Supply Organ Development in Young Broilers in Response to Changing Dietary Fat and Amino Acids in the Starter Period †
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Animals and Housing
2.3. Data Collection
2.4. Histology
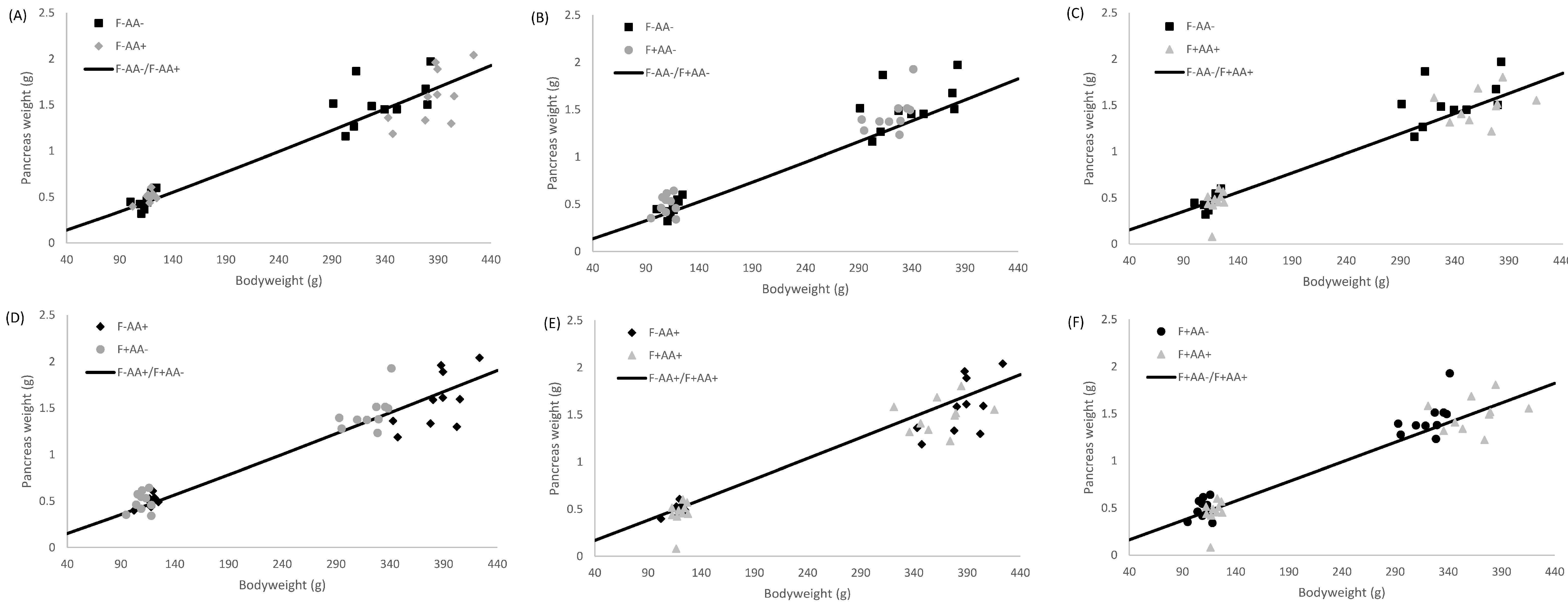
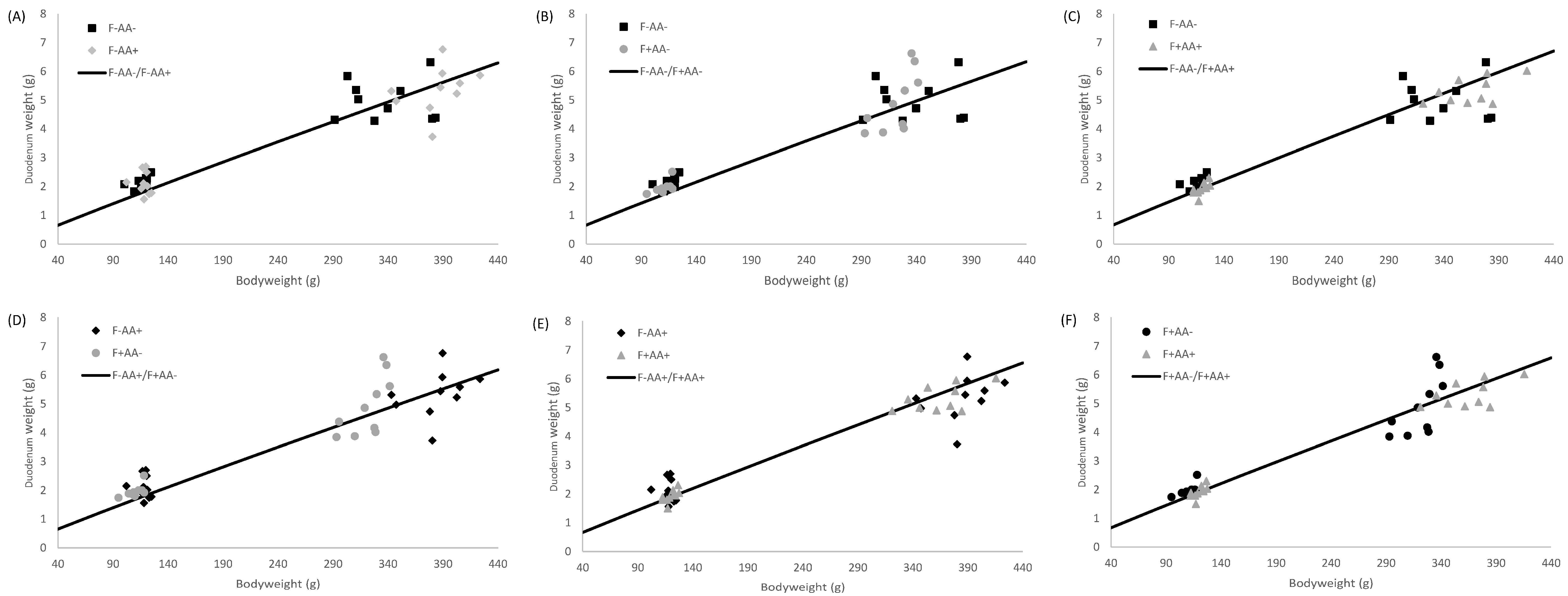
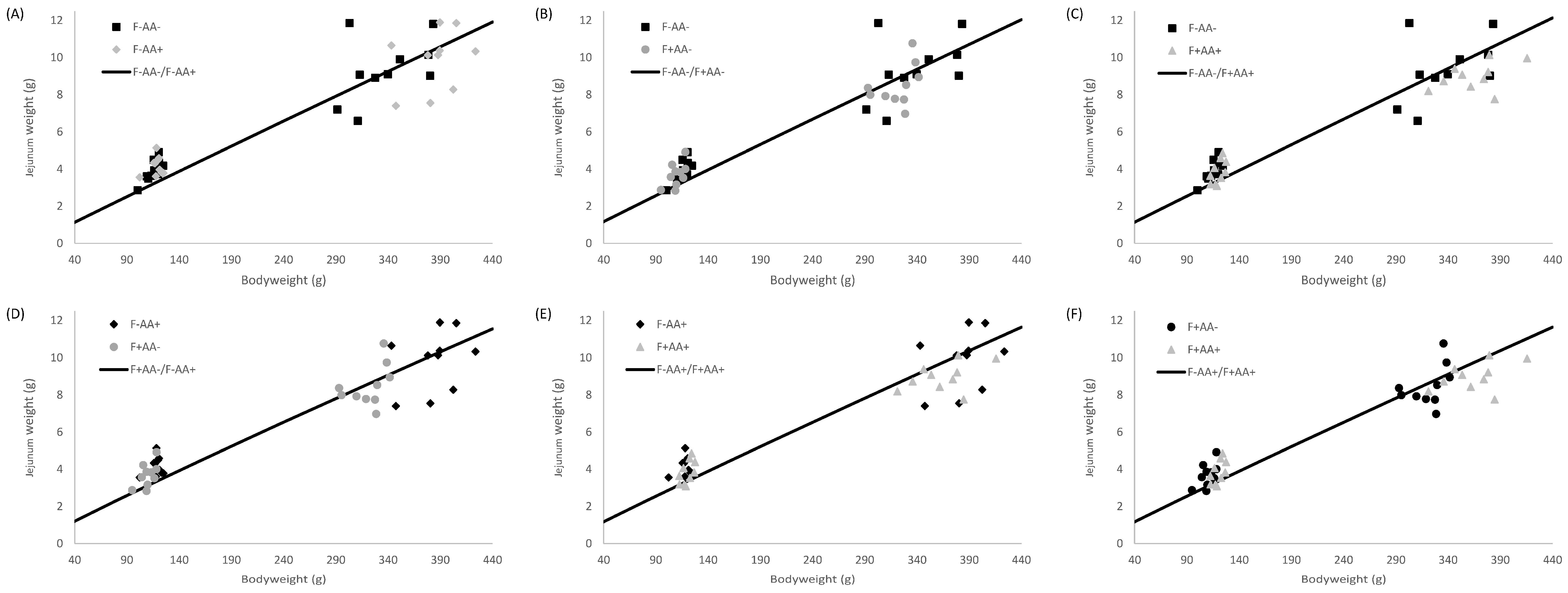
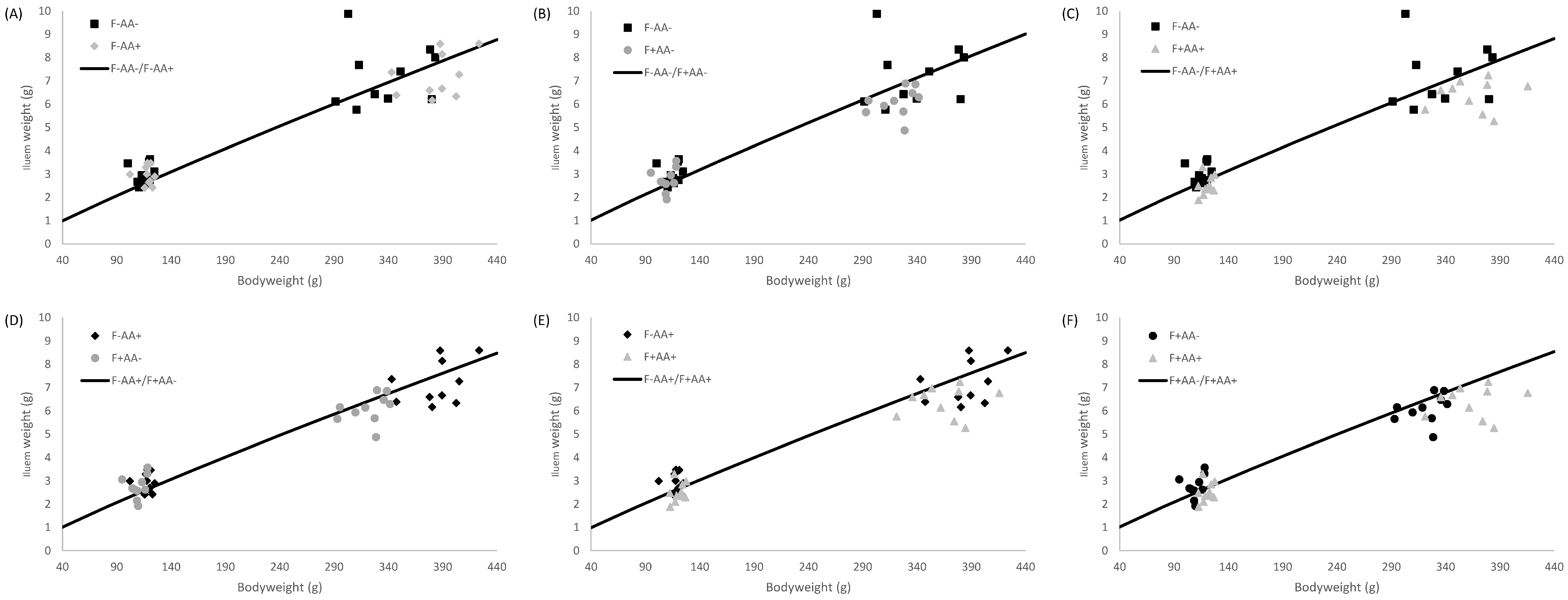
2.5. Allometric Growth Modeling
2.6. Statistical Analysis
3. Results
3.1. Growth Performance and Feed Intake
3.2. Nutrient Intake and Efficiency
3.3. Supply Organ Weight and Intestinal Histo-Morphological Parameters
3.4. Allometric Growth
4. Discussion
4.1. Growth Performance and Nutrient Intake
4.2. Supply Organ Weights, Intestinal Histo-Morphological Parameters, and Allometric Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Amino acids |
| AA− | Low amino acid concentration |
| AA+ | High amino acid concentration |
| AB | Alcian blue |
| AME | Apparent metabolizable energy |
| AVD | Animal experiment permit number (Dutch system) |
| BW | Body weight |
| BWG | Body weight gain |
| CO2 | Carbon dioxide |
| CP | Crude protein |
| CVB | Centraal Veevoeder Bureau (Dutch feed evaluation system) |
| d | Day |
| dig. Lys | Digestible lysine |
| DL | DL-methionine |
| DM | Dry matter |
| F | Dietary AME/fat |
| F− | Low dietary AME/fat |
| F+ | High dietary AME/fat |
| FCR | Feed conversion ratio |
| FI | Feed intake |
| F:G | Feed-to-gain ratio |
| g/cm | Grams per centimeter |
| HE | Hematoxylin and eosin |
| ICP-AES | Inductively coupled plasma atomic emission spectroscopy |
| ISO | International Organization for Standardization |
| LS means | Least square means |
| Lys | Lysine |
| nRMSE | Normalized root mean square error |
| NLIN | Non-linear procedure in SAS |
| P | Phosphorus |
| PROC GLM | General linear model procedure in SAS |
| R2/pseudo-R2 | Coefficient of determination |
| SAS | Statistical Analysis System software |
| SD | Standard deviation |
| SEM | Standard error of the mean |
| SID Lys | Standardized ileal digestible lysine |
| V:C ratio | Villus-to-crypt ratio |
Appendix A
| F | AA | F × AA | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F− | F+ | AA− | AA+ | F−AA− | F−AA+ | F+AA− | F+AA+ | SEM | F | AA | F × AA | |
