Abstract
In 2023, at the Center for Biological Resources (CRB) at the Institute of Genetics and Biophysics (IGB, Naples, Italy) of the National Research Council (CNR), the Breast Cancer Tissues and Organoids Biobank (BCTO BioBank) was founded. This is a new generation Biobank, dedicated to the collection, characterization, storage, and distribution of tissues and their 3D ‘organoid’ patients-derived. Tumor and healthy tissues from breast cancer patients have been collected from surgeons at Monaldi Hospital (Naples, Italy) and used to generate the corresponding tumor and healthy organoids from the same patient. After their establishment in culture, both organoids were characterized for their receptor status on a microfluidic 2-lane OrganoPlate, by immunofluorescence. The resulting data were compared with the expression profile obtained by immunohistochemistry on respective parental tissues. These data allowed us to phenotypically validate the generated organoids and classify them in a dedicated database, where also the clinical data of the corresponding patients were collected. During the six months of activities, we collected and characterized 27 samples. The continuous BCTO BioBank activity is fundamental to generating a high number of samples, for a broader and efficiently elaborated patient stratification at molecular level, biomarker discovery investigations, and for tailored treatment protocols design.
1. Introduction
Breast cancer (BC) remains one of the most diagnosed cancers as well as the leading cause of death among women worldwide (World Health Organization WH. Breast Cancer 2021. (https://www.who.int/news-room/fact-sheets/detail/breast-cancer, accessed on 8 February 2024) accounting for 6.9% of all cancer-related deaths globally in 2020 [1]. A total of 531,000 new BC cases have been registered in Europe in 2020, and an increase to 568,000 is predicted by 2040 (GLOBOCAN 2020: Estimated European Breast Cancer Incidence in 2040. https://gco.iarc.fr/tomorrow/en/dataviz/isotype?types=0&sexes=0&mode=population&group_populations=1&multiple_populations=1&multiple_cancers=0&cancers=20&populations=908&single_unit=50000, accessed on 8 February 2024). Breast cancer is by far the most prevalent cancer type among females in Italy, with almost 56 thousand estimated new cases.
This type of cancer is commonly classified, according to the 13th St. Gallen International Breast Cancer Conference, in five distinct immunohistochemical subtypes based on the expression pattern of various receptors: estrogen (ER), progesterone (PR) and human epidermal growth factor receptor 2 (HER2), and of cell proliferation marker Ki-67 [2].
They are defined as luminal A, luminal B, HER2-enriched, basal-like, and normal-like breast cancer. Luminal A is characterized by a high expression of ER and/or PR, the absence of HER2 and a low expression of cell proliferation marker Ki-67 (less than 20%); they represent about 70% of all BC cases. Luminal B shows ER, and/or PR, HER2 and a high expression level of Ki-67 (more than 20%); they represent about 12% of BC cases. HER2-enriched subtype which is ER and PR negative with a high HER2 expression represents about 5% of BC cases, and triple negative, lacking ER, PR, and HER2 expression with a high expression level of Ki-67 make up about 12% of BC cases.
Although cancer immunohistochemical characterization represents a valid tool for choosing the more appropriate patient’s treatment, often patients do not respond to the therapies and their prognosis are even inconsistent with that one expected based on their molecular features.
For decades, pre-clinical BC research has relied on a cell line system as the in vitro representation of this disease or PDX (Patient-Derived Xenografts) models. However, BC cell lines do not fully capture the large BC spectrum and are not clinically relevant for individual patients [3], while PDX models, although they capture tumor heterogeneity, do not allow drug high-throughput screening (HTS). Specifically, this issue has been tackled by directly treating mice in vivo [4], or by PDX-derived cultures in vitro [5]. However, both methods, although very promising, are limited since the first depends on PDX generation and the second one does not allow extended passaging in vitro.
Furthermore, the development of protocols to establish mammospheres in vitro provides a 3D cell system for preclinical BC research [6]. This system offers a specific advantage over previous models, inasmuch as it does not require the exclusion of cells from the bulk population or require genetic modification prior to study, thus allowing to examine the entire, highly heterogeneous population. Its use has been mainly for the drug HTS.
Recently, the development of near-physiological, self-renewing organoids in culture [7] has provided a valid tool to investigate cancer biology and identify new treatments [8,9,10,11]. Currently, patient-derived organoids (PDOs) from colorectal cancers are used in preclinical and clinical trials like SENSOR studies [12], showing their huge potential.
Thus, we decided to develop a Biobank of breast cancer organoids in Southern Italy. Here, we illustrated the procedures we adopted to generate organoids from breast cancer patients for the purpose of culturing and phenotypically characterization. We also describe the database we developed for tracking all the data related to all the samples stored in the BCTO Biobank.
2. Materials and Methods
2.1. Patient’s Eligibility Criteria
Patients eligible for our Biobank storage were aged ≥18 years <90 years, able to understand and willing to sign the informed consent form (Supplementary Materials, consent form) and to comply with the study visit schedule and other protocol requirements; diagnosed with primary and metastatic breast cancer and patients, under follow-up, back to the hospital (Supplementary Materials, Table S1). Patients with Hepatitis or HIV or hematological malignancies were not included in this study. All enrolled patients were informed about the study and all patients’ written informed consents were obtained prior to any procedure.
2.2. Patient Derived Organoids (PDOs) Establishment
To generate patient-derived organoids (PDOs), tumor and healthy pieces were resected from the surgical specimens derived from the same patient, and placed into a 15 mL tube containing 5 mL of ice-cold PBS and Pen/Strep, assuring to keep it at 4 °C. Both were processed in the same way within 2–48 h from their collection following the protocol below. All procedures were carried out in sterile conditions in a biosafety cabinet using BioSafety Level 2 (BSL2) techniques.
