 31, 2355–2371. [Google Scholar] [CrossRef] [PubMed]
- Vishy, C.E.; Thomas, C.; Vincent, T.; Crawford, D.K.; Goddeeris, M.M.; Freedman, B.S. Genetics of cystogenesis in base-edited human organoids reveal therapeutic strategies for polycystic kidney disease. Cell Stem Cell 2024, 31, 537–553.e5. [Google Scholar] [CrossRef]
- Hejazi, L.; Sharma, S.; Ruiz, A.; Zhang, G.; Tucci, F.C.; Sharma, K. 400-P: Spatial Metabolomics Analysis by MSI-DeepPath Identifies Key Pathways in ZDF Diabetic Kidney Disease Model. Diabetes 2023, 72, 400-P. [Google Scholar] [CrossRef]
- van Smaalen, T.C.; Ellis, S.R.; Mascini, N.E.; Siegel, T.P.; Cillero-Pastor, B.; Hillen, L.M.; van Heurn, L.W.E.; Peutz-Kootstra, C.J.; Heeren, R.M.A. Rapid Identification of Ischemic Injury in Renal Tissue by Mass-Spectrometry Imaging. Anal. Chem. 2019, 91, 3575–3581. [Google Scholar] [CrossRef] [PubMed]
- Bindi, G.; Monza, N.; de Oliveira, G.S.; Denti, V.; Fatahian, F.; Seyed-Golestan, S.J.; L’Imperio, V.; Pagni, F.; Smith, A. Sequential MALDI-HiPLEX-IHC and Untargeted Spatial Proteomics Mass Spectrometry Imaging to Detect Proteomic Alterations Associated with Tumour Infiltrating Lymphocytes. J. Proteome Res. 2025, 24, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fu, W.; Huo, M.; He, B.; Liu, Y.; Tian, L.; Li, W.; Zhou, Z.; Wang, B.; Xia, J.; et al. Spatial-resolved metabolomics reveals tissue-specific metabolic reprogramming in diabetic nephropathy by using mass spectrometry imaging. Acta Pharm. Sin. B 2021, 11, 3665–3677. [Google Scholar] [CrossRef] [PubMed]
- Ransick, A.; Lindström, N.O.; Liu, J.; Zhu, Q.; Guo, J.-J.; Alvarado, G.F.; Kim, A.D.; Black, H.G.; Kim, J.; McMahon, A.P. Single-Cell Profiling Reveals Sex, Lineage, and Regional Diversity in the Mouse Kidney. Dev. Cell 2019, 51, 399–413.e7. [Google Scholar] [CrossRef]
- van den Berg, C.W.; Koudijs, A.; Ritsma, L.; Rabelink, T.J. In Vivo Assessment of Size-Selective Glomerular Sieving in Transplanted Human Induced Pluripotent Stem Cell-Derived Kidney Organoids. J. Am. Soc. Nephrol. 2020, 31, 921–929. [Google Scholar] [CrossRef]
| Gene | Cell Type(s) | Function/Role | Citations |
|---|---|---|---|
| SLC34A1 | Proximal tubule | Phosphate transport | [46,47] |
| NPHS1 | Podocyte | Filtration barrier | |
| UMOD | Thick ascending limb | Salt handling, disease risk | [48,49] |
| SHROOM3 | Multiple nephron cells | Morphogenesis, disease association | [49] |
| Lewis et al., 2021 [6] | Limbutara et al., 2020 [8] | Ransick et al., 2019 [70] | |
|---|---|---|---|
| Approach | SLC-omics of the kidney; Transcriptomics + Proteomics of SLC transporters across nephron segments | Quantitative Proteomics of all 14 renal tubule segments in rat; high-resolution proteomics using mass spectrometry + “proteomic ruler” | Single-Cell Profiling of mouse kidney; Single-Cell RNA sequencing (scRNA-seq) |
| Data set/ Scale | 431 SLC genes curated; mapped expression in 14 nephron segments (RNA-Seq + LC-MS/MS) | ~4234 proteins per segment quantified; 99% proteome coverage | Thousands of cells sequenced from adult mouse kidney |
| Key Findings | Segment-selective transporter expression Sex differences in proximal tubule Importance of accessory subunits Integrated open-access database | Absolute protein counts per cell type; Consistent patterns with prior physiology; Created kidney tubule expression atlas (KTEA) | High-resolution kidney cell atlas; Public interactive atlas; Sex differences in gene expression; Regional/stromal diversity; Lineage insights (e.g., collecting duct cell types) |
| Clinical/ Research Significance | Informs drug targeting (e.g., SGLT2 inhibitors) | Protein-level reference for nephron biology | Basis for precision medicine (sex-specific drug responses) |
| Explains hereditary transporter disorders (e.g., cystinuria, dRTA) | Enables biomarker discovery | Maps cell-specific disease mechanisms | |
| Enables precision medicine approaches to renal transport | Facilitates therapeutic development targeting transporters, enzymes, and channels | Framework for cross-species comparison (mouse versus human) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groeneveld, K.; Mrowka, R. Combining Proteomics and Organoid Research to Unravel the Multifunctional Complexity of Kidney Physiology Enhances the Need for Controlled Organoid Maturation. Organoids 2025, 4, 28. https://doi.org/10.3390/organoids4040028
Groeneveld K, Mrowka R. Combining Proteomics and Organoid Research to Unravel the Multifunctional Complexity of Kidney Physiology Enhances the Need for Controlled Organoid Maturation. Organoids. 2025; 4(4):28. https://doi.org/10.3390/organoids4040028
Chicago/Turabian StyleGroeneveld, Kathrin, and Ralf Mrowka. 2025. "Combining Proteomics and Organoid Research to Unravel the Multifunctional Complexity of Kidney Physiology Enhances the Need for Controlled Organoid Maturation" Organoids 4, no. 4: 28. https://doi.org/10.3390/organoids4040028
APA StyleGroeneveld, K., & Mrowka, R. (2025). Combining Proteomics and Organoid Research to Unravel the Multifunctional Complexity of Kidney Physiology Enhances the Need for Controlled Organoid Maturation. Organoids, 4(4), 28. https://doi.org/10.3390/organoids4040028









