A Concise Review of Organoid Tissue Engineering: Regenerative Applications and Precision Medicine
Abstract
1. Introduction
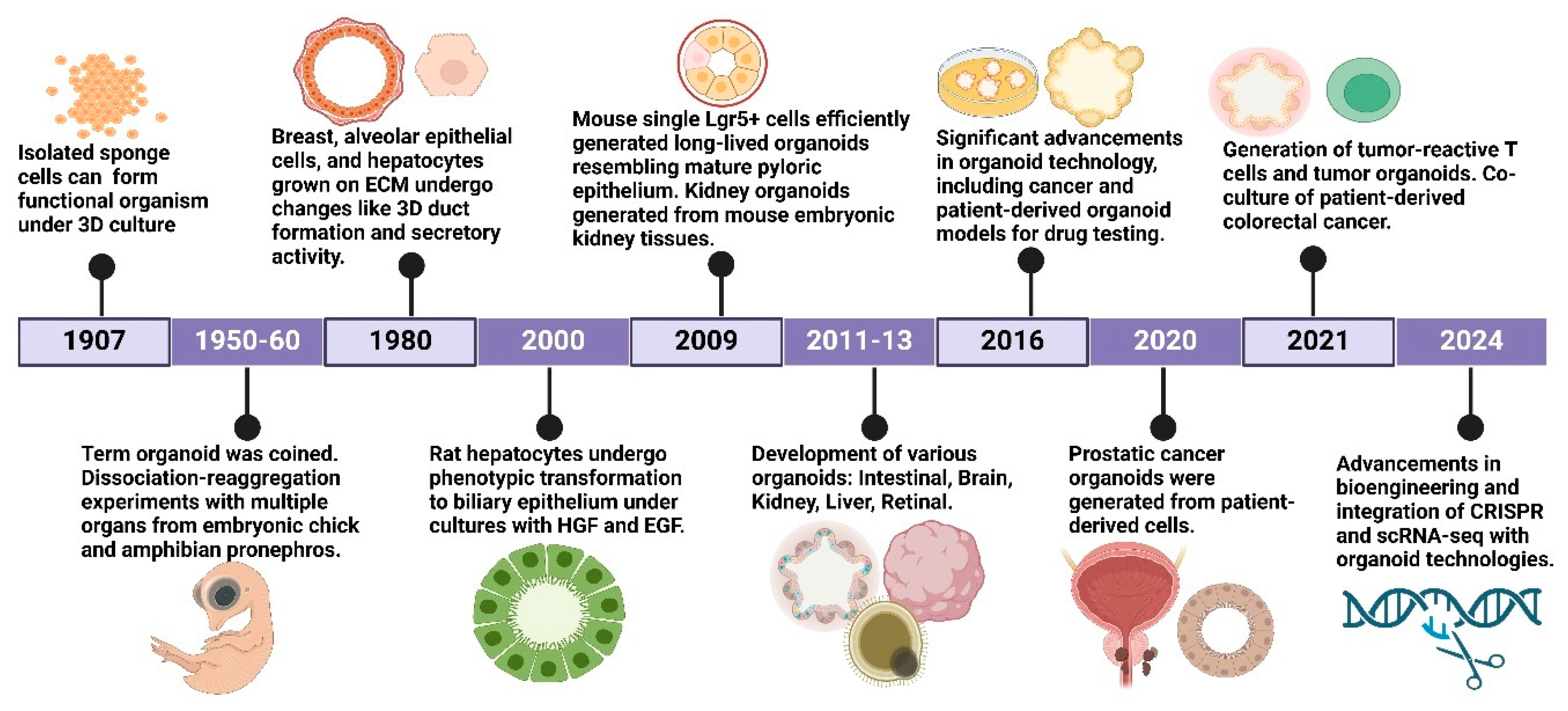
2. History of Organoids
3. Cell Culture Approaches for Organoids
4. Human Organoids as an Upcoming Model for Research
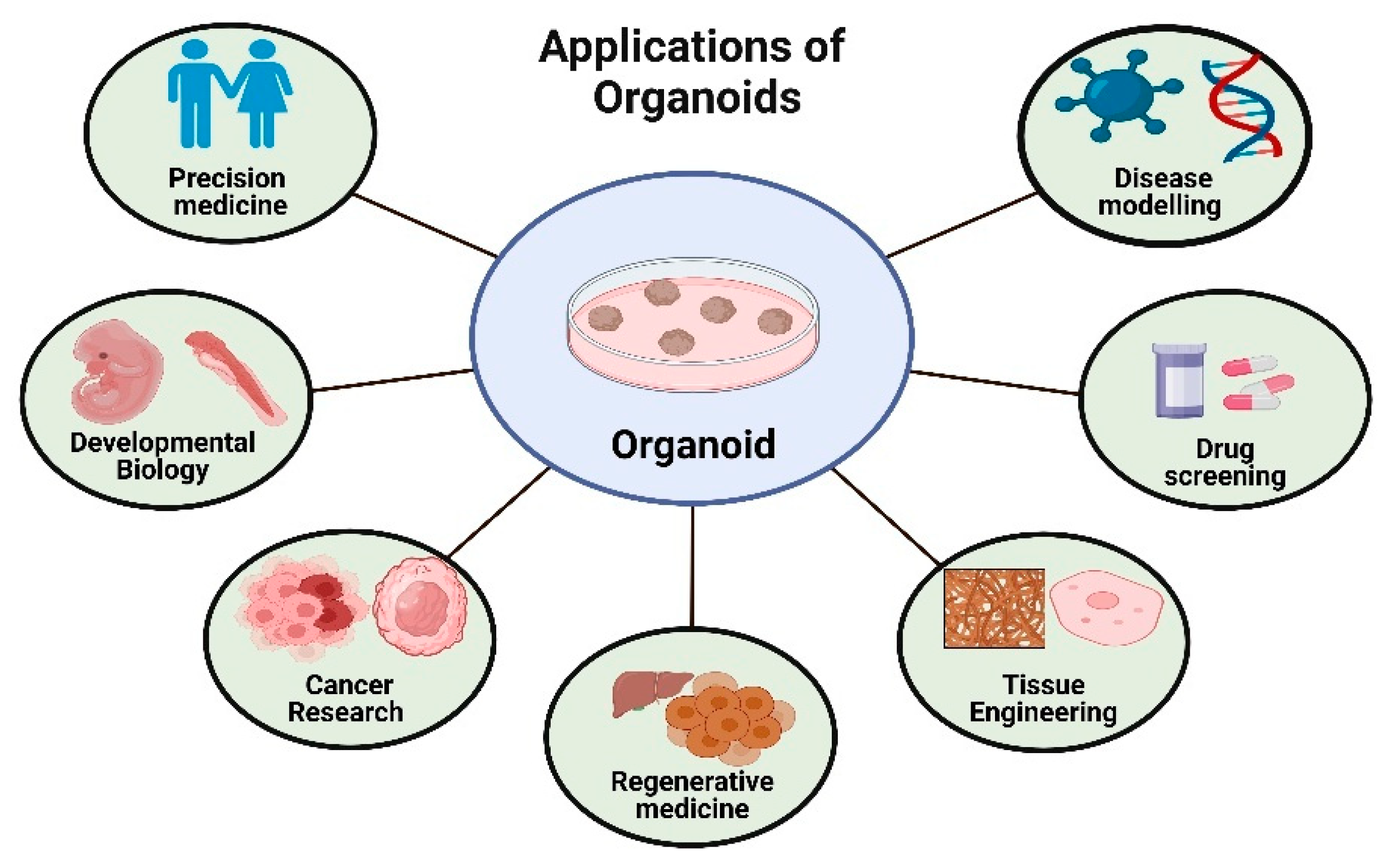
5. Application of Organoids
5.1. Cancer Research
5.2. Drug Development
5.3. Precision Medicine
5.4. Developmental Biology
5.5. Tissue Engineering and Regenerative Medicine
5.6. Emerging Applications of Organoids
6. Genetic Modification in Organoids
7. Next-Generation Organoids
8. Challenges and Limitations in Organoid Research
9. Future Directions
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilson, H.V. A New Method by Which Sponges May Be Artificially Reared. Science 1907, 25, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Heydari, Z.; Moeinvaziri, F.; Agarwal, T.; Pooyan, P.; Shpichka, A.; Maiti, T.K.; Timashev, P.; Baharvand, H.; Vosough, M. Organoids: A novel modality in disease modeling. Biodes Manuf. 2021, 4, 689–716. [Google Scholar] [CrossRef]
- Teriyapirom, I.; Batista-Rocha, A.S.; Koo, B.K. Genetic engineering in organoids. J. Mol. Med. 2021, 99, 555–568. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 168. [Google Scholar] [CrossRef]
- Tang, X.Y.; Wu, S.; Wang, D.; Chu, C.; Hong, Y.; Tao, M.; Hu, H.; Xu, M.; Guo, X.; Liu, Y. Human organoids in basic research and clinical applications. Signal Transduct. Target. Ther. 2022, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Devarasetty, M.; Mazzocchi, A.R.; Skardal, A. Applications of Bioengineered 3D Tissue and Tumor Organoids in Drug Development and Precision Medicine: Current and Future. BioDrugs 2018, 32, 53–68. [Google Scholar] [CrossRef]
- Yamada, K.M.; Doyle, A.D.; Lu, J. Cell-3D matrix interactions: Recent advances and opportunities. Trends Cell Biol. 2022, 32, 883–895. [Google Scholar] [CrossRef]
- Cerneckis, J.; Cai, H.; Shi, Y. Induced pluripotent stem cells (iPSCs): Molecular mechanisms of induction and applications. Signal Transduct. Target. Ther. 2024, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.; Wang, Z.; Liu, Y.; Yu, J.; Wang, W.; Chen, S.; Wu, W.; Wang, J.; Qian, G.; et al. Standardization of organoid culture in cancer research. Cancer Med. 2023, 12, 14375–14386. [Google Scholar] [CrossRef]
- Bartfeld, S.; Clevers, H. Stem cell-derived organoids and their application for medical research and patient treatment. J. Mol. Med. 2017, 95, 729–738. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Z.; Tang, Z.; Chen, Y.; Huang, M.; Liu, H.; Huang, W.; Ye, Q.; Jia, B. Research Progress, Challenges, and Breakthroughs of Organoids as Disease Models. Front. Cell Dev. Biol. 2021, 9, 740574. [Google Scholar] [CrossRef]
- Roman, V.; Mihaila, M.; Radu, N.; Marineata, S.; Diaconu, C.C.; Bostan, M. Cell Culture Model Evolution and Its Impact on Improving Therapy Efficiency in Lung Cancer. Cancers 2023, 15, 4996. [Google Scholar] [CrossRef] [PubMed]
- Silva-Pedrosa, R.; Salgado, A.J.; Ferreira, P.E. Revolutionizing Disease Modeling: The Emergence of Organoids in Cellular Systems. Cells 2023, 12, 930. [Google Scholar] [CrossRef] [PubMed]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Mu, Q.; Bi, H.; Chen, Y.; Wang, Q.; Ma, X.; Lu, L. Interpretation of the past, present, and future of organoid technology: An updated bibliometric analysis from 2009 to 2024. Front. Cell Dev. Biol. 2024, 12, 1433111. [Google Scholar] [CrossRef]
- Corro, C.; Novellasdemunt, L.; Li, V.S.W. A brief history of organoids. Am. J. Physiol. Cell Physiol. 2020, 319, C151–C165. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Cala, G.; Sina, B.; De Coppi, P.; Giobbe, G.G.; Gerli, M.F.M. Primary human organoids models: Current progress and key milestones. Front. Bioeng. Biotechnol. 2023, 11, 1058970. [Google Scholar] [CrossRef]
- Roshanravan, N.; Ghaffari, S.; Bastani, S.; Pahlavan, S.; Asghari, S.; Doustvandi, M.A.; Jalilzadeh-Razin, S.; Dastouri, M. Human cardiac organoids: A recent revolution in disease modeling and regenerative medicine. J. Cardiovasc. Thorac. Res. 2023, 15, 68–72. [Google Scholar] [CrossRef]
- Wensink, G.E.; Elias, S.G.; Mullenders, J.; Koopman, M.; Boj, S.F.; Kranenburg, O.W.; Roodhart, J.M.L. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. NPJ Precis. Oncol. 2021, 5, 30. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, F.; Jin, Y.; Ma, Y. Applications of human organoids in the personalized treatment for digestive diseases. Signal Transduct. Target. Ther. 2022, 7, 336. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. Bioprinting of Cells, Organoids and Organs-on-a-Chip Together with Hydrogels Improves Structural and Mechanical Cues. Cells 2024, 13, 1638. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.F.; Yu, T. CRISPR/Cas9 therapeutics: Progress and prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Kimura, H.; Nishikawa, M.; Kutsuzawa, N.; Tokito, F.; Kobayashi, T.; Kurniawan, D.A.; Shioda, H.; Cao, W.; Shinha, K.; Nakamura, H.; et al. Advancements in Microphysiological systems: Exploring organoids and organ-on-a-chip technologies in drug development -focus on pharmacokinetics related organs. Drug Metab. Pharmacokinet. 2025, 60, 101046. [Google Scholar] [CrossRef]
- Li, P.; Huang, M.; Ma, Y.; Zhang, Y.; Shi, C. Novel research model for in vitro immunotherapy: Co-culturing tumor organoids with peripheral blood mononuclear cells. Cancer Cell Int. 2024, 24, 438. [Google Scholar] [CrossRef]
- Bose, S.; Clevers, H.; Shen, X. Promises and Challenges of Organoid-Guided Precision Medicine. Med 2021, 2, 1011–1026. [Google Scholar] [CrossRef]
- Licata, J.P.; Schwab, K.H.; Har-El, Y.E.; Gerstenhaber, J.A.; Lelkes, P.I. Bioreactor Technologies for Enhanced Organoid Culture. Int. J. Mol. Sci. 2023, 24, 11427. [Google Scholar] [CrossRef]
- Wasson, E.M.; He, W.; Ahlquist, J.; Hynes, W.F.; Triplett, M.G.; Hinckley, A.; Karelehto, E.; Gray-Sherr, D.R.; Friedman, C.F.; Robertson, C.; et al. A perfused multi-well bioreactor platform to assess tumor organoid response to a chemotherapeutic gradient. Front. Bioeng. Biotechnol. 2023, 11, 1193430. [Google Scholar] [CrossRef]