- (a)
- PDOs Establishment Protocol
- I.
- After collecting, the healthy (h) and tumor (t) tissue pieces were transferred separately to a 10 cm cell culture dish, keeping them moist with PBS/Pen/Strep.
- II.
- Each piece of tissue was chopped into small fragments of 1–3 mm, using scissors, making sure to remove non-epithelial components (i.e., adipose tissue, mainly present in breast tissue).
- III.
- One or two fragments, depending on the abundance of material available, from each minced piece, were fixed in 10% (wt/vol) neutral buffered formalin for their paraffin embedding.
- IV.
- Two or more fragments from each minced piece were also snap-frozen for molecular/biochemical analysis at −80 °C.
- V.
- The remaining minced tissue was transferred using a cell lifter to a 15 mL tube, containing the digestion buffer (10 mL/g of tissue; Supplementary Data, File S1) and subjected to enzymatic digestion for 60–120 min at 37 °C.
- VI.
- Digestion was monitored by microscope observation, transferring the digestion mixture to a MW-6 every 10–15 min. Each time the digested tissue was returned to the 15 mL tube, it was pipetted vigorously up and down using a P1000 pipette 30 times and then placed back at 37 °C to continue enzymatic digestion for as long as needed (usually this step takes no more than 60–120 min).
- VII.
- When the tissue was disrupted and the mixture became cloudy, the tissue was further broken up by mechanically pipetting up and down 10–20 times, using a P1000 pipette (Gilson, Milan, Italy).
- VIII.
- adDMEM/F12+++ medium (8 mL; Supplementary Data, File S1) was added to the mixture and then it was centrifuged at 300× g for 5 min at 4 °C. If the pellet contained a lot of fat, one or two additional washing steps were performed by using 10 mL of adDMEM/F12+++ medium.
- IX.
- The pellet was resuspended in 10 mL adDMEM/F12+++ medium and filtered using a 100 μm cell strainer (431752, Corning Gilson, Milan, Italy).
- X.
- If a red layer on the top of the pellet was observed, due to the presence of red blood cells, 1 mL of ACK Lysing Buffer was added and incubated for 10 min in a 37 °C water bath. Then, 10 mL of adDMEM/F12+++ medium was added to neutralize the red lysis buffer and a centrifuge (at 300× g for 5 min at 4 °C) was performed.
- XI.
- After aspirating the supernatant, the pellet was resuspended in Reduced Growth Factor Basement Membrane Extract (RGF BME), Type 2, previously kept on ice, to prevent it from solidifying.
- XII.
- Approximately 10,000 cells were plated in 20 μL of 80% BME (vol/vol) to ensure the formation of solid droplets into MW-48 plate, placing one drop for each well.
- XIII.
- The culture plate, flipped upside down, was then placed into a humidified incubator at 37 °C and 5% (vol/vol) CO2 for 30 min to let the BME solidify.
- XIV.
- The required amount of organoid medium (medium A), containing the growth factors listed in Table S2 (Supplementary Materials, File S1), 10 μM ROCK inhibitor and Primocin (100 μg/mL) was pre-heated in the 37 °C water bath.
- XV.
- Once the BME drops were solidified (~30 min), the plate was flipped upside down and 500 μL of organoid medium was carefully added to each well.
- XVI.
- Then, the culture plate was placed on the OrganoFlow L, Perfusion Rocker (L-191100104, Mimetas, 2342 DH Oegstgeest The Netherlands) in a humidified incubator at 37 °C and 5% (vol/vol) CO2.
- XVII.
- The medium was changed every 2–3 days, by carefully aspirating the medium from the wells and replacing it with fresh, pre-heated medium, containing ROCK inhibitor and Primocin in the culture medium for the first 5 days of the culture.
- XVIII.
- After five days, culture medium A was substituted with the B ones (Supplementary Materials, File S1).
- (b)
- PDOs Culturing Protocol
BC PDOs were generally passed every 2 weeks by only mechanical dissociation in case of the small and non-compact organoids or by a combination of mechanical and enzymatic dissociation for big and compact organoids, as described below.
PDOs mechanical dissociation
- I.
- BME drops were dissolved, and the organoids were dissociated, by pipetting 20 times up and down the medium inside of each well into the plate with a P1000 filter tip with a (non-filtered) P100 tip on top to aid the disaggregation of the organoids. Then, they were transferred into a 15 mL tube.
- II.
- Each well was further washed twice using the ice-cold adDMEM/F12+++ medium
- III.
- Both washes were collected into the same 15 mL tube with the previously transferred BME drops containing the disaggregated organoids, and centrifuged at 300× g, T = 8 °C for 5 min.
- IV.
- The supernatant was discarded.
- V.
- The pellet was resuspended into the appropriate volume of 80% BME (vol/vol) to expand the culture 1:2–1:3, by following the seeding procedure described above.
- VI.
- Then, the plate was placed on the Perfusion Rocker, in a humidified incubator at 37 °C and 5% (vol/vol) CO2 for PDOs culturing.
PDOs enzymatic dissociation
- I.
- After the washing step of the PDOs mechanical dissociation procedure, the pellets were further dissociated using TrypLE dissociation buffer. Thus, after the centrifugation at step 3, 1 mL of TrypLE buffer was added.
- II.
