Contraindicated Drug Responses in Dravet Syndrome Brain Organoids Utilizing Micro Electrode Array Assessment Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. DS Patient-Derived and Healthy Control Brain Organoids
2.2. Immunostaining
2.3. MEA Measurements
2.4. Pharmacological Testing
2.5. Measurement of DS Brain Organoid Using CMOS-MEA
2.6. Detection of Oscillations
2.7. Waveform Potential Analysis
2.8. Statistical Analysis
3. Results
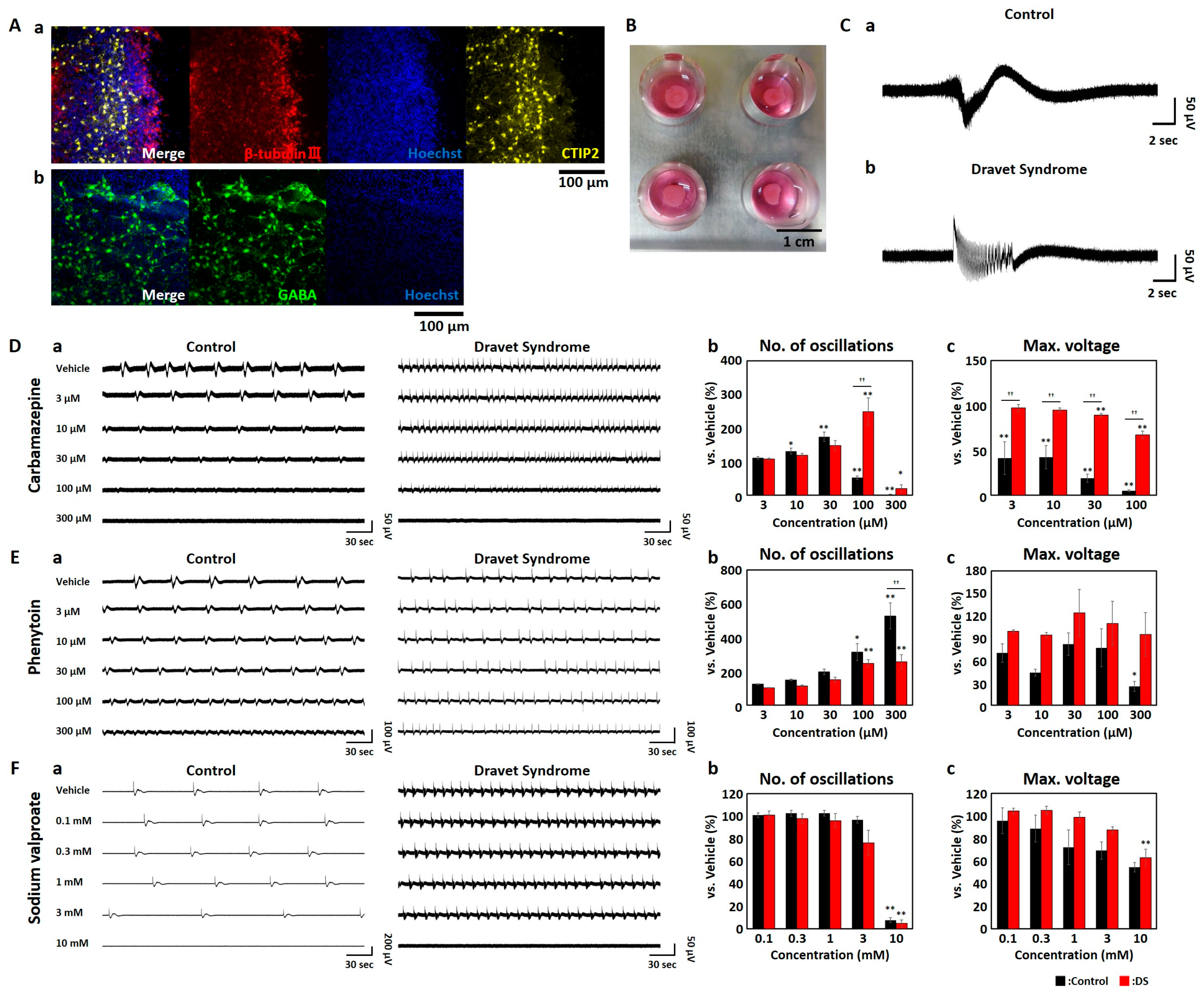
3.1. MEA Measurments of Cerebral Brain Organoids Derived from iPSCs of DS Patients
3.2. Contraindicated Drug Responses in DS Brain Organoids
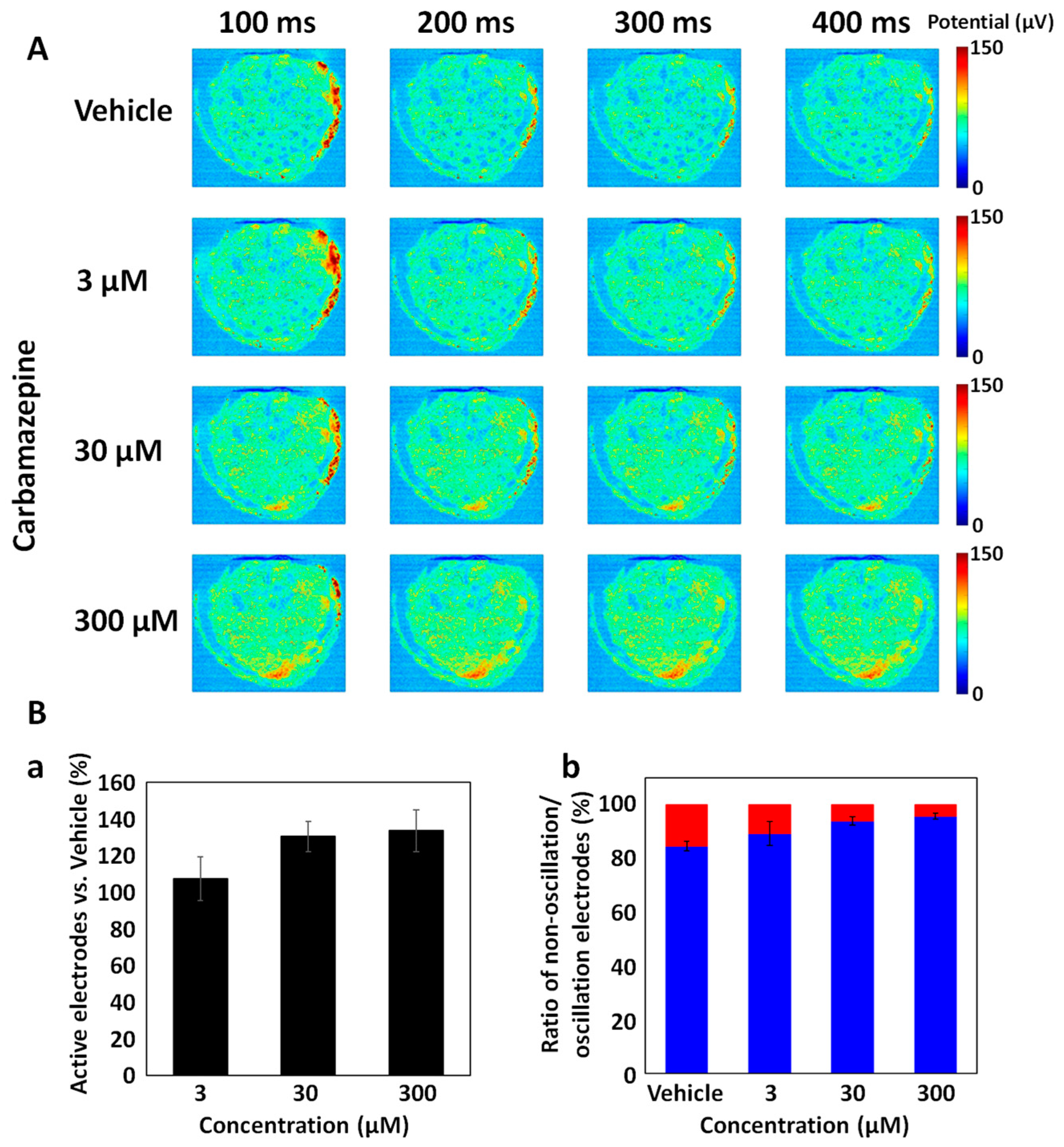
3.3. Carbamazepine Responses in DS Brain Organoids Using CMOS-MEA
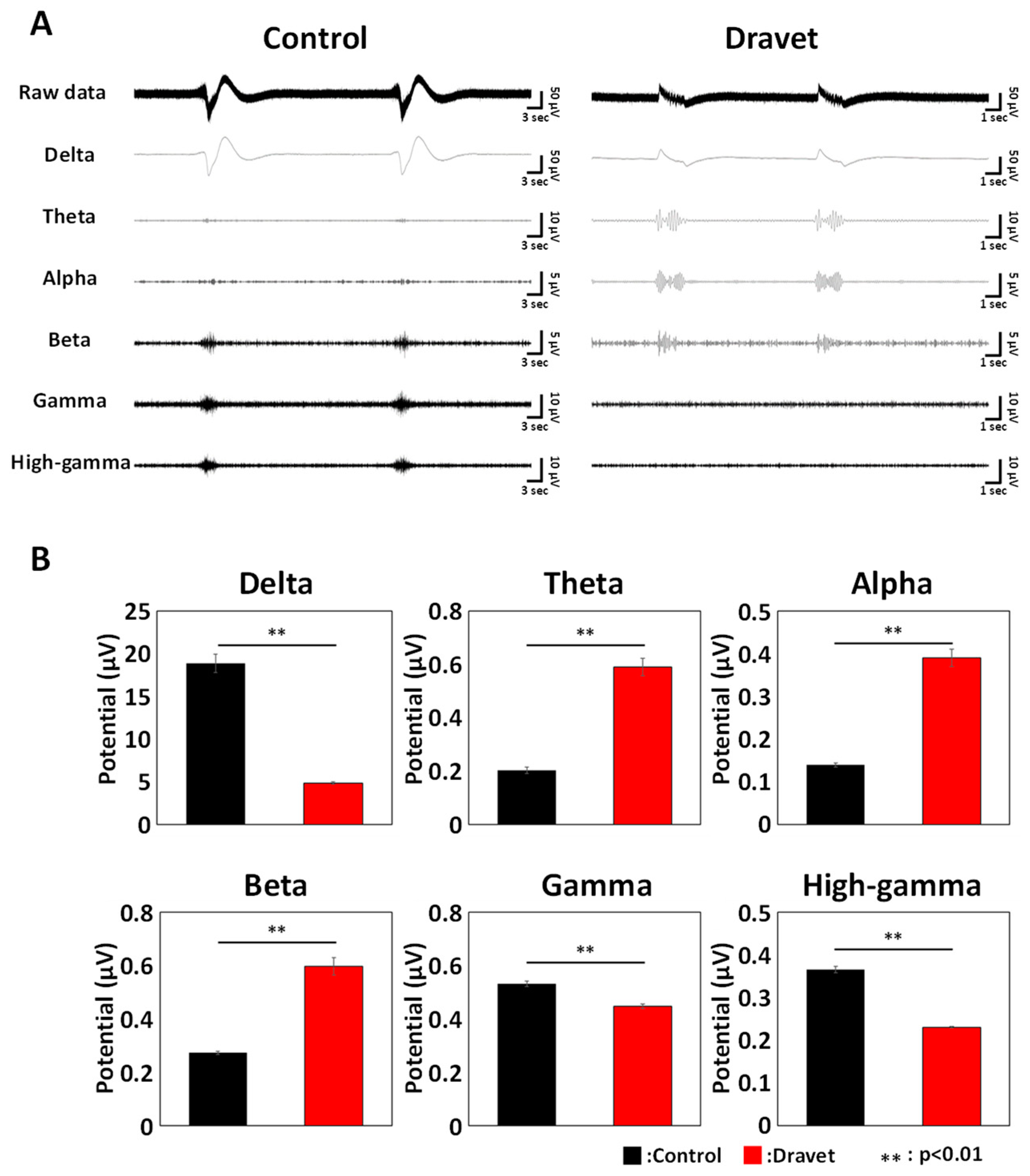
3.4. Frequency Characteristics of DS Brain Organoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Liu, Y.; Lopez-Santiago, L.F.; Yuan, Y.; Jones, J.M.; Zhang, H.; O’Malley, H.A.; Patino, G.A.; O’Brien, J.E.; Rusconi, R.; Gupta, A.; et al. Dravet syndrome patient-derived neurons suggest a novel epilepsy mechanism. Ann. Neurol. 2013, 74, 128–139. [Google Scholar] [CrossRef]
- Jiao, J.; Yang, Y.; Shi, Y.; Chen, J.; Gao, R.; Fan, Y.; Yao, H.; Liao, W.; Sun, X.F.; Gao, S. Modeling Dravet syndrome using induced pluripotent stem cells (iPSCs) and directly converted neurons. Hum. Mol. Genet. 2013, 22, 4241–4252. [Google Scholar] [CrossRef] [PubMed]
- Marchetto, M.C.; Carromeu, C.; Acab, A.; Yu, D.; Yeo, G.W.; Mu, Y.; Chen, G.; Gage, F.H.; Muotri, A.R. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 2010, 143, 527–539. [Google Scholar] [CrossRef]
- Hotta, A.; Cheung, A.Y.; Farra, N.; Vijayaragavan, K.; Séguin, C.A.; Draper, J.S.; Pasceri, P.; Maksakova, I.A.; Mager, D.L.; Rossant, J.; et al. Isolation of human iPS cells using EOS lentiviral vectors to select for pluripotency. Nat. Methods 2009, 6, 370–376. [Google Scholar] [CrossRef]
- Chamberlain, S.J.; Chen, P.F.; Ng, K.Y.; Bourgois-Rocha, F.; Lemtiri-Chlieh, F.; Levine, E.S.; Lalande, M. Induced pluripotent stem cell models of the genomic imprinting disorders Angelman and Prader-Willi syndromes. Proc. Natl. Acad. Sci. USA 2010, 107, 17668–17673. [Google Scholar] [CrossRef]
- Stanurova, J.; Neureiter, A.; Hiber, M.; de Oliveira Kessler, H.; Stolp, K.; Goetzke, R.; Klein, D.; Bankfalvi, A.; Klump, H.; Steenpass, L. Angelman syndrome-derived neurons display late onset of paternal UBE3A silencing. Sci. Rep. 2016, 6, 30792. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef]
- Giandomenico, S.L.; Sutcliffe, M.; Lancaster, M.A. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat. Protoc. 2021, 16, 579–602. [Google Scholar] [CrossRef] [PubMed]