- Kim, M.B.; Hwangbo, S.; Jang, S.; Jo, Y.K. Bioengineered Co-culture of organoids to recapitulate host-microbe interactions. Mater. Today Bio 2022, 16, 100345. [Google Scholar] [CrossRef] [PubMed]
- Price, S.; Bhosle, S.; Goncalves, E.; Li, X.; McClurg, D.P.; Barthorpe, S.; Beck, A.; Hall, C.; Lightfoot, H.; Farrow, L.; et al. A suspension technique for efficient large-scale cancer organoid culturing and perturbation screens. Sci. Rep. 2022, 12, 5571. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ahmed, I.; Brown, J.; Khosrotehrani, K.; Shafiee, A. The empowering influence of air-liquid interface culture on skin organoid hair follicle development. Burns Trauma 2025, 13, tkae070. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Deng, C.; Wen, L.; Luo, G.; Meng, Y. CRISPR-Cas and CRISPR-based screening system for precise gene editing and targeted cancer therapy. J. Transl. Med. 2024, 22, 516. [Google Scholar] [CrossRef]
- Hong, Z.X.; Zhu, S.T.; Li, H.; Luo, J.Z.; Yang, Y.; An, Y.; Wang, X.; Wang, K. Bioengineered skin organoids: From development to applications. Mil. Med. Res. 2023, 10, 40. [Google Scholar] [CrossRef]
- Jeong, S.; Na, Y.; Nam, H.M.; Sung, G.Y. Skin-on-a-chip strategies for human hair follicle regeneration. Exp. Dermatol. 2023, 32, 13–23. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Y.; Ma, Y.; Wang, P.; Yao, R. Converging bioprinting and organoids to better recapitulate the tumor microenvironment. Trends Biotechnol. 2024, 42, 648–663. [Google Scholar] [CrossRef]
- Porter, R.J.; Murray, G.I.; McLean, M.H. Current concepts in tumour-derived organoids. Br. J. Cancer 2020, 123, 1209–1218. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Grebenyuk, S.; Abdel Fattah, A.R.; Kumar, M.; Toprakhisar, B.; Rustandi, G.; Vananroye, A.; Salmon, I.; Verfaillie, C.; Grillo, M.; Ranga, A. Large-scale perfused tissues via synthetic 3D soft microfluidics. Nat. Commun. 2023, 14, 193. [Google Scholar] [CrossRef]
- Saorin, G.; Caligiuri, I.; Rizzolio, F. Microfluidic organoids-on-a-chip: The future of human models. Semin. Cell Dev. Biol. 2023, 144, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Dellaquila, A.; Le Bao, C.; Letourneur, D.; Simon-Yarza, T. In Vitro Strategies to Vascularize 3D Physiologically Relevant Models. Adv. Sci. 2021, 8, e2100798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.; Chen, Y.G. Generation of 3D human gastrointestinal organoids: Principle and applications. Cell Regen. 2020, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cheng, Y.; Liu, X.; Ding, W.; Liu, J.; Ling, Z.; Wu, L. Advances in the Development and Application of Human Organoids: Techniques, Applications, and Future Perspectives. Cell Transplant. 2025, 34, 9636897241303271. [Google Scholar] [CrossRef]
- Yao, Q.; Cheng, S.; Pan, Q.; Yu, J.; Cao, G.; Li, L.; Cao, H. Organoids: Development and applications in disease models, drug discovery, precision medicine, and regenerative medicine. MedComm 2024, 5, e735. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Yang, S.; Hu, H.; Kung, H.; Zou, R.; Dai, Y.; Hu, Y.; Wang, T.; Lv, T.; Yu, J.; Li, F. Organoids: The current status and biomedical applications. MedComm 2023, 4, e274. [Google Scholar] [CrossRef]
- Sieben, C.J.; Harris, P.C. Experimental Models of Polycystic Kidney Disease: Applications and Therapeutic Testing. Kidney360 2023, 4, 1155–1173. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernandez-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and potential in organoid research. Nat. Rev. Genet. 2018, 19, 671–687. [Google Scholar] [CrossRef]
- Heinzelmann, E.; Piraino, F.; Costa, M.; Roch, A.; Norkin, M.; Garnier, V.; Homicsko, K.; Brandenberg, N. iPSC-derived and Patient-Derived Organoids: Applications and challenges in scalability and reproducibility as pre-clinical models. Curr. Res. Toxicol. 2024, 7, 100197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yuan, F.; Zuo, X.; Li, M. Breakthroughs and challenges of organoid models for assessing cancer immunotherapy: A cutting-edge tool for advancing personalised treatments. Cell Death Discov. 2025, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Konoe, R.; Morizane, R. Strategies for Improving Vascularization in Kidney Organoids: A Review of Current Trends. Biology 2023, 12, 503. [Google Scholar] [CrossRef]
- Munsie, M.; Hyun, I.; Sugarman, J. Ethical issues in human organoid and gastruloid research. Development 2017, 144, 942–945. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Suhito, I.R.; Kim, T.-H. Recent advances and challenges in organoid-on-a-chip technology. Organoid 2022, 2, e4. [Google Scholar] [CrossRef]
- Aazmi, A.; Zhang, D.; Mazzaglia, C.; Yu, M.; Wang, Z.; Yang, H.; Huang, Y.Y.S.; Ma, L. Biofabrication methods for reconstructing extracellular matrix mimetics. Bioact. Mater. 2024, 31, 475–496. [Google Scholar] [CrossRef]
- Gan, Z.; Qin, X.; Liu, H.; Liu, J.; Qin, J. Recent advances in defined hydrogels in organoid research. Bioact. Mater. 2023, 28, 386–401. [Google Scholar] [CrossRef]
- Passaniti, A.; Kleinman, H.K.; Martin, G.R. Matrigel: History/background, uses, and future applications. J. Cell Commun. Signal 2022, 16, 621–626. [Google Scholar] [CrossRef]
- Shi, J.; Yu, L.; Ding, J. PEG-based thermosensitive and biodegradable hydrogels. Acta Biomater. 2021, 128, 42–59. [Google Scholar] [CrossRef]
- Zhan, Y.; Jiang, W.; Liu, Z.; Wang, Z.; Guo, K.; Sun, J. Utilizing bioprinting to engineer spatially organized tissues from the bottom-up. Stem Cell Res. Ther. 2024, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Guo, Y.; Liu, T.; Xu, R.; Mao, S.; Mo, X.; Zhang, T.; Ouyang, L.; Xiong, Z.; Sun, W. Advances in 3D Bioprinting. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100011. [Google Scholar] [CrossRef]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef]