- The pellet was then resuspended by pipetting up and down for 20 times, using a P1000 filter tip with a (non-filtered) P10 tip on top, followed by an incubation of 7 min at 37 °C.
- III.
- PDOs shearing was checked under the light microscope, to make sure that organoids were disaggregated.
- IV.
- Then, 10 mL of the adDMEM/F12+++ medium was added to neutralize TrypLE, and the sheared organoids were centrifuged at 300× g, T = 8 °C for 5 min.
- V.
- The supernatant was discarded.
- VI.
- The pellet was resuspended into the appropriate volume of 80% BME (vol/vol) to expand the organoid culture 1:2–1:3, by following the seeding procedure described above.
- VII.
- Then, the plate was placed on the Perfusion Rocker, in a humidified incubator at 37 °C and 5% (vol/vol) CO2 for PDOs culturing.
- (c)
- PDOs Freezing Protocol
PDO cultures were tested for mycoplasma, by using a PCR Mycoplasma Detection Kit (G238, abm), before their freezing and storage into the Biobank.
- I.
- BME drops containing organoids from 12 wells of a 48-well plate were pooled in a 15 mL conical tube.
- II.
- To dissolve the BME gel, 10 mL of the ice-cold adDMEM/F12+++ medium was added, pipetting up and down several times with a 10 mL serological pipette in the tube placed in ice.
- III.
- The sample was incubated in ice for 10 min.
- IV.
- Then, the organoids were centrifuged at 300× g for 5 min at 4 °C.
- V.
- The supernatant was aspirated without disturbing the pellet.
- VI.
- The organoids were resuspended in 500 μL of the freezing medium (Supplementary Materials, File S1) in a sterile cryogenic vial.
- VII.
- The cryogenic vial was placed into a freezing container and stored at −80 °C for 24 h.
- VIII.
- Then, the cryo-vial was transferred into the liquid nitrogen tank (approximately −180 °C).
- (d)
- PDOs Thawing Protocol
- I.
- A 15 mL conical tube with 10 mL of the adDMEM/F12+++ medium was prepared at room temperature, before collecting the cryovial in dry ice from the liquid nitrogen tank.
- II.
- The cryogenic vial was incubated for 1–3 min in a water bath at 37 °C.
- III.
- A total of 1 mL of adDMEM/F12+++ medium was added dropwise into the cryogenic vial while mixing, and then the entire volume with the organoids in the vial was transferred to the 15 mL tube, previously prepared.
- IV.
- The organoids were centrifuged at 300× g for 5 min at 4 °C.
- V.
- The supernatant was removed without disturbing the pellet.
- VI.
- Then, organoids were resuspended into the appropriate volume of 80% BME (vol/vol) by following the seeding procedure described above.
2.3. Formalin-Fixed Paraffin Embedded (FFPE) PDOs Procedure
To PDOs paraffin embedding, the medium from each well of a 48-well plate was removed and the organoids were washed twice with sterile 1× phosphate-buffered saline (PBS). Then, they were fixed with 10% neutral buffered formalin (500 μL) fixative for 30 min at room temperature. After washing the organoids twice with sterile 1× phosphate-buffered saline (PBS) they were collected in a 1.5 mL centrifuge tube, using a lifter and placed in a mold for the paraffin embedding procedure using a Thermo Scientific HistoStar Embedding Workstation (GMI, Ramsey, MN 55303, USA). Paraffin blocks were cut to obtain 3 μm thick slices, which were mounted on DFrost Plus Microscope slides positively charged (060SFPD, DiaPath, Bergamo, Italy). Tissues and PDOs slices were deparaffinized in xylene and rehydrated through graded decreasing concentrations of alcohol and used for immunofluorescence studies and H&E staining.
2.4. PDOs if on a Microfluidic OrganoPlate
BC organoids were grown in a microfluidic 2-lane OrganoPlate® (9605-400-B, Mimetas, 2342 DH Oegstgeest, The Netherlands) platform for extracellular matrix (ECM) embedded cancer culture under perfusion, following the manufacturer’s seeding protocol. Briefly, organoids (2 µL for each well) were seeded in the gel channel of a 2-lane OrganoPlate in 80% BME gel for their culturing. To investigate the receptor status of the samples, PDOs in each well were fixed using 10% neutral buffered formalin for 30 min at room temperature. After washing twice with 1× phosphate-buffered saline (PBS), PDOs were permeabilized only for the detection of nuclear and cytoplasmatic antigens using PBS containing 0.5% Triton X100 for 7 min. This step of permeabilization was performed twice. Then, PDOs-on-chip were washed with PBS and successively a blocking step was carried out by using 2% NGS, 2% BSA, and 0.1% Tween20 in PBS for 45 min at room temperature (RT). After this step, the primary antibody was added, and the microplate was placed on the rocker platform at RT, overnight. After the incubation with the primary antibody, the PDOs-on-chip were washed twice with 2% NGS in PBS for 3 min and then incubated with the fluorescent secondary antibodies for 4 h in the dark at RT. The PDOs were washed twice with 2% NGS in PBS and once with PBS (3 min for each washing step). Then, the nuclei were stained using Hoectsh, according to the manufacturer’s protocol, for 30 min in the dark at RT. A washing step with PBS was performed for 5 min at room temperature, before microscopy observation and image analysis by a Leica DMI6000 inverted microscope (Leica, Mannheim, Germany). Quantitative analysis of positivity for biomarker expression was performed by calculating the number of biomarker-positive stained cells relative to the total number of cells, calculated by counting Hoecths stained cells. The measurements were performed on at least three organoids and by two different operators.