| n | 16 | 16 | 16 | 16 | 8 | 8 | 8 | 8 | ||||
| Duodenum | ||||||||||||
| Total goblet cells per villus | 80 | 71 | 80 | 72 | 82 | 77 | 78 | 66 | 4.8 | 0.184 | 0.196 | 0.051 |
| Acidic goblet cells per villus | 62 | 59 | 64 | 59 | 65 | 61 | 62 | 57 | 4.7 | 0.638 | 0.715 | 0.203 |
| Goblet cells surface per 1 mm of villus | 1921 | 1700 | 1952 | 1671 | 2029 | 1817 | 1875 | 1525 | 121 | 0.397 | 0.466 | 0.101 |
| Goblet cell size (µm) | 24 | 23 | 24 | 23 | 24 | 23 | 24 | 22 | 0.6 | 0.869 | 0.843 | 0.461 |
| Goblet cell surface as % of villus | 1.34 | 1.32 | 1.45 | 1.21 | 1.45 | 1.24 | 1.46 | 1.19 | 0.10 | 0.930 | 0.127 | 0.116 |
| Jejunum | ||||||||||||
| Total goblet cells per villus | 55 | 62 | 57 | 60 | 54 | 56 | 61 | 65 | 2.9 | 0.217 | 0.604 | 0.192 |
| Acidic goblet cells per villus | 44 | 59 | 50 | 53 | 42 | 45 | 59 | 60 | 3.0 | 0.213 | 0.897 | 0.188 |
| Goblet cells surface per 1 mm of villus | 1347 | 1410 | 1345 | 1412 | 1355 | 1338 | 1334 | 1487 | 89 | 0.618 | 0.575 | 0.206 |
| Goblet cell size (µm) | 24 | 22 | 23 | 23 | 24 | 24 | 22 | 23 | 0.7 | 0.719 | 0.973 | 0.651 |
| Goblet cell surface as % of villus | 2.08 | 2.23 | 2.17 | 2.14 | 2.13 | 2.02 | 2.21 | 2.26 | 0.12 | 0.382 | 0.604 | 0.237 |
| Ileum | ||||||||||||
| Total goblet cells per villus | 60 | 60 | 59 | 61 | 61 | 58 | 56 | 64 | 3.5 | 0.969 | 0.902 | 0.210 |
| Acidic goblet cells per villus | 47 | 45 | 45 | 46 | 50 | 43 | 41 | 49 | 3.3 | 0.756 | 0.843 | 0.428 |
| Goblet cells surface per 1 mm of villus | 1251 | 1177 | 1133 | 1294 | 1167 | 1334 | 1100 | 1254 | 66.9 | 0.644 | 0.717 | 0.619 |
| Goblet cell size (µm) | 23 | 20 | 20 | 22 | 21 | 24 | 20 | 20 | 0.9 | 0.489 | 0.508 | 0.337 |
| Goblet cell surface as % of villus | 2.87 | 3.09 | 2.82 | 3.14 | 2.71 | 3.02 | 2.93 | 3.25 | 0.17 | 0.552 | 0.427 | 0.199 |
| F | AA | F × AA | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F− | F+ | AA− | AA+ | F−AA− | F−AA+ | F+AA− | F+AA+ | SEM | F | AA | F × AA | |
| n | 16 | 16 | 16 | 16 | 8 | 8 | 8 | 8 | ||||
| Duodenum | ||||||||||||
| Total goblet cells per villus | 174 | 197 | 179 | 193 | 160 | 188 | 198 | 198 | 8.1 | 0.117 | 0.312 | 0.104 |
| Acidic goblet cells per villus | 125 | 135 | 126 | 134 | 116 | 134 | 136 | 135 | 7.7 | 0.764 | 0.801 | 0.225 |