- Li, S.; Dan, X.; Chen, H.; Li, T.; Liu, B.; Ju, Y.; Li, Y.; Lei, L.; Fan, X. Developing fibrin-based biomaterials/scaffolds in tissue engineering. Bioact. Mater. 2024, 40, 597–623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Brislinger, D.; Fuchs, J.; Lyons, A.; Langthaler, S.; Hauser, C.A.E.; Baumgartner, C. Vascularised organoids: Recent advances and applications in cancer research. Clin. Transl. Med. 2025, 15, e70258. [Google Scholar] [CrossRef]
- Driehuis, E.; Clevers, H. CRISPR/Cas 9 genome editing and its applications in organoids. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G257–G265. [Google Scholar] [CrossRef]
- Xu, H.; Lyu, X.; Yi, M.; Zhao, W.; Song, Y.; Wu, K. Organoid technology and applications in cancer research. J. Hematol. Oncol. 2018, 11, 116. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Huang, L.; Huang, J.; Liang, D.; Liao, L.; Deng, Y.; Zhang, L.; Zhang, B.; Tang, W. Organoid: Next-Generation Modeling of Cancer Research and Drug Development. Front. Oncol. 2021, 11, 826613. [Google Scholar] [CrossRef]
- Fang, Z.; Li, P.; Du, F.; Shang, L.; Li, L. The role of organoids in cancer research. Exp. Hematol. Oncol. 2023, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Love, J.R.; Karthaus, W.R. Next-Generation Modeling of Cancer Using Organoids. Cold Spring Harb. Perspect. Med. 2024, 14, a041380. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, B.A.; Tsegaw, T.; Demessie, Y.; Getnet, K.; Bitew, A.B.; Kinde, M.Z.; Beirhun, A.M.; Mebratu, A.S.; Mekasha, Y.T.; Feleke, M.G.; et al. Comprehensive review of drug resistance in mammalian cancer stem cells: Implications for cancer therapy. Cancer Cell Int. 2024, 24, 406. [Google Scholar] [CrossRef]
- Zuo, J.; Fang, Y.; Wang, R.; Liang, S. High-throughput solutions in tumor organoids: From culture to drug screening. Stem Cells 2025, 43, sxae070. [Google Scholar] [CrossRef] [PubMed]
- Thorel, L.; Perreard, M.; Florent, R.; Divoux, J.; Coffy, S.; Vincent, A.; Gaggioli, C.; Guasch, G.; Gidrol, X.; Weiswald, L.B.; et al. Patient-derived tumor organoids: A new avenue for preclinical research and precision medicine in oncology. Exp. Mol. Med. 2024, 56, 1531–1551. [Google Scholar] [CrossRef] [PubMed]
- Soto-Gamez, A.; Gunawan, J.P.; Barazzuol, L.; Pringle, S.; Coppes, R.P. Organoid-based personalized medicine: From tumor outcome prediction to autologous transplantation. Stem Cells 2024, 42, 499–508. [Google Scholar] [CrossRef]
- Seppala, T.T.; Burkhart, R.A. Can Pancreatic Organoids Help in the Treatment of Pancreatic Cancer? Adv. Surg. 2021, 55, 215–229. [Google Scholar] [CrossRef]
- Yuki, K.; Cheng, N.; Nakano, M.; Kuo, C.J. Organoid Models of Tumor Immunology. Trends Immunol. 2020, 41, 652–664. [Google Scholar] [CrossRef]
- Sun, C.P.; Lan, H.R.; Fang, X.L.; Yang, X.Y.; Jin, K.T. Organoid Models for Precision Cancer Immunotherapy. Front. Immunol. 2022, 13, 770465. [Google Scholar] [CrossRef]
- Fontoura, J.C.; Viezzer, C.; Dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110264. [Google Scholar] [CrossRef]
- Peng, Z.; Lv, X.; Sun, H.; Zhao, L.; Huang, S. 3D tumor cultures for drug resistance and screening development in clinical applications. Mol. Cancer 2025, 24, 93. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, X.; Song, W. Tumor organoid biobank-new platform for medical research. Sci. Rep. 2023, 13, 1819. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tao, X.; Zhu, J.; Dai, Z.; Du, Y.; Xie, Y.; Chu, X.; Fu, G.; Lei, Z. Tumor organoid-immune co-culture models: Exploring a new perspective of tumor immunity. Cell Death Discov. 2025, 11, 195. [Google Scholar] [CrossRef]
- Zhou, L.; Luo, D.; Lu, W.; Han, J.; Zhao, M.; Li, X.; Shen, T.; Jin, Z.; Zeng, J.; Wen, Y. Gastrointestinal tract organoids as novel tools in drug discovery. Front. Pharmacol. 2024, 15, 1463114. [Google Scholar] [CrossRef] [PubMed]
- Kondo, J.; Inoue, M. Application of Cancer Organoid Model for Drug Screening and Personalized Therapy. Cells 2019, 8, 470. [Google Scholar] [CrossRef]
- Zhao, B.; Hemann, M.T.; Lauffenburger, D.A. Intratumor heterogeneity alters most effective drugs in designed combinations. Proc. Natl. Acad. Sci. USA 2014, 111, 10773–10778. [Google Scholar] [CrossRef]
- Vandana, J.J.; Manrique, C.; Lacko, L.A.; Chen, S. Human pluripotent-stem-cell-derived organoids for drug discovery and evaluation. Cell Stem Cell 2023, 30, 571–591. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Organoids for Drug Discovery and Personalized Medicine. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 447–462. [Google Scholar] [CrossRef]
- Tentler, J.J.; Tan, A.C.; Weekes, C.D.; Jimeno, A.; Leong, S.; Pitts, T.M.; Arcaroli, J.J.; Messersmith, W.A.; Eckhardt, S.G. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 2012, 9, 338–350. [Google Scholar] [CrossRef]
- Muniyandi, P.; O’Hern, C.; Popa, M.A.; Aguirre, A. Biotechnological advances and applications of human pluripotent stem cell-derived heart models. Front. Bioeng. Biotechnol. 2023, 11, 1214431. [Google Scholar] [CrossRef]
- Afonso, M.B.; Marques, V.; van Mil, S.W.C.; Rodrigues, C.M.P. Human liver organoids: From generation to applications. Hepatology 2024, 79, 1432–1451. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, H.; Chen, K. Living biobank-based cancer organoids: Prospects and challenges in cancer research. Cancer Biol. Med. 2022, 19, 965–982. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kowalczewski, A.; Vu, D.; Liu, X.; Salekin, A.; Yang, H.; Ma, Z. Organoid intelligence: Integration of organoid technology and artificial intelligence in the new era of in vitro models. Med. Nov. Technol. Devices 2024, 21, 5271. [Google Scholar] [CrossRef]
- Schuster, B.; Junkin, M.; Kashaf, S.S.; Romero-Calvo, I.; Kirby, K.; Matthews, J.; Weber, C.R.; Rzhetsky, A.; White, K.P.; Tay, S. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat. Commun. 2020, 11, 5271. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, L.; Lacko, L.A.; Chen, S. Human organoid models to study SARS-CoV-2 infection. Nat. Methods 2022, 19, 418–428. [Google Scholar] [CrossRef]
- Gorshkov, K.; Chen, C.Z.; Marshall, R.E.; Mihatov, N.; Choi, Y.; Nguyen, D.T.; Southall, N.; Chen, K.G.; Park, J.K.; Zheng, W. Advancing precision medicine with personalized drug screening. Drug Discov. Today 2019, 24, 272–278. [Google Scholar] [CrossRef]