2.5. Reagents
- –
- RGF BME Type 2, Cultrex reduced growth factor BME type 2 (BIOTECHNE, Milan, Italy; cat. no. 3533-010-02);
- –
- BSA (Sigma-Aldrich, Milan, Italy; cat. no. A9418);
- –
- Dispase (Gibco, cat. no. 17105-041);
- –
- Collagenase type 2 (Wortington, cat. no. LS004176);
- –
- GlutaMAX supplement, 100× (Thermo Fisher Scientific, Milan, Italy, cat. no. 35050-061);
- –
- PBS (Sigma-Aldrich, cat. no. P4417);
- –
- DMSO (Sigma-Aldrich, cat. no. D2650);
- –
- TrypLE Express Enzyme (1×), phenol red (Thermo Fisher Scientific, cat. no. 12605-010);
- –
- Hydrocortisone (Sigma-Aldrich, cat. no. H0135);
- –
- Gentamycin (Euroclone, Milan, Italy; cat. no. ECM0011B);
- –
- ACK Lysing Buffer (Invitrogen, Milan, Italy; cat. no. A1049201);
- –
- Rabbit monoclonal Estrogen Receptor (SP1) antibody (Ventana, Basel, Switzerland; (92) 790-4324);
- –
- Progepharm mouse Progesteron Receptor antibody (Dako, Santa Clara, CA, USA; cat. no. 10128534);
- –
- Rabbit anti-human HER2 protein (HerceptTestTM, Agilent, Santa Clara, CA, USA; cat. no. SK001);
- –
- Rabbit Polyclonal Ki-67/MK167 antibody (Novus, Milan, Italy; cat. no. NB500-170);
- –
- Mouse Cytokeratin, Multi (AE1/AE3) antibody (Leica, Milan, Italy; cat. no. PA0094 AE1/AE3);
- –
- Mouse Monoclonal E-Cadherin antibody [4A2] (Abcam, Cambridge, UK; cat. no. ab231303);
- –
- AlexafluorTM 488 GAR (Invitrogen, cat. no. R37116);
- –
- AlexafluorTM 594 GAR (Invitrogen, cat. no. R37117);
- –
- AlexafluorTM 488 GAM (Invitrogen, cat. no. R37120);
- –
- AlexafluorTM 594 GAM (Invitrogen, cat. no. R37121);
- –
- Hoectsh 33342 (Invitrogen, Milan, Italy; cat. no. H3570);
- –
- Fetal Bovin Serum (Gibco cat. no. A5670701);
- –
- Normal Goat Serum (NGS, Gibco, cat. no. 16210-064).
3. Results
3.1. Patient Samples and Clinical Data
A total of 27 breast cancer tissues were obtained for biobanking, of which twenty-six were from female patients and one from a male patient, all subjected to surgery at the Senology Surgery Unit (U.O.C) of Monaldi-Colli Hospital, from October 2023 to May 2024. After obtaining their informed consent, the tissues were first anonymized, to be successively processed and biobanked, as described below.
All enrolled patients were affected by different types of breast cancer and at any stage, as reported in Figure 1.
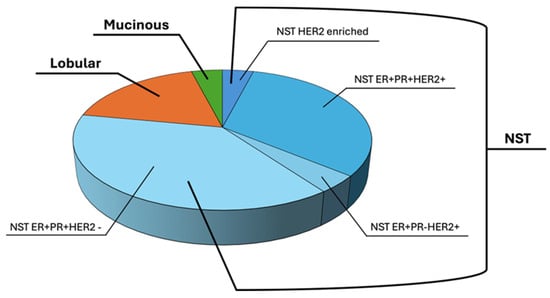
Figure 1.
Breast cancer type percentages into BCTO Biobank. NST, lobular and mucinous percentages of BC types affecting patients enrolled in this study were reported. NST were further classified, based on their receptor status.
About 78% of patients were diagnosed with No Special Type (NST) breast cancer, the most common type of invasive BC, among which 18% were Lobular BC and 4% were Mucinous type breast cancer (MBC), representing a rare breast cancer histological type.
Among NST breast cancers, about 42% expressed ER, PR, and HER2, while 48% expressed ER, PR but did not express HER2, 5% expressed ER and HER2, and another 5% were HER2-enriched BC subtypes, as determined by immunohistochemical examination by anatomy pathologists at the Pathological Anatomy and Histology Unit, of Monaldi-Colli Hospital, according to the College of American Pathologists (CAP) guidelines, released on March, 2023 (https://documents.cap.org/documents/Breast.Bmk_1.5.0.1.REL_CAPCP.pdf?_gl=1*1o5li43*_ga*MjA5ODkyNzc1Mi4xNzM0NDI3NDMz*_ga_97ZFJSQQ0X*MTczNDQyNzQzMy4xLjEuMTczNDQyNzYzMS4wLjAuMA, accessed on 8 February 2024).
The clinical and histological characteristics are reported in Table S1, Supplementary Materials.
Moreover, the clinical data from each patient were integrated with the anamnestic data reported into the ‘Data Patient Form’ (Supplementary Materials, File S2) and collected for the BCTO Biobank database, as successively described in the BCTO-Database section.
3.2. Establishment of Organoids for Their Storage in Breast Cancer Tissues and Organoids Biobank (BCTO BioBank)
To establish healthy (h) and tumor (t) patient-derived organoids (hPDOs and tPDOs) from breast cancer patients, the surgical specimens from each patient were examined by anatomy pathologists to separate healthy from tumor tissues, respectively. Both were then collected and transported to the research laboratory to be processed as schematically shown in Figure 2.