- Kanton, S.; Boyle, M.J.; He, Z.; Santel, M.; Weigert, A.; Sanchís-Calleja, F.; Guijarro, P.; Sidow, L.; Fleck, J.S.; Han, D.; et al. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature 2019, 574, 418–422. [Google Scholar] [CrossRef]
- Velasco, S.; Kedaigle, A.J.; Simmons, S.K.; Nash, A.; Rocha, M.; Quadrato, G.; Paulsen, B.; Nguyen, L.; Adiconis, X.; Regev, A.; et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 2019, 570, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.N.; Bright, F.; Morey, N.; Au, C.; Ittner, L.M.; Ke, Y.D. Efficient Gene Expression in Human Stem Cell Derived-Cortical Organoids Using Adeno Associated Virus. Cells 2022, 11, 3194. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.G.; Badsha, F.; Florio, M.; Kanton, S.; Gerber, T.; Wilsch-Bräuninger, M.; Lewitus, E.; Sykes, A.; Hevers, W.; Lancaster, M.; et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl. Acad. Sci. USA 2015, 112, 15672–15677. [Google Scholar] [CrossRef]
- Luo, C.; Lancaster, M.A.; Castanon, R.; Nery, J.R.; Knoblich, J.A.; Ecker, J.R. Cerebral Organoids Recapitulate Epigenomic Signatures of the Human Fetal Brain. Cell Rep. 2016, 17, 3369–3384. [Google Scholar] [CrossRef]
- Gordon, A.; Yoon, S.J.; Tran, S.S.; Makinson, C.D.; Park, J.Y.; Andersen, J.; Valencia, A.M.; Horvath, S.; Xiao, X.; Huguenard, J.R.; et al. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat. Neurosci. 2021, 24, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Mokhtari, R.; Pedrosa, E.; Kirschenbaum, M.; Bayrak, C.; Zheng, D.; Lachman, H.M. CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in cerebral organoids derived from iPS cells. Mol. Autism 2017, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Coppola, G.; Zhang, P.; Abyzov, A.; Provini, L.; Tomasini, L.; Amenduni, M.; Szekely, A.; Palejev, D.; Wilson, M.; et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell 2015, 162, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, M.; Delepine, C.; Feldman, D.; Pham, V.A.; Chou, S.; Ip, J.; Nott, A.; Tsai, L.H.; Ming, G.L.; So, P.T.C.; et al. Label-free three-photon imaging of intact human cerebral organoids for tracking early events in brain development and deficits in Rett syndrome. eLife 2022, 11, e78079. [Google Scholar] [CrossRef]
- Sabate-Soler, S.; Nickels, S.L.; Saraiva, C.; Berger, E.; Dubonyte, U.; Barmpa, K.; Lan, Y.J.; Kouno, T.; Jarazo, J.; Robertson, G.; et al. Microglia integration into human midbrain organoids leads to increased neuronal maturation and functionality. Glia 2022, 70, 1267–1288. [Google Scholar] [CrossRef]
- Saleem, A.; Santos, A.C.; Aquilino, M.S.; Sivitilli, A.A.; Attisano, L.; Carlen, P.L. Modelling hyperexcitability in human cerebral cortical organoids: Oxygen/glucose deprivation most effective stimulant. Heliyon 2023, 9, e14999. [Google Scholar] [CrossRef]