- Takagi, K.; Takagi, M.; Hiyama, G.; Goda, K. A deep-learning model for characterizing tumor heterogeneity using patient-derived organoids. Sci. Rep. 2024, 14, 22769. [Google Scholar] [CrossRef]
- Duarte, D.; Vale, N. Evaluation of synergism in drug combinations and reference models for future orientations in oncology. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100110. [Google Scholar] [CrossRef]
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A.; Burczynski, M.E.; Crean, C.; Edwards, R.; Gaudilliere, B.; et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nat. Rev. Neurol. 2020, 16, 381–400. [Google Scholar] [CrossRef]
- Zuieva, A.; Can, S.; Boelke, F.; Reuter, S.; Schattscheider, S.; Topfer, E.; Westphal, A.; Mrowka, R.; Wolfl, S. Real-time monitoring of immediate drug response and adaptation upon repeated treatment in a microfluidic chip system. Arch. Toxicol. 2022, 96, 1483–1487. [Google Scholar] [CrossRef]
- Li, C.; Fleck, J.S.; Martins-Costa, C.; Burkard, T.R.; Themann, J.; Stuempflen, M.; Peer, A.M.; Vertesy, A.; Littleboy, J.B.; Esk, C.; et al. Single-cell brain organoid screening identifies developmental defects in autism. Nature 2023, 621, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Seppala, T.T.; Zimmerman, J.W.; Suri, R.; Zlomke, H.; Ivey, G.D.; Szabolcs, A.; Shubert, C.R.; Cameron, J.L.; Burns, W.R.; Lafaro, K.J.; et al. Precision Medicine in Pancreatic Cancer: Patient-Derived Organoid Pharmacotyping Is a Predictive Biomarker of Clinical Treatment Response. Clin. Cancer Res. 2022, 28, 3296–3307. [Google Scholar] [CrossRef]
- Ono, M.; Ono, Y.; Nakamura, T.; Tsuchikawa, T.; Kuraya, T.; Kuwabara, S.; Nakanishi, Y.; Asano, T.; Matsui, A.; Tanaka, K.; et al. Predictors of Long-Term Survival in Pancreatic Ductal Adenocarcinoma after Pancreatectomy: TP53 and SMAD4 Mutation Scoring in Combination with CA19-9. Ann. Surg. Oncol. 2022, 29, 5007–5019. [Google Scholar] [CrossRef]
- Ukai, S.; Honma, R.; Sakamoto, N.; Yamamoto, Y.; Pham, Q.T.; Harada, K.; Takashima, T.; Taniyama, D.; Asai, R.; Fukada, K.; et al. Molecular biological analysis of 5-FU-resistant gastric cancer organoids; KHDRBS3 contributes to the attainment of features of cancer stem cell. Oncogene 2020, 39, 7265–7278. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Yu, Y.; Wang, M.; Liu, J.; He, G.; Yu, S.; Yan, H.; Yang, L.; Li, H.; Peng, X. Advancing cancer research through organoid technology. J. Transl. Med. 2024, 22, 1007. [Google Scholar] [CrossRef] [PubMed]
- Shelton, S.E.; Nguyen, H.T.; Barbie, D.A.; Kamm, R.D. Engineering approaches for studying immune-tumor cell interactions and immunotherapy. iScience 2021, 24, 101985. [Google Scholar] [CrossRef]
- Gronholm, M.; Feodoroff, M.; Antignani, G.; Martins, B.; Hamdan, F.; Cerullo, V. Patient-Derived Organoids for Precision Cancer Immunotherapy. Cancer Res. 2021, 81, 3149–3155. [Google Scholar] [CrossRef]
- Gu, Z.; Wu, Q.; Shang, B.; Zhang, K.; Zhang, W. Organoid co-culture models of the tumor microenvironment promote precision medicine. Cancer Innov. 2024, 3, e101. [Google Scholar] [CrossRef]
- Luksik, A.S.; Yazigi, E.; Shah, P.; Jackson, C.M. CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. Cancers 2023, 15, 1414. [Google Scholar] [CrossRef]
- Shin, J.H.; Jeong, J.; Maher, S.E.; Lee, H.W.; Lim, J.; Bothwell, A.L.M. Colon cancer cells acquire immune regulatory molecules from tumor-infiltrating lymphocytes by trogocytosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2110241118. [Google Scholar] [CrossRef]
- Beekman, J.M. Individualized medicine using intestinal responses to CFTR potentiators and correctors. Pediatr. Pulmonol. 2016, 51, S23–S34. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.C.; Kong, D.F.; Zhao, J.; Faber, K.N.; Xia, Q.; He, K. Liver organoids: Established tools for disease modeling and drug development. Hepatol. Commun. 2023, 7, e0105. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, P.M.; Wu, J.; Luo, Z.G. Advances and Applications of Brain Organoids. Neurosci. Bull. 2023, 39, 1703–1716. [Google Scholar] [CrossRef]
- Zahid, M.U.; Mohsin, N.; Mohamed, A.S.R.; Caudell, J.J.; Harrison, L.B.; Fuller, C.D.; Moros, E.G.; Enderling, H. Forecasting Individual Patient Response to Radiation Therapy in Head and Neck Cancer With a Dynamic Carrying Capacity Model. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Kastner, C.; Hendricks, A.; Deinlein, H.; Hankir, M.; Germer, C.T.; Schmidt, S.; Wiegering, A. Organoid Models for Cancer Research-From Bed to Bench Side and Back. Cancers 2021, 13, 4812. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Kulkarni, P.; Thilakan, J.; Munisamy, M.; Malhotra, A.G.; Singh, J.; Kumar, A.; Rangnekar, V.M.; Arya, N.; Rao, M. Integration of pan-omics technologies and three-dimensional in vitro tumor models: An approach toward drug discovery and precision medicine. Mol. Cancer 2024, 23, 50. [Google Scholar] [CrossRef]
- Liang, W.H.; Federico, S.M.; London, W.B.; Naranjo, A.; Irwin, M.S.; Volchenboum, S.L.; Cohn, S.L. Tailoring Therapy for Children With Neuroblastoma on the Basis of Risk Group Classification: Past, Present, and Future. JCO Clin. Cancer Inform. 2020, 4, 895–905. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef]
- McCauley, H.A.; Wells, J.M. Pluripotent stem cell-derived organoids: Using principles of developmental biology to grow human tissues in a dish. Development 2017, 144, 958–962. [Google Scholar] [CrossRef]
- Corsini, N.S.; Knoblich, J.A. Human organoids: New strategies and methods for analyzing human development and disease. Cell 2022, 185, 2756–2769. [Google Scholar] [CrossRef]