Figure 2.
Processing of tissue samples from Breast Surgery Unit to BCTO Biobank. The specimens from the Surgery Unit were first sent to the Pathological Anatomy and Histology Unit, where h- and t-tissues were prepared, collected in 15 mL tubes and sent to the BCTO Biobank. Here, they were recorded, subjected to anonymization and then sent to the BCTO-laboratory for processing.
Briefly, tissue clinical data were anonymized and recorded and each tissue was further divided into three parts: one was snap-frozen to be stored in the BCTO Biobank liquid-nitrogen tank, one was fixed in formalin, and the third one was used for the PDOs establishment, as carefully described in Materials and Methods Section. In particular, the last one was subjected to further mechanical fragmentation by using razor blades or scissors, then to enzymatic digestion by the incubation of the obtained tissue fragments with a mixture of collagenase and dispase enzymes to obtain cell preparations and then they were plated into drops of an ECM hydrogel (BME) and cultured, using a medium rich in growth factors, in a humidified incubator at 37 °C and 5% (vol/vol) CO2 on a rocker platform, to ensure the media perfusion into the gel.
We observed that among the 27 healthy samples collected, 10 were not seeded due to lack of starting tissue, 8 were seeded but failed to result in PDO cultures, although the starting tissue was enough and 9 resulted in PDO cultures (Figure 3a), corresponding to patients P#1, P#2, P#4, P#5, P#6, P#7, P#8, P#13, and P#21.
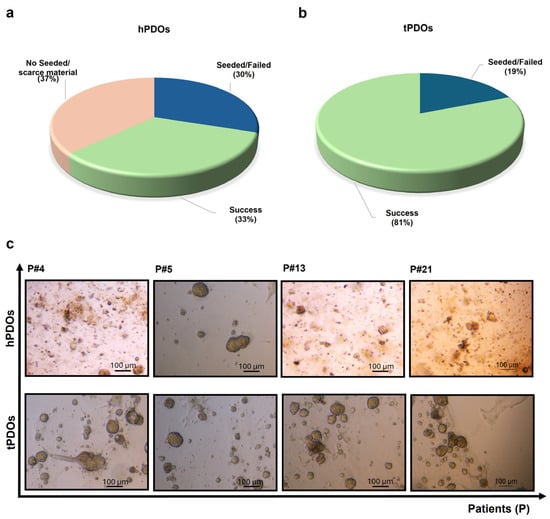
Figure 3.
Healthy and tumor PDOs establishment. (a) The obtained hPDO percentages and (b) tPDO percentages were reported; (c) brightfield images of organoids derived from different patients from the healthy and tumor surgical specimen, after 7 days in culture (magnification, 10× scale bar: 100 μm).
For tumor tissue samples, 19% (5/27) of the processed samples did not result in a PDO culture (Figure 3b), corresponding to patients P#1, P#9, P#16, P#20, and P#24. No patient’s tissue was excluded for seeding, due to lack of material or bacterial contamination.
As shown in Figure 3c, their morphology was different among and inside of the same PDOs cultures, we observed organoids with different sizes and shapes, reflecting the cancer heterogeneity. Generally, between 10 and 15 days the organoids were passed and at least two cryovials at the early passages (between the first (P1) and third passage (P3) and two at the late passages (between the fourth (P4) and sixth passage (P6)) were stored into the BCTO liquid nitrogen tank. We will perform marker expression and karyotype analysis for PDOs at late passages (P10) to ensure their identity and eventually observe changes over time in the culture, such as their ability to respond to estrogen treatment, which will be noted in our database.
Before biobanking, all the PDO cultures were examined for mycoplasma presence, as described in the Materials and Methods Section. All were found to be mycoplasma negative and classified and stored in the liquid nitrogen tank inside of the BCTO Biobank (BC-Tiss and Org BioBank, https://www.igb.cnr.it/index.php/bcto-biobank/, accessed on 8 February 2024).
Furthermore, we established a procedure for PDOs paraffin embedding, as described in the Materials and Methods Section for their storage in the BCTO Biobank. These could be used along with the starting tissue for further investigations. Brightfield images obtained after hematoxylin and eosin staining of the tissue and of the corresponding organoids are reported in Figure 4.

Figure 4.
A representative hematoxylin and eosin staining of the initial tissue (A) and of the corresponding organoids (B) were illustrated (10× and 40×, magnification). The images were acquired by Nikon Eclipse Ci microscope.
3.3. Organoids Characterization for Their Receptor Status by Immunofluorescence on Microfluidic 2-Lane OrganoPlate
To compare the phenotype of the PDOs with that one shown by the starting tissues from which they were derived, we analyzed the expression profile of the ER, PR, and HER2 biomarkers, as well as of Cytokeratins and ki67 proliferation marker in PDOs, from both, early and late passages of culture, by immunofluorescence. We also included the examination of E-cadherin expression, a cell adhesion glycoprotein, frequently inactivated in breast cancer, whose loss is indicative of cancer cell invasion and metastasis [13,14].
To this end, PDOs were seeded on microfluidic 2-lane OrganoPlate and, after 7–10 days from their seeding, were analyzed by immunofluorescence, directly on the plate, as described in Section 2.
As shown in Figure 5A, PDOs from patient #2 at early passage (P1) exhibited a high expression of ER and PR comparable to the one observed in the starting tissue by IHS (Table S1, Supplementary Materials) as well as a comparable Ki-67 expression (Figure 5B).