| Goblet cells surface per 1 mm of villus | 4159 | 4823 | 4314 | 4667 | 3665 | 4652 | 4964 | 4682 | 224 | 0.163 | 0.356 | 0.082 |
| Goblet cell size (µm) | 23 | 24 | 23 | 24 | 22 | 24 | 24 | 23 | 0.7 | 0.874 | 0.791 | 0.814 |
| Goblet cell surface as % of villus | 1.60 | 1.69 | 1.55 | 1.74 | 1.42 | 1.77 | 1.68 | 1.70 | 0.08 | 0.653 | 0.469 | 0.181 |
| Jejunum | ||||||||||||
| Total goblet cells per villus | 139 | 139 | 129 | 149 | 130 | 148 | 127 | 151 | 8.9 | 0.956 | 0.679 | 0.548 |
| Acidic goblet cells per villus | 105 | 103 | 95 | 113 | 96 | 114 | 94 | 112 | 7.6 | 0.971 | 0.365 | 0.296 |
| Goblet cells surface per 1 mm of villus | 2988 | 3456 | 2947 | 3497 | 2655 | 3321 | 3239 | 3673 | 243 | 0.348 | 0.384 | 0.165 |
| Goblet cell size (µm) | 22 | 24 | 23 | 23 | 21 | 22 | 24 | 23 | 0.6 | 0.785 | 0.917 | 0.696 |
| Goblet cell surface as % of villus | 2.16 | 2.31 | 2.09 | 2.39 | 1.95 | 2.39 | 2.23 | 2.40 | 0.14 | 0.761 | 0.517 | 0.498 |
| Ileum | ||||||||||||
| Total goblet cells per villus | 115 | 112 | 126 | 102 | 132 | 98 | 119 | 106 | 7.3 | 0.951 | 0.296 | 0.117 |
| Acidic goblet cells per villus | 86 | 87 | 94 | 78 | 99 | 75 | 93 | 81 | 7.6 | 0.916 | 0.137 | 0.192 |
| Goblet cells surface per 1 mm of villus | 1708 | 1395 | 1567 | 1535 | 1852 | 1564 | 1282 | 1507 | 109 | 0.145 | 0.864 | 0.286 |
| Goblet cell size (µm) | 16 | 13 | 13 | 16 | 15 | 17 | 11 | 14 | 0.8 | 0.349 | 0.371 | 0.318 |
| Goblet cell surface as % of villus | 2.29 | 2.25 | 2.46 | 2.08 | 2.40 | 2.17 | 2.52 | 1.99 | 0.11 | 0.865 | 0.349 | 0.318 |
Appendix B
| Organ | Diet Treatment | a | a (95% CI) | b | b (95% CI) | Pseudo-R2 |
|---|---|---|---|---|---|---|
| Heart | F−AA− | 0.005 | 0.004–0.006 | 1.129 | 1.099–1.166 | 0.91 |
| F−AA+ | 0.005 | 0.004–0.006 | 1.121 | 1.087–1.154 | 0.90 | |
| F+AA− | 0.006 | 0.005–0.007 | 1.095 | 1.063–1.124 | 0.93 | |
| F+AA+ | 0.005 | 0.004–0.006 | 1.152 | 1.118–1.189 | 0.92 | |
| Liver | F−AA− | 0.003 | 0.002–0.004 | 1.580 | 1.525–1.633 | 0.88 |
| F−AA+ | 0.003 | 0.002–0.004 | 1.594 | 1.546–1.639 | 0.87 | |
| F+AA− | 0.005 | 0.004–0.006 | 1.450 | 1.411–1.481 | 0.91 | |
| F+AA+ | 0.005 | 0.004–0.006 | 1.443 | 1.402–1.482 | 0.92 | |