- Nasser, H.; Vera, L.; Elmaleh-Berges, M.; Steindl, K.; Letard, P.; Teissier, N.; Ernault, A.; Guimiot, F.; Afenjar, A.; Moutard, M.L.; et al. CDK5RAP2 primary microcephaly is associated with hypothalamic, retinal and cochlear developmental defects. J. Med. Genet. 2020, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Coppola, G.; Zhang, P.; Abyzov, A.; Provini, L.; Tomasini, L.; Amenduni, M.; Szekely, A.; Palejev, D.; Wilson, M.; et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell 2015, 162, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Barre-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Future Sci. OA 2015, 1, FSO63. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Paik, D.T.; Chandy, M.; Wu, J.C. Patient and Disease-Specific Induced Pluripotent Stem Cells for Discovery of Personalized Cardiovascular Drugs and Therapeutics. Pharmacol. Rev. 2020, 72, 320–342. [Google Scholar] [CrossRef]
- Marcos, L.F.; Wilson, S.L.; Roach, P. Tissue engineering of the retina: From organoids to microfluidic chips. J. Tissue Eng. 2021, 12, 20417314211059876. [Google Scholar] [CrossRef]
- Pollen, A.A.; Bhaduri, A.; Andrews, M.G.; Nowakowski, T.J.; Meyerson, O.S.; Mostajo-Radji, M.A.; Di Lullo, E.; Alvarado, B.; Bedolli, M.; Dougherty, M.L.; et al. Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution. Cell 2019, 176, 743–756 e717. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Peng, B.; Tian, D.; Zhang, D.; Li, Y.; Feng, X.; Liu, J.; Li, J.; Zhang, T.; et al. Generating viable mice with heritable embryonically lethal mutations using the CRISPR-Cas9 system in two-cell embryos. Nat. Commun. 2019, 10, 2883. [Google Scholar] [CrossRef]
- Frum, T.; Spence, J.R. hPSC-derived organoids: Models of human development and disease. J. Mol. Med. 2021, 99, 463–473. [Google Scholar] [CrossRef]
- Gabriel, E.; Ramani, A.; Altinisik, N.; Gopalakrishnan, J. Human Brain Organoids to Decode Mechanisms of Microcephaly. Front. Cell. Neurosci. 2020, 14, 115. [Google Scholar] [CrossRef]
- Santos, J.L.S.; Araujo, C.A.; Rocha, C.A.G.; Costa-Ferro, Z.S.M.; Souza, B.S.F. Modeling Autism Spectrum Disorders with Induced Pluripotent Stem Cell-Derived Brain Organoids. Biomolecules 2023, 13, 260. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Park, J.; Moon, J.H.; Shin, D.; Li, L.; O’Shea, H.; Hwang, S.U.; Lee, H.J.; Brimble, E.; Lee, J.W.; et al. The patient-specific mouse model with Foxg1 frameshift mutation provides insights into the pathophysiology of FOXG1 syndrome. Nat. Commun. 2025, 16, 4760. [Google Scholar] [CrossRef]
- Arjmand, B.; Rabbani, Z.; Soveyzi, F.; Tayanloo-Beik, A.; Rezaei-Tavirani, M.; Biglar, M.; Adibi, H.; Larijani, B. Advancement of Organoid Technology in Regenerative Medicine. Regen. Eng. Transl. Med. 2023, 9, 83–96. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, X.; Xia, X.; Han, M.; Li, F.; Li, C.; Li, Y.; Gao, D. Organoid technology for tissue engineering. J. Mol. Cell Biol. 2020, 12, 569–579. [Google Scholar] [CrossRef]
- Septiana, W.L.; Pawitan, J.A. Potential Use of Organoids in Regenerative Medicine. Tissue Eng. Regen. Med. 2024, 21, 1125–1139. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Ko, E.; Kim, E.M.; Ballance, W.C.; Ito, J.D.; Chalifoux, M.; Kim, Y.J.; Bashir, R.; Kong, H. Extracellular Microenvironmental Control for Organoid Assembly. Tissue Eng. Part. B Rev. 2022, 28, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Lin, B.; Chen, J.T.; Tang, W.C.; Browne, A.W.; Seiler, M.J. The Prospects for Retinal Organoids in Treatment of Retinal Diseases. Asia Pac. J. Ophthalmol. 2022, 11, 314–327. [Google Scholar] [CrossRef]
- Miura, S.; Suzuki, A. Generation of Mouse and Human Organoid-Forming Intestinal Progenitor Cells by Direct Lineage Reprogramming. Cell Stem Cell 2017, 21, 456–471 e5. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, W.; Gao, Y.; Yi, Z.; Chen, Z.; Qu, C.; Huang, J.; Liu, F.; Liu, Z. Application of Organoids in Regenerative Medicine. Stem Cells 2023, 41, 1101–1112. [Google Scholar] [CrossRef]
- Wojciechowski, D.; Wiseman, A. Long-Term Immunosuppression Management: Opportunities and Uncertainties. Clin. J. Am. Soc. Nephrol. 2021, 16, 1264–1271. [Google Scholar] [CrossRef]
- Su, X.; Yue, P.; Kong, J.; Xu, X.; Zhang, Y.; Cao, W.; Fan, Y.; Liu, M.; Chen, J.; Liu, A.; et al. Human Brain Organoids as an In Vitro Model System of Viral Infectious Diseases. Front. Immunol. 2021, 12, 792316. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Xu, R.; Yi, G.; Li, Z.; Zhang, H.; Qi, S.; Huang, G. Patient-derived organoids in human cancer: A platform for fundamental research and precision medicine. Mol. Biomed. 2024, 5, 6. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Roper, J.; Yilmaz, O.H. Breakthrough Moments: Genome Editing and Organoids. Cell Stem Cell 2019, 24, 841–842. [Google Scholar] [CrossRef]
- Suhito, I.R.; Sunil, C.; Tay, A. Engineering human immune organoids for translational immunology. Bioact. Mater. 2025, 44, 164–183. [Google Scholar] [CrossRef]
- Yousefi, F.; Foster, L.A.; Selim, O.A.; Zhao, C. Integrating Physical and Biochemical Cues for Muscle Engineering: Scaffolds and Graft Durability. Bioengineering 2024, 11, 1245. [Google Scholar] [CrossRef]
- Lin, B.; McLelland, B.T.; Aramant, R.B.; Thomas, B.B.; Nistor, G.; Keirstead, H.S.; Seiler, M.J. Retina Organoid Transplants Develop Photoreceptors and Improve Visual Function in RCS Rats With RPE Dysfunction. Investig. Ophthalmol. Vis. Sci. 2020, 61, 34. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, J.; Zaalberg, I.C.; Maurice, M.M. Organoid-based modeling of intestinal development, regeneration, and repair. Cell Death Differ. 2021, 28, 95–107. [Google Scholar] [CrossRef]
- Michalska, N.; Toton, E.; Kopczynski, P.; Jankowska-Wajda, M.; Rubis, B. Alternative Therapies in Transplantology as a Promising Perspective in Medicine. Ann. Transplant. 2024, 29, e943387. [Google Scholar] [CrossRef]
- Matsumoto, K.; Fujimori, N.; Ichihara, K.; Takeno, A.; Murakami, M.; Ohno, A.; Kakehashi, S.; Teramatsu, K.; Ueda, K.; Nakata, K.; et al. Patient-derived organoids of pancreatic ductal adenocarcinoma for subtype determination and clinical outcome prediction. J. Gastroenterol. 2024, 59, 629–640. [Google Scholar] [CrossRef]