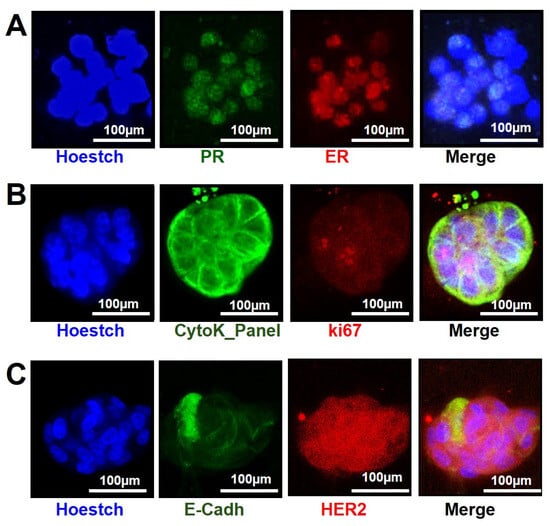
Figure 5.
PDOs immunofluorescence on microfluidic 2lane OrganoPlate. Representative immunofluorescence staining of PR (green), ER (red) (A), Cytokeratin panel (green), Ki67 (red) (B), E-cadherin (green) and HER2 (red) (C) in PDOs. Hoestch counterstain (blue) indicated the nuclei (magnification, ×20; scale bar: 100 μm).
We also found a high expression of HER2 on PDOs from patient #4 (Figure 5C), at late passage (P4), as observed by IHS on the origin tissue (Table S1, Supplementary Materials). The ER, PR, and Ki67 marker expression data obtained on all examined PDOs by immunofluorescence was summarized in Table S3, Supplementary Materials. Moreover, since cultured ER-positive PDOs may remain ER-positive but no longer respond to estrogen treatment, we tested their ability to respond to ER and noted the results in our database. These data could also be a reflection of tumor evolution over time and thus, need to be recorded [15]. Interestingly, we observed the E-cadherin expression onto the membranes of some cells, as well as intracellular inside of a close group of cells, suggesting a potential loss of this marker in these PDOs from patient #4. These data seemed to confirm the invasive features of the original tumor, further supporting how a more detailed PDOs characterization will be critical to increase our knowledge on a patient-specific tumor.
3.4. BCTO BioBank Database
All the data related to the samples into the BCTO Biobank, were collected into a database, to integrate information about collection of biological materials, and phenotypic and clinical data of the Biobank samples, as illustrated in Figure 6.

Figure 6.
BCTO Biobank database. A general scheme of the database adopted from BCTO Biobank is reported.
Personal data and patient data were collected and stored in the BCTO database with different codes, with the purpose of disaggregating the data and protecting the personal identity of all participants in this study. Only authorized personnel had access to the database using provided personal credentials/authorizations. Stringent quality control measures were also implemented throughout data collection and analysis processes to ensure the reliability of findings; this included regular audits and validation of data entry procedures.
We developed a database where a wide range of data, including clinical data, phenotypic data, and biological sample data, were recorded.
Phenotypic data included basic demographic information such as age, gender, ethnicity, and other sociodemographic variables that may influence phenotype expression, physical characteristics based on clinical data medical history such as family history of diseases, current health conditions, and treatments, presence of specific symptoms or signs related to disease or phenotype.
Biological samples data concerned the information about types of biological materials collected and about collection details such as date and time of sample collection, methods, and procedures used to collect and process the sample, information on the quality and integrity of the sample (e.g., whether it is viable, frozen, or degraded), sample storage information, storage conditions (temperature, environment, storage media, and location of biological samples within biorepositories).
Furthermore, consent forms and ethical approval documentation were collected, in compliance with research ethics and privacy laws, and stored into the database.
3.5. BCTO BioBank Data and Samples Access
The “Breast Cancer Tissues and Organoids” Biobank also BCTO BioBank is located at the Center for Biological Resources (CRB) at the Institute of Genetics and Biophysics of Naples, belonging to the network of Biobanks and Biological Resource Centers of the BBMRI (https://www.igb.cnr.it/index.php/about-the-bcto-biobank-english/, accessed on 8 February 2024), where other Biobanks are also located.
The human biological material and the data connected to it inside of the BCTO Biobank are managed by the Management Committee in accordance with established and documented operational specifications which reflect the resolution of the Federico II Ethics Committee and operate in accordance with current regulations regarding quality and respect for privacy, as described in the Regulation of the BCTO Biobank (https://www.igb.cnr.it/index.php/about-the-bcto-biobank-english/, accessed on 8 February 2024).
Briefly, institutions interested in accessing biomaterials must submit an application to the Head of the BCTO BioBank, accompanied by a description of the Research Project they intend to carry out on the requested material. The application must specify the Researcher Responsible for the Project (Principal Investigator, PI), with the curriculum vitae, a summary description of the attached Project and a copy of the approval of the Ethics Committee relating to the proposed Project. The samples will be transferred, in coded form, to researchers working at national and international research institutes after the evaluation of the proposed project by the Scientific Committee of the BCTO BioBank.
Data access will be allowed, after the approval of the project by the BCTO Biobank Scientific Committee, through the use of controlled access systems where approved researchers can visualize the de-identified data, on secure, cloud-based platforms. Once access is granted, the approved researchers are expected to comply with data protection laws, including maintaining confidentiality, using the data solely for the approved purpose, and reporting any publications or findings that result from the use of the data.
4. Discussion
Despite intense efforts in cancer research, breast cancer remains one of the types of cancer with the highest mortality in Europe, UK, and the US.