| Proventriculus | F−AA− | 0.090 | 0.082–0.097 | 0.561 | 0.533–0.590 | 0.83 |
| F−AA+ | 0.079 | 0.072–0.085 | 0.596 | 0.568–0.628 | 0.85 | |
| F+AA− | 0.096 | 0.087–0.103 | 0.544 | 0.515–0.575 | 0.81 | |
| F+AA+ | 0.079 | 0.072–0.086 | 0.594 | 0.563–0.623 | 0.87 | |
| Gizzard | F−AA− | 0.007 | 0.006–0.008 | 1.379 | 1.328–1.422 | 0.86 |
| F−AA+ | 0.008 | 0.007–0.009 | 1.341 | 1.297–1.385 | 0.88 | |
| F+AA− | 0.007 | 0.006–0.008 | 1.370 | 1.323–1.418 | 0.85 | |
| F+AA+ | 0.005 | 0.004–0.006 | 1.451 | 1.405–1.492 | 0.89 | |
| Pancreas | F−AA− | 0.001 | 0.001–0.002 | 1.241 | 1.189–1.291 | 0.80 |
| F−AA+ | 0.001 | 0.001–0.002 | 1.268 | 1.215–1.322 | 0.79 | |
| F+AA− | 0.001 | 0.000–0.002 | 1.264 | 1.219–1.315 | 0.81 | |
| F+AA+ | 0.001 | 0.000–0.002 | 1.351 | 1.301–1.398 | 0.85 | |
| Duodenum | F−AA− | 0.004 | 0.003–0.004 | 1.321 | 1.278–1.365 | 0.84 |
| F−AA+ | 0.005 | 0.004–0.006 | 1.281 | 1.242–1.322 | 0.88 | |
| F+AA− | 0.004 | 0.003–0.005 | 1.316 | 1.272–1.359 | 0.85 | |
| F+AA+ | 0.003 | 0.002–0.004 | 1.395 | 1.341–1.441 | 0.80 | |
| Jejunum | F−AA− | 0.003 | 0.002–0.004 | 1.488 | 1.432–1.539 | 0.82 |
| F−AA+ | 0.004 | 0.003–0.005 | 1.482 | 1.427–1.534 | 0.83 | |
| F+AA− | 0.003 | 0.002–0.004 | 1.569 | 1.503–1.628 | 0.81 | |
| F+AA+ | 0.003 | 0.002–0.004 | 1.515 | 1.457–1.567 | 0.82 | |
| Ileum | F−AA− | 0.010 | 0.009–0.011 | 1.196 | 1.159–1.237 | 0.83 |
| F−AA+ | 0.013 | 0.012–0.015 | 1.142 | 1.111–1.178 | 0.81 | |
| F+AA− | 0.009 | 0.008–0.010 | 1.220 | 1.179–1.257 | 0.82 | |
| F+AA+ | 0.008 | 0.007–0.009 | 1.251 | 1.208–1.292 | 0.82 | |
| Ceca | F−AA− | 0.000 | 0.000–0.001 | 1.706 | 1.623–1.783 | 0.78 |
| F−AA+ | 0.000 | 0.000–0.001 | 1.766 | 1.681–1.844 | 0.79 | |
| F+AA− | 0.000 | 0.000–0.001 | 1.697 | 1.624–1.772 | 0.77 | |
| F+AA+ | 0.000 | 0.000–0.001 | 1.923 | 1.849–2.001 | 0.76 |
| Organ | Diet Treatment | a | a (95% CI) | b | b (95% CI) | Pseudo-R2 |
|---|---|---|---|---|---|---|
| Heart | F−AA− | 0.026 | 0.023–0.029 | 0.790 | 0.752–0.828 | 0.87 |
| F−AA+ | 0.024 | 0.021–0.027 | 0.800 | 0.764–0.838 | 0.88 | |
| F+AA− | 0.020 | 0.019–0.022 | 0.838 | 0.803–0.874 | 0.89 | |
| F+AA+ | 0.029 | 0.026–0.032 | 0.771 | 0.737–0.806 | 0.86 | |
| Liver | F−AA− | 0.096 | 0.087–0.105 | 0.854 | 0.819–0.890 | 0.90 |