- Caipa Garcia, A.L.; Arlt, V.M.; Phillips, D.H. Organoids for toxicology and genetic toxicology: Applications with drugs and prospects for environmental carcinogenesis. Mutagenesis 2022, 37, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Wagar, L.E.; Salahudeen, A.; Constantz, C.M.; Wendel, B.S.; Lyons, M.M.; Mallajosyula, V.; Jatt, L.P.; Adamska, J.Z.; Blum, L.K.; Gupta, N.; et al. Modeling human adaptive immune responses with tonsil organoids. Nat. Med. 2021, 27, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Schwank, G.; Koo, B.-K.; Sasselli, V.; Dekkers, J.F.; Heo, I.; Demircan, T.; Sasaki, N.; Boymans, S.; Cuppen, E.; van der Ent, C.K.; et al. Functional Repair of CFTR by CRISPR/Cas9 in Intestinal Stem Cell Organoids of Cystic Fibrosis Patients. Cell Stem Cell 2013, 13, 653–658. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Liu, Y.; Shin, S.; Mattson, M.P.; Rao, M.S.; Zhan, M. Cross-species transcriptional profiles establish a functional portrait of embryonic stem cells. Genomics 2007, 89, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Clevers, H. Organoid culture systems to study host-pathogen interactions. Curr. Opin. Immunol. 2017, 48, 15–22. [Google Scholar] [CrossRef]
- Bonaiti, E.; Muraro, M.G.; Robert, P.A.; Jakscha, J.; Dirnhofer, S.; Martin, I.; Berger, C.T. Tonsil explants as a human in vitro model to study vaccine responses. Front. Immunol. 2024, 15, 1425455. [Google Scholar] [CrossRef]
- Verissimo, C.S.; Overmeer, R.M.; Ponsioen, B.; Drost, J.; Mertens, S.; Verlaan-Klink, I.; Gerwen, B.V.; van der Ven, M.; Wetering, M.V.; Egan, D.A.; et al. Targeting mutant RAS in patient-derived colorectal cancer organoids by combinatorial drug screening. Elife 2016, 5, e18489. [Google Scholar] [CrossRef]
- de Poel, E.; Lefferts, J.W.; Beekman, J.M. Intestinal organoids for Cystic Fibrosis research. J. Cyst. Fibros. 2020, 19 (Suppl. S1), S60–S64. [Google Scholar] [CrossRef]
- Saint-Criq, V.; Gray, M.A. Role of CFTR in epithelial physiology. Cell. Mol. Life Sci. 2017, 74, 93–115. [Google Scholar] [CrossRef]
- Lomunova, M.A.; Gershovich, P.M. Gene Therapy for Cystic Fibrosis: Recent Advances and Future Prospects. Acta Naturae 2023, 15, 20–31. [Google Scholar] [CrossRef]
- Aalbers, B.L.; Brunsveld, J.E.; van der Ent, C.K.; van den Eijnden, J.C.; Beekman, J.M.; Heijerman, H.G.M. Forskolin induced swelling (FIS) assay in intestinal organoids to guide eligibility for compassionate use treatment in a CF patient with a rare genotype. J. Cyst. Fibros. 2022, 21, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Geurts, M.H.; de Poel, E.; Amatngalim, G.D.; Oka, R.; Meijers, F.M.; Kruisselbrink, E.; van Mourik, P.; Berkers, G.; de Winter-de Groot, K.M.; Michel, S.; et al. CRISPR-Based Adenine Editors Correct Nonsense Mutations in a Cystic Fibrosis Organoid Biobank. Cell Stem Cell 2020, 26, 503–510 e7. [Google Scholar] [CrossRef] [PubMed]
- Altunel, E.; Roghani, R.S.; Chen, K.Y.; Kim, S.Y.; McCall, S.; Ware, K.E.; Shen, X.; Somarelli, J.A.; Hsu, D.S. Development of a precision medicine pipeline to identify personalized treatments for colorectal cancer. BMC Cancer 2020, 20, 592. [Google Scholar] [CrossRef] [PubMed]
- St John, J.; Powell, K.; Conley-Lacomb, M.K.; Chinni, S.R. TMPRSS2-ERG Fusion Gene Expression in Prostate Tumor Cells and Its Clinical and Biological Significance in Prostate Cancer Progression. J. Cancer Sci. Ther. 2012, 4, 94–101. [Google Scholar] [CrossRef]
- Menche, C.; Farin, H.F. Strategies for genetic manipulation of adult stem cell-derived organoids. Exp. Mol. Med. 2021, 53, 1483–1494. [Google Scholar] [CrossRef]
- Andreatta, F.; Hendriks, D.; Artegiani, B. Human Organoids as an Emerging Tool for Genome Screenings. Annu. Rev. Biomed. Eng. 2025, 27, 157–183. [Google Scholar] [CrossRef]
- Bock, C.; Datlinger, P.; Chardon, F.; Coelho, M.A.; Dong, M.B.; Lawson, K.A.; Lu, T.; Maroc, L.; Norman, T.M.; Song, B.; et al. High-content CRISPR screening. Nat. Rev. Methods Primers 2022, 2, 8. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef]
- Yu, F.; Hunziker, W.; Choudhury, D. Engineering Microfluidic Organoid-on-a-Chip Platforms. Micromachines 2019, 10, 165. [Google Scholar] [CrossRef]
- Augustine, R.; Kalva, S.N.; Ahmad, R.; Zahid, A.A.; Hasan, S.; Nayeem, A.; McClements, L.; Hasan, A. 3D Bioprinted cancer models: Revolutionizing personalized cancer therapy. Transl. Oncol. 2021, 14, 101015. [Google Scholar] [CrossRef] [PubMed]
- LeSavage, B.L.; Suhar, R.A.; Broguiere, N.; Lutolf, M.P.; Heilshorn, S.C. Next-generation cancer organoids. Nat. Mater. 2022, 21, 143–159. [Google Scholar] [CrossRef]
- Liu, Y.; Gan, Y.; AiErken, N.; Chen, W.; Zhang, S.; Ouyang, J.; Zeng, L.; Tang, D. Combining Organoid Models with Next-Generation Sequencing to Reveal Tumor Heterogeneity and Predict Therapeutic Response in Breast Cancer. J. Oncol. 2022, 2022, 9390912. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Liu, X.; Shi, Q.; He, B.; Zheng, C.; Meng, X. Breast cancer organoids and their applications for precision cancer immunotherapy. World J. Surg. Oncol. 2023, 21, 343. [Google Scholar] [CrossRef]
- Garg, P.; Malhotra, J.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Emerging Therapeutic Strategies to Overcome Drug Resistance in Cancer Cells. Cancers 2024, 16, 2478. [Google Scholar] [CrossRef] [PubMed]
- Werschler, N.; Quintard, C.; Nguyen, S.; Penninger, J. Engineering next generation vascularized organoids. Atherosclerosis 2024, 398, 118529. [Google Scholar] [CrossRef]
- Chhibber, T.; Bagchi, S.; Lahooti, B.; Verma, A.; Al-Ahmad, A.; Paul, M.K.; Pendyala, G.; Jayant, R.D. CNS organoids: An innovative tool for neurological disease modeling and drug neurotoxicity screening. Drug Discov. Today 2020, 25, 456–465. [Google Scholar] [CrossRef]
- Hartung, T.; Morales Pantoja, I.E.; Smirnova, L. Brain organoids and organoid intelligence from ethical, legal, and social points of view. Front. Artif. Intell. 2023, 6, 1307613. [Google Scholar] [CrossRef]
- Taurin, S.; Alzahrani, R.; Aloraibi, S.; Ashi, L.; Alharmi, R.; Hassani, N. Patient-derived tumor organoids: A preclinical platform for personalized cancer therapy. Transl. Oncol. 2025, 51, 102226. [Google Scholar] [CrossRef]
- Ge, J.Y.; Wang, Y.; Li, Q.L.; Liu, F.K.; Lei, Q.K.; Zheng, Y.W. Trends and challenges in organoid modeling and expansion with pluripotent stem cells and somatic tissue. PeerJ 2024, 12, e18422. [Google Scholar] [CrossRef]