- Wu, W.; Yao, H.; Negraes, P.D.; Wang, J.; Trujillo, C.A.; de Souza, J.S.; Muotri, A.R.; Haddad, G.G. Neuronal hyperexcitability and ion channel dysfunction in CDKL5-deficiency patient iPSC-derived cortical organoids. Neurobiol. Dis. 2022, 174, 105882. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wu, W.; Cerf, I.; Zhao, H.W.; Wang, J.; Negraes, P.D.; Muotri, A.R.; Haddad, G.G. Methadone interrupts neural growth and function in human cortical organoids. Stem Cell Res. 2020, 49, 102065. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.R.; Fernandes, T.G.; Vaz, S.H.; Silva, T.P.; Bekman, E.P.; Xapelli, S.; Duarte, S.; Ghazvini, M.; Gribnau, J.; Muotri, A.R.; et al. Modeling Rett Syndrome with Human Patient-Specific Forebrain Organoids. Front. Cell Dev. Biol. 2020, 8, 610427. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Chiuppesi, F.; Chen, X.; Wang, C.; Tian, E.; Nguyen, J.; Kha, M.; Trinh, D.; Zhang, H.; Marchetto, M.C.; et al. Modeling Human Cytomegalovirus-Induced Microcephaly in Human iPSC-Derived Brain Organoids. Cell Rep. Med. 2020, 1, 100002. [Google Scholar] [CrossRef]
- Chen, X.; Sun, G.; Tian, E.; Zhang, M.; Davtyan, H.; Beach, T.G.; Reiman, E.M.; Blurton-Jones, M.; Holtzman, D.M.; Shi, Y. Modeling Sporadic Alzheimer’s Disease in Human Brain Organoids under Serum Exposure. Adv. Sci. 2021, 8, e2101462. [Google Scholar] [CrossRef]
- Kathuria, A.; Lopez-Lengowski, K.; Vater, M.; McPhie, D.; Cohen, B.M.; Karmacharya, R. Transcriptome analysis and functional characterization of cerebral organoids in bipolar disorder. Genome Med. 2020, 12, 34. [Google Scholar] [CrossRef]
- Trujillo, C.A.; Rice, E.S.; Schaefer, N.K.; Chaim, I.A.; Wheeler, E.C.; Madrigal, A.A.; Buchanan, J.; Preissl, S.; Wang, A.; Negraes, P.D.; et al. Reintroduction of the archaic variant of NOVA1 in cortical organoids alters neurodevelopment. Science 2021, 371, eaax2537. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, W.; Wang, X.; Jiao, C.; Xu, S.; Liu, C.; Tang, B.; Chen, C. Human forebrain organoids reveal connections between valproic acid exposure and autism risk. Transl. Psychiatry 2022, 12, 130. [Google Scholar] [CrossRef]
- Phouphetlinthong, O.; Partiot, E.; Bernou, C.; Sebban, A.; Gaudin, R.; Charlot, B. Protruding cantilever microelectrode array to monitor the inner electrical activity of cerebral organoids. Lab Chip 2023, 23, 3603–3614. [Google Scholar] [CrossRef]
- Fagerlund, I.; Dougalis, A.; Shakirzyanova, A.; Gómez-Budia, M.; Pelkonen, A.; Konttinen, H.; Ohtonen, S.; Fazaludeen, M.F.; Koskuvi, M.; Kuusisto, J.; et al. Microglia-like Cells Promote Neuronal Functions in Cerebral Organoids. Cells 2021, 11, 124. [Google Scholar] [CrossRef]