| F−AA+ | 0.123 | 0.111–0.134 | 0.814 | 0.781–0.846 | 0.88 | |
| F+AA− | 0.087 | 0.079–0.096 | 0.858 | 0.822–0.894 | 0.91 | |
| F+AA+ | 0.061 | 0.056–0.067 | 0.930 | 0.892–0.969 | 0.94 | |
| Proventriculus | F−AA− | 0.057 | 0.051–0.063 | 0.636 | 0.606–0.668 | 0.82 |
| F−AA+ | 0.073 | 0.067–0.080 | 0.611 | 0.581–0.644 | 0.83 | |
| F+AA− | 0.046 | 0.040–0.051 | 0.701 | 0.663–0.743 | 0.80 | |
| F+AA+ | 0.067 | 0.060–0.073 | 0.629 | 0.598–0.661 | 0.85 | |
| Gizzard | F−AA− | 0.195 | 0.179–0.210 | 0.687 | 0.657–0.719 | 0.81 |
| F−AA+ | 0.197 | 0.183–0.210 | 0.673 | 0.643–0.704 | 0.82 | |
| F+AA− | 0.267 | 0.244–0.291 | 0.615 | 0.582–0.650 | 0.78 | |
| F+AA+ | 0.230 | 0.213–0.248 | 0.663 | 0.633–0.694 | 0.83 | |
| Pancreas | F−AA− | 0.003 | 0.002–0.003 | 1.072 | 1.024–1.121 | 0.84 |
| F−AA+ | 0.004 | 0.003–0.005 | 1.000 | 0.957–1.046 | 0.83 | |
| F+AA− | 0.005 | 0.004–0.006 | 0.973 | 0.932–1.015 | 0.85 | |
| F+AA+ | 0.012 | 0.010–0.013 | 0.799 | 0.764–0.837 | 0.81 | |
| Duodenum | F−AA− | 0.057 | 0.050–0.063 | 0.765 | 0.728–0.804 | 0.86 |
| F−AA+ | 0.050 | 0.044–0.056 | 0.785 | 0.746–0.823 | 0.87 | |
| F+AA− | 0.068 | 0.061–0.075 | 0.737 | 0.696–0.779 | 0.85 | |
| F+AA+ | 0.058 | 0.052–0.064 | 0.779 | 0.742–0.819 | 0.88 | |
| Jejunum | F−AA− | 0.084 | 0.077–0.093 | 0.809 | 0.772–0.848 | 0.80 |
| F−AA+ | 0.127 | 0.115–0.139 | 0.731 | 0.692–0.772 | 0.79 | |
| F+AA− | 0.196 | 0.179–0.212 | 0.660 | 0.621–0.703 | 0.77 | |
| F+AA+ | 0.134 | 0.121–0.147 | 0.726 | 0.686–0.766 | 0.80 | |
| Ileum | F−AA− | 0.077 | 0.070–0.084 | 0.779 | 0.742–0.820 | 0.85 |
| F−AA+ | 0.077 | 0.070–0.085 | 0.762 | 0.726–0.802 | 0.84 | |
| F+AA− | 0.110 | 0.100–0.120 | 0.709 | 0.669–0.751 | 0.82 | |
| F+AA+ | 0.128 | 0.116–0.140 | 0.679 | 0.641–0.718 | 0.81 | |
| Ceca | F−AA− | 0.021 | 0.018–0.024 | 0.788 | 0.741–0.839 | 0.78 |
| F−AA+ | 0.024 | 0.021–0.027 | 0.746 | 0.699–0.793 | 0.77 | |
| F+AA− | 0.020 | 0.018–0.023 | 0.799 | 0.754–0.845 | 0.79 | |
| F+AA+ | 0.027 | 0.024–0.030 | 0.773 | 0.729–0.816 | 0.76 |
References
- Iji, P.A.; Saki, A.; Tivey, D.R. Body and Intestinal Growth of Broiler Chicks on a Commercial Starter Diet. 1. Intestinal Weight and Mucosal Development. Br. Poult. Sci. 2001, 42, 505–513. [Google Scholar] [CrossRef]