- Tsuruta, S.; Uchida, H.; Akutsu, H. Intestinal Organoids Generated from Human Pluripotent Stem Cells. JMA J. 2020, 3, 9–19. [Google Scholar] [PubMed]
- Funata, M.; Nio, Y.; Erion, D.M.; Thompson, W.L.; Takebe, T. The promise of human organoids in the digestive system. Cell Death Differ. 2021, 28, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Mollaki, V. Ethical Challenges in Organoid Use. BioTech 2021, 10, 12. [Google Scholar] [CrossRef]
- de Jongh, D.; Massey, E.K.; VANGUARD Consortium; Bunnik, E.M. Organoids: A systematic review of ethical issues. Stem Cell Res. Ther. 2022, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Jeziorski, J.; Brandt, R.; Evans, J.H.; Campana, W.; Kalichman, M.; Thompson, E.; Goldstein, L.; Koch, C.; Muotri, A.R. Brain organoids, consciousness, ethics and moral status. Semin. Cell Dev. Biol. 2023, 144, 97–102. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.M.; Kumbar, S.G.; Nukavarapu, S.P. Decellularized extracellular matrix biomaterials for regenerative therapies: Advances, challenges and clinical prospects. Bioact. Mater. 2024, 32, 98–123. [Google Scholar] [CrossRef]
- Xiang, N.; Ni, Z. Innovations in Microfluidics to Enable Novel Biomedical Applications. Biosensors 2024, 14, 507. [Google Scholar] [CrossRef]
- Zahmatkesh, E.; Khoshdel-Rad, N.; Mirzaei, H.; Shpichka, A.; Timashev, P.; Mahmoudi, T.; Vosough, M. Evolution of organoid technology: Lessons learnt in Co-Culture systems from developmental biology. Dev. Biol. 2021, 475, 37–53. [Google Scholar] [CrossRef]
- Reina-Mahecha, A.; Beers, M.J.; van der Veen, H.C.; Zuhorn, I.S.; van Kooten, T.G.; Sharma, P.K. A Review of the Role of Bioreactors for iPSCs-Based Tissue-Engineered Articular Cartilage. Tissue Eng. Regen. Med. 2023, 20, 1041–1052. [Google Scholar] [CrossRef]
- Bleijs, M.; van de Wetering, M.; Clevers, H.; Drost, J. Xenograft and organoid model systems in cancer research. EMBO J. 2019, 38, e101654. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- De Spirito, M.; Palmieri, V.; Perini, G.; Papi, M. Bridging the Gap: Integrating 3D Bioprinting and Microfluidics for Advanced Multi-Organ Models in Biomedical Research. Bioengineering 2024, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Puigvert, J.; Spjuth, O. Artificial intelligence for high content imaging in drug discovery. Curr. Opin. Struct. Biol. 2024, 87, 102842. [Google Scholar] [CrossRef]
- Jin, H.; Xue, Z.; Liu, J.; Ma, B.; Yang, J.; Lei, L. Advancing Organoid Engineering for Tissue Regeneration and Biofunctional Reconstruction. Biomater. Res. 2024, 28, 0016. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Z.; Zhang, Y.; Zhong, H.; Cai, X.; Guan, R. Recent progress on the organoids: Techniques, advantages and applications. Biomed. Pharmacother. 2025, 185, 117942. [Google Scholar] [CrossRef]


| Application Area | Description | References |
|---|---|---|
| Pharmaceutical Development | Used by biotech companies to develop and optimize novel drug combinations and therapeutic strategies. | [71] |
| Translational Research | Preserve histological and molecular features of primary tumors, improving the relevance of preclinical studies. | [72] |
| Personalized Medicine | Allow drug testing on patient-specific tumor organoids to guide therapy decisions based on individual responses. | [75] |
| Tumor Modeling | Recapitulate 3D tumor architecture, genetic heterogeneity, and cell–cell interactions more accurately than 2D cultures. | [80] |
| Drug Resistance Studies | Enable long-term drug exposure studies to understand mechanisms of acquired resistance. | [81] |
| High-Throughput Drug Screening | Facilitate rapid testing of multiple drugs across various tumor subtypes using organoid biobanks. | [74,82] |
| Immuno-Oncology | Co-culture with immune cells to evaluate immunotherapy responses, such as CAR T-cell therapy and immune checkpoint inhibitors. | [83] |
| Application Area | Description | References |
|---|---|---|
| Drug Screening | Provide physiologically relevant models for screening drug response, toxicity, and efficacy. | [74] |
| Comparative Studies (PDX vs. Organoids) | Assess drug sensitivity differences and complement PDX models in preclinical testing. | [79] |
| Hepatotoxicity and Nephrotoxicity | Liver and kidney organoids outperform traditional cell lines and animal models in predicting drug toxicity. | [81] |
| Infectious Disease Drug Testing | Enable antiviral drug evaluation using vascular organoids, e.g., for SARS-CoV-2. | [95] |
| Personalized Drug Testing (PDOs) | Enable patient-specific drug response prediction and therapy selection. | [96] |
| Tumor Heterogeneity Analysis | Model diverse genetic and phenotypic profiles of tumors to inform treatment strategies. | [97] |
| Combination Chemotherapy | Facilitate studies to assess synergistic effects between multiple drug treatments. | [98] |
| Cardiotoxicity Modeling | Cardiac organoids help evaluate cardiotoxic effects of drugs with greater physiological relevance than 2D cultures. | [98] |
| Functional Biomarker Discovery | Enable discovery of biomarkers predicting drug efficacy beyond genetic markers. | [99] |
| Microfluidics Integration | Microfluidic platforms improve real-time drug response monitoring. | [100] |
| Application Area | Description | References |
|---|---|---|
| Longitudinal Disease Monitoring | Monitor tumor evolution and resistance mechanisms through serial PDO generation and testing. | [81] |
| Immunotherapy Prediction (PDO + Immune Cells) | Co-culture organoids with autologous immune cells to evaluate immune response and predict efficacy of checkpoint inhibitors and CAR-T therapy. | [83] |
| Functional Precision Oncology | Provide functional validation of treatment strategies in real time, complementing genomic approaches. | [85] |
| Patient-Derived Organoids (PDOs) | Enable personalized treatment by testing therapies on patient-specific tumor organoids. | [88,89] |
| Rapid Clinical Decision Support | Reduce turnaround time from biopsy to treatment planning using organoid-based testing workflows. | [89] |