It has been demonstrated that tumor organoids, or patient-derived organoids (PDOs), are reliable cancer models, retaining the aspects of the tumor structure and heterogeneity, and providing a more physiologically, and pathologically realistic platform against which to screen drugs/combinations than two-dimensional cultures or cancer cell lines [16,17,18].
Moreover, PDOs cultures offer important advantages over the PDX models, in terms of costs and time in the pre-clinical research [15,16]. Interestingly, it has been demonstrated that PDOs able to respond to drug treatment were only those derived from patients who had responded to that treatment. This PDO behavior makes them critical in clinician decision-making processes for already approved drugs and makes them a useful tool for screening new anti-tumor compounds in pre-clinical research [19,20].
In this study, we created a Breast Cancer Tissue and Organoids Biobank after the Ethics Committee approval of the related project and of the protocols we were going to use during the process from the surgery specimen collection to its manipulation until its delivery to the Biobank for PDOs generation and storage as a frozen sample in liquid nitrogen tanks or as paraffin embedded samples. Thus, we integrated the established protocols from Clever [10,21], Mazzucchelli [22], and Aggarwal [23] to generate BC PDOs for storage in our Biobank.
However, although generating organoids from a surgical specimen is not complicated, growing and replicating them for Biobank storage requires experience and self-confidence with cell biology and, in particular, 3D culturing techniques.
Each patient specimen, used to derivate the corresponding organoids, is unique. Thus, the procedure for processing them is not the same for each sample but it requires continuous adjustments and modifications, when necessary. First, each surgical specimen has a different size, composition in stroma, fat, extracellular matrix, as well as a different level of necrosis, and amount of red blood cells. All this needs to be monitored in order to apply the right procedure, in terms of steps and times to modify, to obtain successfully organoids from them.
Furthermore, during the process of digestion, tissue samples need to be observed by microscopy to make sure that long-time and short-time digestion will not compromise the quality of the cell preparation. In our hands, small tissue fragments of 1–3 mm did not require a digestion time longer than 120 min with our enzyme combination to obtain a good quality cell preparation to generate organoids.
Moreover, once organoids are established, we could enrich them from stroma, inflammatory cells, and debries during culturing, by adjusting procedures; for example, by adding a differential centrifugation step and/or using cell strainers. This provides a further tool to investigate the role of the tumor microenvironment in breast cancer biology and in its progression.
Furthermore, after five days, the initial medium rich in factors promoting stemness, such as R-Spondin 1, neuregulin-1, Noggin, and ROCK inhibitor, needs to be substituted with the ones including growth factors, such as insulin and hydrocortisone, to support the proliferation of mammary epithelial cells.
To all this, we need to consider the time parameter: the time between the surgery and the collection of the specimen by the anatomy pathologist as well as between the preparation of two pieces (h and t samples) to their processing at the BCTO Biobank. This parameter is not the same among all the processed samples.
PDOs characterization is then the successive step, to make sure that the obtained PDOs retained the same phenotype compared to the one obtained from the corresponding patient tissue, so that they may be used for further studies.
Here, we decided to adopt the immunofluorescence on PDOs seeded on a microfluidic OrganoPlate, as a method to define the biomarker expression of the generated PDOs. This system allowed us to analyze the ER, PR, HER2, and Ki67 expression profiles on whole organoids, by using a small amount of starting material. It also allowed us to avoid unmasking steps, often responsible for losing the slices on the microscope slides, during on-slices immunohistochemical (IHS) and immunofluorescence (IF) staining procedures. Moreover, by using on-chip immunofluorescence we were able to examine two different biomarkers at the same time more easily and the required amount of antibody was very small (20 µL of diluted antibody) compared to the one (at least 100 µL) needed by on-slices immunohistochemistry (IHS) and immunofluorescence (IF) staining procedure.
Moreover, we experienced difficulties in nuclear biomarker profiling by immunofluorescence on paraffin embedded PDO slices. For instance, we had to modify the on-slices immunofluorescence by introducing the same two permeabilization steps used into the on-chip immunofluorescence procedure, after the alcohol hydration steps. This adjustment allowed us to observe these intracellular receptors also on paraffin embedded PDO slices.
All experimental observations during the surgical specimen process were noted in the Biobank database with the goal of tracking all the biological samples across the various stages of research, from collection to storage and eventual analysis, ensuring data integrity and traceability.
This Biobank along with those developed worldwide will have a tremendous impact on the translational research in breast cancer [24], filling current scientific gaps and at the same time being relevant to studies aimed at defining the PDO’s ability to respond to certain pharmacological therapies before their use on patients.
5. Conclusions
The continuous development of the BCTO Biobank is relevant to public health, providing more insight into the field of breast cancer development and progression.
Furthermore, it offers an important human-relevant tool in place of animal models in compliance with the European Union Directive 2010/63/EU, aimed at replacing them in scientific research [25].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/organoids4010005/s1, Table S1: List of enrolled BC patients.; Table S2: Media and Buffer; Table S3: Data Patient Form.