- Sklan, D. Development of the Digestive Tract of Poultry. Worlds Poult. Sci. J. 2001, 57, 415–428. [Google Scholar] [CrossRef]
- Buyse, J.; Geypens, B.; Malheiros, R.D.; Moraes, V.M.; Swennen, Q.; Decuypere, E. Assessment of Age-Related Glucose Oxidation Rates of Broiler Chickens by Using Stable Isotopes. Life Sci. 2004, 75, 2245–2255. [Google Scholar] [CrossRef]
- Noy, Y.; Sklan, D. Digestion and Absorption in the Young Chick. Poult. Sci. 1995, 74, 366–373. [Google Scholar] [CrossRef]
- Noy, Y.; Sklan, D. Yolk Utilisation in the Newly Hatched Poult. Br. Poult. Sci. 1998, 39, 446–451. [Google Scholar] [CrossRef]
- Uni, Z.; Noy, Y.; Sklan, D. Posthatch Development of Small Intestinal Function in the Poult. Poult. Sci. 1999, 78, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Batal, A.B.; Parsons, C.M. Effects of Age on Nutrient Digestibility in Chicks Fed Different Diets. Poult. Sci. 2002, 81, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.V.; Ravindran, V.; Ravindran, G. Nutrient Digestibility and Energy Utilisation of Diets Based on Wheat, Sorghum or Maize by the Newly Hatched Broiler Chick. Br. Poult. Sci. 2008, 49, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Tancharoenrat, P.; Ravindran, V.; Zaefarian, F.; Ravindran, G. Digestion of Fat and Fatty Acids along the Gastrointestinal Tract of Broiler Chickens. Poult. Sci. 2014, 93, 371–379. [Google Scholar] [CrossRef]
- Lamot, D.M.; Sapkota, D.; Wijtten, P.J.A.; Van Den Anker, I.; Heetkamp, M.J.W.; Kemp, B.; Van Den Brand, H. Diet Density during the First Week of Life: Effects on Growth Performance, Digestive Organ Weight, and Nutrient Digestion of Broiler Chickens. Poult. Sci. 2019, 98, 789–795. [Google Scholar] [CrossRef]
- Swennen, Q.; Everaert, N.; Debonne, M.; Verbaeys, I.; Careghi, C.; Tona, K.; Janssens, G.P.J.; Decuypere, E.; Bruggeman, V.; Buyse, J. Effect of Macronutrient Ratio of the Pre-starter Diet on Broiler Performance and Intermediary Metabolism. J. Anim. Physiol. Anim. Nutr. 2010, 94, 375–384. [Google Scholar] [CrossRef]
- Diehl, J.J.E.; van Eerden, E.; Duijster, M.; Kwakkel, R.P. Supply Organ Development of Young Broilers in Response to Increased Carbohydrates and Amino Acids in the Starter Period. Poult. Sci. 2024, 103, 104092. [Google Scholar] [CrossRef] [PubMed]