| Companion Diagnostic Development | Assist in developing biomarkers or tests to predict which patients will benefit from specific treatments. | [102] |
| Therapy Response Prediction | Accurately forecast patient response to chemotherapies, targeted agents, and radiation. | [114] |
| Rare Cancer Models | Facilitate treatment decision-making for patients with rare or atypical cancers using PDO-based drug response data. | [115] |
| Molecular Profiling Integration | Combine organoid testing with genomic, transcriptomic, and proteomic data to tailor therapy. | [116] |
| Pediatric Oncology Applications | Offer a viable model for tailoring therapies in pediatric tumors with limited treatment options. | [117] |
| Application Area | Description | References |
|---|---|---|
| PSC-Derived Organoids | Generated from ESCs or iPSCs, capable of forming all three germ layers to model early development stages. | [107] |
| Human-Specific Developmental Modeling | Enable modeling of human-specific biological processes not reproducible in animal models due to species differences in physiology and genetics. | [123] |
| Directed Differentiation | Use of growth factors and cytokines to guide germ layer formation and cell maturation into complex tissues. | [124] |
| Patient-Specific Disease Models | iPSC-derived organoids allow the creation of individualized models to study genetic disorders and patient-specific pathologies. | [53,125] |
| Hard-to-Obtain Tissue Modeling | Facilitate the engineering of inaccessible tissues such as brain and retina. | [126] |
| Neurodevelopmental Research | Reveal human-specific features by comparing brain organoids from humans and primates at the single-cell level. | [127] |
| Overcoming Embryonic Lethality | Enable knockout studies of essential genes that would be lethal in animal embryos. | [128] |
| Embryonic and Fetal Development Insights | PSC-derived organoids model early- to mid-gestation stages, aiding the study of human development and pregnancy-related diseases. | [129] |
| Neuropsychiatric Disease Modeling | Allow investigation of disorders such as microcephaly and autism via patient-derived brain organoids. | [130,131] |
| Genetic Pathway Analysis | Enable identification of gene dysregulation (e.g., FoxG1 upregulation in autism), providing insights into developmental gene networks. | [132] |
| Application Area | Description | References |
|---|---|---|
| Organ-on-Chip Systems | Integration with microfluidics allows the creation of organoids-on-chips to study organ function in dynamic and controlled conditions. | [25,36] |
| Genetic Correction and Autologous Repair | Combine with genetic editing to enable patient-specific therapies with reduced risk of immune rejection. | [44] |
| Disease Modeling | Used to study organ-specific diseases, including neurological and psychiatric conditions. | [70] |
| Functional 3D Tissue Models | Self-assemble into complex, stable, and functional tissue-like architectures, unlike traditional 2D cell cultures. | [4,118] |
| Regenerative Medicine | Mimic the structure and function of native tissues, offering potential for repair or replacement of damaged organs. | [133] |
| Tissue Engineering Integration | Combine stem cells, scaffolds, and biochemical cues to create bioengineered tissues that replicate physiological conditions. | [146] |
| Transplantation Potential | Demonstrate regenerative ability in animal models (e.g., retinal sheets and intestinal organoids restoring tissue function). | [147,148] |
| Overcoming Transplantation Barriers | Offer an alternative to donor-dependent organ transplantation, eliminating issues like donor shortage and immune rejection. | [149] |
| Personalized Therapeutics | Enable individualized treatment development through patient-specific organoids for drug screening and toxicity assessment. | [18,150] |
| Application Area | Description | References |
|---|---|---|
| Environmental Toxicology | Cerebral organoids model neurotoxicant effects (e.g., methyl-mercury, bisphenol A), disrupting cortical development and synaptogenesis. | [17] |
| Infectious Disease Modeling | Lung and intestinal organoids simulate host-pathogen interactions, e.g., SARS-CoV-2, rotavirus, norovirus, Helicobacter pylori. | [155] |
| Vaccine Development and Immunology | Tonsil-derived organoids mimic germinal center formation and antigen-specific B cell activation to evaluate vaccine efficacy in vitro. | [156] |
| Gene-Therapy Testing | Organoids serve as platforms for gene-editing validation, such as CRISPR correction of CFTR mutations in cystic fibrosis organoids. | [153] |
| Comparative Evolutionary Biology | Cross-species organoids from human, primate, and rodent stem cells allow the study of species-specific development and gene regulation. | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makesh, K.Y.; Navaneethan, A.; Ajay, M.; Munuswamy-Ramanujam, G.; Chinnasamy, A.; Gnanasampanthapandian, D.; Palaniyandi, K. A Concise Review of Organoid Tissue Engineering: Regenerative Applications and Precision Medicine. Organoids 2025, 4, 16. https://doi.org/10.3390/organoids4030016
Makesh KY, Navaneethan A, Ajay M, Munuswamy-Ramanujam G, Chinnasamy A, Gnanasampanthapandian D, Palaniyandi K. A Concise Review of Organoid Tissue Engineering: Regenerative Applications and Precision Medicine. Organoids. 2025; 4(3):16. https://doi.org/10.3390/organoids4030016
Chicago/Turabian StyleMakesh, Karnika Yogeswari, Abilash Navaneethan, Mrithika Ajay, Ganesh Munuswamy-Ramanujam, Arulvasu Chinnasamy, Dhanavathy Gnanasampanthapandian, and Kanagaraj Palaniyandi. 2025. "A Concise Review of Organoid Tissue Engineering: Regenerative Applications and Precision Medicine" Organoids 4, no. 3: 16. https://doi.org/10.3390/organoids4030016
APA StyleMakesh, K. Y., Navaneethan, A., Ajay, M., Munuswamy-Ramanujam, G., Chinnasamy, A., Gnanasampanthapandian, D., & Palaniyandi, K. (2025). A Concise Review of Organoid Tissue Engineering: Regenerative Applications and Precision Medicine. Organoids, 4(3), 16. https://doi.org/10.3390/organoids4030016