- Adams, J.W.; Negraes, P.D.; Truong, J.; Tran, T.; Szeto, R.A.; Guerra, B.S.; Herai, R.H.; Teodorof-Diedrich, C.; Spector, S.A.; Del Campo, M.; et al. Impact of alcohol exposure on neural development and network formation in human cortical organoids. Mol. Psychiatry 2023, 28, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Silvosa, M.J.; Mercado, N.R.; Merlock, N.; Vidhate, S.; Mejia-Alvarez, R.; Yuan, T.T.; Willis, A.M.; Lybrand, Z.R. Understanding Primary Blast Injury: High Frequency Pressure Acutely Disrupts Neuronal Network Dynamics in Cerebral Organoids. J. Neurotrauma 2022, 39, 1575–1590. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, C.A.; Gao, R.; Negraes, P.D.; Gu, J.; Buchanan, J.; Preissl, S.; Wang, A.; Wu, W.; Haddad, G.G.; Chaim, I.A.; et al. Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell 2019, 25, 558–569.e557. [Google Scholar] [CrossRef] [PubMed]
- Morelli, K.H.; Jin, W.; Shathe, S.; Madrigal, A.A.; Jones, K.L.; Schwartz, J.L.; Bridges, T.; Mueller, J.R.; Shankar, A.; Chaim, I.A.; et al. MECP2-related pathways are dysregulated in a cortical organoid model of myotonic dystrophy. Sci. Transl. Med. 2022, 14, eabn2375. [Google Scholar] [CrossRef] [PubMed]
- Foliaki, S.T.; Schwarz, B.; Groveman, B.R.; Walters, R.O.; Ferreira, N.C.; Orrù, C.D.; Smith, A.; Wood, A.; Schmit, O.M.; Freitag, P.; et al. Neuronal excitatory-to-inhibitory balance is altered in cerebral organoid models of genetic neurological diseases. Mol. Brain 2021, 14, 156. [Google Scholar] [CrossRef]
- Ghatak, S.; Dolatabadi, N.; Trudler, D.; Zhang, X.; Wu, Y.; Mohata, M.; Ambasudhan, R.; Talantova, M.; Lipton, S.A. Mechanisms of hyperexcitability in Alzheimer’s disease hiPSC-derived neurons and cerebral organoids vs. isogenic controls. eLife 2019, 8, e50333. [Google Scholar] [CrossRef]
- Kathuria, A.; Lopez-Lengowski, K.; Jagtap, S.S.; McPhie, D.; Perlis, R.H.; Cohen, B.M.; Karmacharya, R. Transcriptomic Landscape and Functional Characterization of Induced Pluripotent Stem Cell-Derived Cerebral Organoids in Schizophrenia. JAMA Psychiatry 2020, 77, 745–754. [Google Scholar] [CrossRef]
- Trujillo, C.A.; Adams, J.W.; Negraes, P.D.; Carromeu, C.; Tejwani, L.; Acab, A.; Tsuda, B.; Thomas, C.A.; Sodhi, N.; Fichter, K.M.; et al. Pharmacological reversal of synaptic and network pathology in human MECP2-KO neurons and cortical organoids. EMBO Mol. Med. 2021, 13, e12523. [Google Scholar] [CrossRef]
- Yokoi, R.; Shibata, M.; Odawara, A.; Ishibashi, Y.; Nagafuku, N.; Matsuda, N.; Suzuki, I. Analysis of signal components < 500 Hz in brain organoids coupled to microelectrode arrays: A reliable test-bed for preclinical seizure liability assessment of drugs and screening of antiepileptic drugs. Biochem. Biophys. Rep. 2021, 28, 101148. [Google Scholar] [CrossRef]