- Cemin, H.S.; Vieira, S.L.; Stefanello, C.; Kipper, M.; Kindlein, L.; Helmbrecht, A. Digestible Lysine Requirements of Male Broilers from 1 to 42 Days of Age Reassessed. PLoS ONE 2017, 12, e0179665. [Google Scholar] [CrossRef]
- Hirai, R.A.; Dennehy, D.G.; Mejia, L.; Coto, C.; McDaniel, C.D.; Wamsley, K.G.S. Evaluating the Digestible Lysine Requirements of Male Cobb MV× Cobb 500 Broilers during the First Fourteen Days of Age and the Carryover Effect of Feeding Varying Levels of Digestible Lysine on Performance and Processing. J. Appl. Poult. Res. 2022, 31, 100274. [Google Scholar] [CrossRef]
- Dozier, W.A., III; Payne, R.L. Digestible Lysine Requirements of Female Broilers from 1 to 15 Days of Age. J. Appl. Poult. Res. 2012, 21, 348–357. [Google Scholar] [CrossRef]
- Garcia, A.; Batal, A.B. Changes in the Digestible Lysine and Sulfur Amino Acid Needs of Broiler Chicks during the First Three Weeks Posthatching. Poult. Sci. 2005, 84, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U. The ARRIVE Guidelines 2019: Updated Guidelines for Reporting Animal Research. bioRxiv 2019. bioRxiv:703181. [Google Scholar] [CrossRef]
- Cobb-Vantress. Cobb500 Broiler European Nutrition Supplement (White Paper); Cobb-Vantress: Siloam Springs, AR, USA, 2018. [Google Scholar]
- Cobb-Vantress. Cobb500 Broiler Performance & Nutrition Supplement; Cobb-Vantress: Siloam Springs, AR, USA, 2018. [Google Scholar]
- ISO 6496:1999; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 5984:2002; Animal Feeding Stuffs—Determination of Crude Ash. International Organization for Standardization: Geneva, Switzerland, 2002.
- ISO 16634-1:2008; Food Products—Determination of the Total Nitrogen Content by Combustion According to the Dumas Principle and Calculation of the Crude Protein Content—Part 1: Oilseeds and Animal Feeding Stuffs. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 6492:2002; Animal Feeding Stuffs—Determination of Fat Content. International Organization for Standardization: Geneva, Switzerland, 2002.
- ISO 6493:2000; Animal Feeding Stuffs—Determination of Starch Content—Polarimetric Method. International Organization for Standardization: Geneva, Switzerland, 2000.