Author Contributions
Conceptualization, E.C.; methodology, V.M., C.A. and S.A.; software, T.N.; formal analysis, E.C., S.M. and L.P.; investigation, L.M. (Lucia Miranda), S.M., C.C., I.D.R., R.L., F.D.R., P.S. and L.M. (Luigi Mandrich); data curation, E.C., L.P. and T.N.; writing—original draft preparation, E.C. and L.M. (Luigi Mandrich); writing—review and editing, E.C. and L.M. (Luigi Mandrich); supervision, E.C.; funding acquisition, E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Regione Campania to E.C., grant “SATIN” POR FESR 2014–2020.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of “Università Federico II” (protocol code # 482/21; 4 February 2022) and by the Ethics Committee of Università degli Studi della Campania “Luigi Vanvitelli”—Azienda Ospedaliera Universitaria “Luigi Vanvitelli”—A.O.R.N. “Ospedali dei Colli” (protocol code #AOC-0012341-2023; 14 April 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Acknowledgments
We are grateful to Bernard Loeffler and Francesca Varrone for their excellent. editing assistance and to the IT-core of the IGB for their excellent work in Biobank website development and maintenance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.V.; Haber, D.A.; Settleman, J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat. Rev. Cancer 2010, 10, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Korn, J.M.; Ferretti, S.; Monahan, J.E.; Wang, Y.; Singh, M.; Zhang, C.; Schnell, C.; Yang, G.; Zhang, Y.; et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 2015, 21, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Bruna, A.; Rueda, O.M.; Greenwood, W.; Batra, A.S.; Callari, M.; Batra, R.N.; Pogrebniak, K.; Sandoval, J.; Cassidy, J.W.; Tufegdzic-Vidakovic, A.; et al. A Biobank of Breast Cancer Explants with Preserved Intra-tumor Heterogeneity to Screen Anticancer Compounds. Cell 2016, 167, 260–274.e22. [Google Scholar] [CrossRef]
- Fitzpatrick, P.A.; Akrap, N.; Söderberg, E.M.V.; Harrison, H.; Thomson, G.J.; Landberg, G. Robotic Mammosphere Assay for High-Throughput Screening in Triple-Negative Breast Cancer. SLAS Discov. 2017, 22, 827–836. [Google Scholar] [CrossRef]
- Muthuswamy, S.K. Bringing together the organoid field: From early beginnings to the road ahead. Development 2017, 144, 963–967. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Seino, T.; Kawasaki, S.; Shimokawa, M.; Tamagawa, H.; Toshimitsu, K.; Fujii, M.; Ohta, Y.; Matano, M.; Nanki, K.; Kawasaki, K.; et al. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell 2018, 22, 454–467.e6. [Google Scholar] [CrossRef]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef]
- Ooft, S.N.; Weeber, F.; Schipper, L.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; van de Haar, J.; Prevoo, W.; van Werkhoven, E.; Snaebjornsson, P.; et al. Prospective experimental treatment of colorectal cancer patients based on organoid drug responses. ESMO Open 2021, 6, 100103. [Google Scholar] [CrossRef]
- Padmanaban, V.; Krol, I.; Suhail, Y.; Szczerba, B.M.; Aceto, N.; Bader, J.S.; Ewald, A.J. E-cadherin is required for metastasis in multiple models of breast cancer. Nature 2019, 573, 439–444. [Google Scholar] [CrossRef]
- Bajrami, I.; Marlow, R.; van de Ven, M.; Brough, R.; Pemberton, H.N.; Frankum, J.; Song, F.; Rafiq, R.; Konde, A.; Krastev, D.B.; et al. E-Cadherin/ROS1 Inhibitor Synthetic Lethality in Breast Cancer. Cancer Discov. 2018, 8, 498–515. [Google Scholar] [CrossRef] [PubMed]
- Malainou, C.P.; Stachika, N.; Damianou, A.K.; Anastopoulos, A.; Ploumaki, I.; Triantafyllou, E.; Drougkas, K.; Gomatou, G.; Kotteas, E. Estrogen-Receptor-Low-Positive Breast Cancer: Pathological and Clinical Perspectives. Curr. Oncol. 2023, 30, 9734–9745. [Google Scholar] [CrossRef]
- Fan, H.; Demirci, U.; Chen, P. Emerging organoid models: Leaping forward in cancer research. J. Hematol. Oncol. 2019, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Silva-Almeida, C.; Ewart, M.A.; Wilde, C. 3D gastrointestinal models and organoids to study metabolism in human colon cancer. Semin. Cell Dev. Biol. 2020, 98, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Sleire, L.; Forde, H.E.; Netland, I.A.; Leiss, L.; Skeie, B.S.; Enger, P.O. Drug repurposing in cancer. Pharmacol. Res. 2017, 124, 74–91. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef]
- Driehuis, E.; Kretzschmar, K.; Clevers, H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat. Protoc. 2020, 15, 3380–3409. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, S.; Piccotti, F.; Allevi, R.; Truffi, M.; Sorrentino, L.; Russo, L.; Agozzino, M.; Signati, L.; Bonizzi, A.; Villani, L.; et al. Establishment and Morphological Characterization of Patient-Derived Organoids from Breast Cancer. Biol. Proced. Online 2019, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, D.; Russo, S.; Naik, P.; Bhatia, S.; Spector, D.L. Establishment and Culture of Patient-Derived Breast Organoids. J. Vis. Exp. 2023, 192, 10-3791. [Google Scholar] [CrossRef]
- Mazzucchelli, S.; Sinati, L.; Messa, L.; Franceschini, A.; Bonizzi, A.; Castagnoli, L.; Gasparini, P.; Consolandi, C.; Mangano, E.; Pelucchi, P.; et al. Breast cancer patient-derived organoids for the investigation of patient-specific tumour evolution. Cancer Cell Int. 2024, 24, 220. [Google Scholar] [CrossRef] [PubMed]
- Sellick, J. Enhancing the protection of animals used for scientific purposes. Environ. Law Manag. 2011, 23, 75–82. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).