- Dravet, C. Dravet syndrome history. Dev. Med. Child Neurol. 2011, 53 (Suppl. S2), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, I.; Miyamoto, H.; Morita, N.; Atapour, N.; Mazaki, E.; Inoue, I.; Takeuchi, T.; Itohara, S.; Yanagawa, Y.; Obata, K.; et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: A circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 5903–5914. [Google Scholar] [CrossRef] [PubMed]
- Dutton, S.B.; Makinson, C.D.; Papale, L.A.; Shankar, A.; Balakrishnan, B.; Nakazawa, K.; Escayg, A. Preferential inactivation of Scn1a in parvalbumin interneurons increases seizure susceptibility. Neurobiol. Dis. 2013, 49, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Fadila, S.; Quinn, S.; Turchetti Maia, A.; Yakubovich, D.; Ovadia, M.; Anderson, K.L.; Giladi, M.; Rubinstein, M. Convulsive seizures and some behavioral comorbidities are uncoupled in the Scn1a(A1783V) Dravet syndrome mouse model. Epilepsia 2020, 61, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, L.; Qian, W.; Ray, S.; Lu, Z.; Liu, T.; Zou, Y.Y.; Naumann, R.K.; Wang, H. A novel rat model of Dravet syndrome recapitulates clinical hallmarks. Neurobiol. Dis. 2023, 184, 106193. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.S.; Cleary, C.M.; LoTurco, J.J.; Chen, X.; Mulkey, D.K. Disordered breathing in a mouse model of Dravet syndrome. eLife 2019, 8, e43387. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sone, T.; Higurashi, N.; Sakuma, T.; Suzuki, S.; Ishikawa, M.; Yamamoto, T.; Mitsui, J.; Tsuji, H.; Okano, H.; et al. Generation of D1-1 TALEN isogenic control cell line from Dravet syndrome patient iPSCs using TALEN-mediated editing of the SCN1A gene. Stem Cell Res. 2018, 28, 100–104. [Google Scholar] [CrossRef]
- Kim, H.W.; Quan, Z.; Kim, Y.B.; Cheong, E.; Kim, H.D.; Cho, M.; Jang, J.; Yoo, Y.R.; Lee, J.S.; Kim, J.H.; et al. Differential effects on sodium current impairments by distinct SCN1A mutations in GABAergic neurons derived from Dravet syndrome patients. Brain Dev. 2018, 40, 287–298. [Google Scholar] [CrossRef]
- Schuster, J.; Fatima, A.; Sobol, M.; Norradin, F.H.; Laan, L.; Dahl, N. Generation of three human induced pluripotent stem cell (iPSC) lines from three patients with Dravet syndrome carrying distinct SCN1A gene mutations. Stem Cell Res. 2019, 39, 101523. [Google Scholar] [CrossRef]
- Sun, Y.; Paşca, S.P.; Portmann, T.; Goold, C.; Worringer, K.A.; Guan, W.; Chan, K.C.; Gai, H.; Vogt, D.; Chen, Y.J.; et al. A deleterious Nav1.1 mutation selectively impairs telencephalic inhibitory neurons derived from Dravet Syndrome patients. eLife 2016, 5, e13073. [Google Scholar] [CrossRef]
- Sun, Y.; Dolmetsch, R.E. Investigating the Therapeutic Mechanism of Cannabidiol in a Human Induced Pluripotent Stem Cell (iPSC)-Based Model of Dravet Syndrome. Cold Spring Harb. Symp. Quant. Biol. 2018, 83, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Tanaka, Y.; Shirasu, N.; Yasunaga, S.; Higurashi, N.; Hirose, S. Establishment of human induced pluripotent stem cells derived from skin cells of a patient with Dravet syndrome. Stem Cell Res. 2020, 47, 101857. [Google Scholar] [CrossRef] [PubMed]
- Zayat, V.; Kuczynska, Z.; Liput, M.; Metin, E.; Rzonca-Niewczas, S.; Smyk, M.; Mazurczak, T.; Goszczanska-Ciuchta, A.; Leszczynski, P.; Hoffman-Zacharska, D.; et al. The Generation of Human iPSC Lines from Three Individuals with Dravet Syndrome and Characterization of Neural Differentiation Markers in iPSC-Derived Ventral Forebrain Organoid Model. Cells 2023, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I.; Matsuda, N.; Han, X.; Noji, S.; Shibata, M.; Nagafuku, N.; Ishibashi, Y. Large-Area Field Potential Imaging Having Single Neuron Resolution Using 236 880 Electrodes CMOS-MEA Technology. Adv. Sci. 2023, 10, e2207732. [Google Scholar] [CrossRef] [PubMed]
- Sharf, T.; van der Molen, T.; Glasauer, S.M.K.; Guzman, E.; Buccino, A.P.; Luna, G.; Cheng, Z.; Audouard, M.; Ranasinghe, K.G.; Kudo, K.; et al. Functional neuronal circuitry and oscillatory dynamics in human brain organoids. Nat. Commun. 2022, 13, 4403. [Google Scholar] [CrossRef]
- Schröter, M.; Wang, C.; Terrigno, M.; Hornauer, P.; Huang, Z.; Jagasia, R.; Hierlemann, A. Functional imaging of brain organoids using high-density microelectrode arrays. MRS Bull. 2022, 47, 530–544. [Google Scholar] [CrossRef]
- Okon, E.A.; Oshie, N.C.; Ubong, I.A.; Kelechi, O.S. The Effect of Carbamazepine on EEG Tracings of People with Seizure Disorders in Calabar, Nigeria. Saudi J. Med. Pharm. Sci. 2017, 3, 73–84. [Google Scholar]
- Quinn, S.; Brusel, M.; Ovadia, M.; Rubinstein, M. Acute effect of antiseizure drugs on background oscillations in Scn1a (A1783V) Dravet syndrome mouse model. Front. Pharmacol. 2023, 14, 1118216. [Google Scholar] [CrossRef]
- Holmes, G.L.; Bender, A.C.; Wu, E.X.; Scott, R.C.; Lenck-Santini, P.P.; Morse, R.P. Maturation of EEG oscillations in children with sodium channel mutations. Brain Dev. 2012, 34, 469–477. [Google Scholar] [CrossRef]
- Akiyama, M.; Kobayashi, K.; Yoshinaga, H.; Ohtsuka, Y. A long-term follow-up study of Dravet syndrome up to adulthood. Epilepsia 2010, 51, 1043–1052. [Google Scholar] [CrossRef]
- Sanchez-Carpintero, R.; Urrestarazu, E.; Cieza, S.; Alegre, M.; Artieda, J.; Crespo-Eguilaz, N.; Valencia, M. Abnormal brain gamma oscillations in response to auditory stimulation in Dravet syndrome. Eur. J. Paediatr. Neurol. Off. J. Eur. Paediatr. Neurol. Soc. 2020, 24, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Kuki, T.; Fujihara, K.; Miwa, H.; Tamamaki, N.; Yanagawa, Y.; Mushiake, H. Contribution of parvalbumin and somatostatin-expressing GABAergic neurons to slow oscillations and the balance in beta-gamma oscillations across cortical layers. Front. Neural Circuits 2015, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Kalume, F.; Oakley, J.C.; Westenbroek, R.E.; Gile, J.; de la Iglesia, H.O.; Scheuer, T.; Catterall, W.A. Sleep impairment and reduced interneuron excitability in a mouse model of Dravet Syndrome. Neurobiol. Dis. 2015, 77, 141–154. [Google Scholar] [CrossRef]
- Cardin, J.A. Inhibitory Interneurons Regulate Temporal Precision and Correlations in Cortical Circuits. Trends Neurosci. 2018, 41, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Cardin, J.A. Snapshots of the Brain in Action: Local Circuit Operations through the Lens of γ Oscillations. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 10496–10504. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Wang, X.J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef]
- Besser, R.; Hornung, K.; Theisohn, M.; Rothacher, G.; Krämer, G. EEG changes in patients during the introduction of carbamazepine. Electroencephalogr. Clin. Neurophysiol. 1992, 83, 19–23. [Google Scholar] [CrossRef]
- Salinsky, M.C.; Oken, B.S.; Morehead, L. Intraindividual analysis of antiepileptic drug effects on EEG background rhythms. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 186–193. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, C.H. Quantitative pharmaco-EEG of carbamazepine in volunteers and epileptics. Clin. Electroencephalogr. 1996, 27, 40–45. [Google Scholar] [CrossRef]
- Salinsky, M.C.; Binder, L.M.; Oken, B.S.; Storzbach, D.; Aron, C.R.; Dodrill, C.B. Effects of gabapentin and carbamazepine on the EEG and cognition in healthy volunteers. Epilepsia 2002, 43, 482–490. [Google Scholar] [CrossRef]
- Clemens, B.; Ménes, A.; Nagy, Z. Objective assessment of neurotoxicity while shifting from carbamazepine to oxcarbazepine. Acta Neurol. Scand. 2004, 109, 324–329. [Google Scholar] [CrossRef]
- Clemens, B.; Ménes, A.; Piros, P.; Bessenyei, M.; Altmann, A.; Jerney, J.; Kollár, K.; Rosdy, B.; Rózsavölgyi, M.; Steinecker, K.; et al. Quantitative EEG effects of carbamazepine, oxcarbazepine, valproate, lamotrigine, and possible clinical relevance of the findings. Epilepsy Res. 2006, 70, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Meador, K.J.; Loring, D.W.; Boyd, A.; Echauz, J.; LaRoche, S.; Velez-Ruiz, N.; Korb, P.; Byrnes, W.; Dilley, D.; Borghs, S.; et al. Randomized double-blind comparison of cognitive and EEG effects of lacosamide and carbamazepine. Epilepsy Behav. 2016, 62, 267–275. [Google Scholar] [CrossRef]
- Fink, M.; Irwin, P.; Sannita, W.; Papakostas, Y.; Green, M.A. Phenytoin: EEG effects and plasma levels in volunteers. Ther. Drug Monit. 1979, 1, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; McEvoy, L.K.; Smith, M.E.; Gevins, A.; Meador, K.; Laxer, K.D. Task-related EEG and ERP changes without performance impairment following a single dose of phenytoin. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2002, 113, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Salinsky, M.C.; Spencer, D.C.; Oken, B.S.; Storzbach, D. Effects of oxcarbazepine and phenytoin on the EEG and cognition in healthy volunteers. Epilepsy Behav. 2004, 5, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Herkes, G.K.; Lagerlund, T.D.; Sharbrough, F.W.; Eadie, M.J. Effects of antiepileptic drug treatment on the background frequency of EEGs in epileptic patients. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 1993, 10, 210–216. [Google Scholar] [CrossRef]
- Arzy, S.; Allali, G.; Brunet, D.; Michel, C.M.; Kaplan, P.W.; Seeck, M. Antiepileptic drugs modify power of high EEG frequencies and their neural generators. Eur. J. Neurol. 2010, 17, 1308–1312. [Google Scholar] [CrossRef]
- Clemens, B. Valproate decreases EEG synchronization in a use-dependent manner in idiopathic generalized epilepsy. Seizure 2008, 17, 224–233. [Google Scholar] [CrossRef]
- Béla, C.; Mónika, B.; Márton, T.; István, K. Valproate selectively reduces EEG activity in anterior parts of the cortex in patients with idiopathic generalized epilepsy. A low resolution electromagnetic tomography (LORETA) study. Epilepsy Res. 2007, 75, 186–191. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoi, R.; Nagafuku, N.; Ishibashi, Y.; Matsuda, N.; Suzuki, I. Contraindicated Drug Responses in Dravet Syndrome Brain Organoids Utilizing Micro Electrode Array Assessment Methods. Organoids 2023, 2, 177-191. https://doi.org/10.3390/organoids2040014
Yokoi R, Nagafuku N, Ishibashi Y, Matsuda N, Suzuki I. Contraindicated Drug Responses in Dravet Syndrome Brain Organoids Utilizing Micro Electrode Array Assessment Methods. Organoids. 2023; 2(4):177-191. https://doi.org/10.3390/organoids2040014
Chicago/Turabian StyleYokoi, Remi, Nami Nagafuku, Yuto Ishibashi, Naoki Matsuda, and Ikuro Suzuki. 2023. "Contraindicated Drug Responses in Dravet Syndrome Brain Organoids Utilizing Micro Electrode Array Assessment Methods" Organoids 2, no. 4: 177-191. https://doi.org/10.3390/organoids2040014
APA StyleYokoi, R., Nagafuku, N., Ishibashi, Y., Matsuda, N., & Suzuki, I. (2023). Contraindicated Drug Responses in Dravet Syndrome Brain Organoids Utilizing Micro Electrode Array Assessment Methods. Organoids, 2(4), 177-191. https://doi.org/10.3390/organoids2040014