- ISO 27085:2009; Animal Feeding Stuffs—Determination of Calcium, Sodium, Phosphorus, Magnesium, Potassium, Iron, Zinc, Copper, Manganese and Cobalt by ICP-AES. International Organization for Standardization: Geneva, Switzerland, 2009.
- Layton, C.; Bancroft, J.D.; Suvarna, S.K. Fixation of Tissues. In Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 40–63. [Google Scholar]
- Huxley, J.S.; Teissier, G. Terminology of Relative Growth. Nature 1936, 137, 780–781. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Ransnas, L.A. Fitting Curves to Data Using Nonlinear Regression: A Practical and Nonmathematical Review. FASEB J. 1987, 1, 365–374. [Google Scholar] [CrossRef]
- Tancharoenrat, P.; Ravindran, V.; Zaefarian, F.; Ravindran, G. Influence of Age on the Apparent Metabolisable Energy and Total Tract Apparent Fat Digestibility of Different Fat Sources for Broiler Chickens. Anim. Feed Sci. Technol. 2013, 186, 186–192. [Google Scholar] [CrossRef]
- Plumstead, P.W.; Romero-Sanchez, H.; Paton, N.D.; Spears, J.W.; Brake, J. Effects of Dietary Metabolizable Energy and Protein on Early Growth Responses of Broilers to Dietary Lysine. Poult. Sci. 2007, 86, 2639–2648. [Google Scholar] [CrossRef]
- Decuypere, M.P.; Buyse, J.; Cokelaere, M.; Swennen, Q.; Everaert, N.; Debonne, M.; Hendriks, W.H.; Kwakkel, R.P.; van Krimpen, M.M. Fundamentele en Toegepaste Aspecten van Voeropnameregulatie Bij Pluimvee; Animal Sciences Group: Wageningen, The Netherlands, 2007. [Google Scholar]
- Richards, M.P.; Proszkowiec-Weglarz, M. Mechanisms Regulating Feed Intake, Energy Expenditure, and Body Weight in Poultry. Poult. Sci. 2007, 86, 1478–1490. [Google Scholar] [CrossRef]
- Griffin, H.D.; Windsor, D.; Whitehead, C.C. Changes in Lipoprotein Metabolism and Body Composition in Chickens in Response to Divergent Selection for Plasma Very Low Density Lipoprotein Concentration. Br. Poult. Sci. 1991, 32, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Hirai, R.A.; Mejia, L.; Coto, C.; Caldas, J.; McDaniel, C.D.; Wamsley, K.G.S. Impact of Varying Starter Amino Acid Density and Energy on 42 d Male Cobb MV× Cobb 500 Broiler Performance and Processing. J. Appl. Poult. Res. 2020, 29, 1004–1019. [Google Scholar] [CrossRef]
- Chen, C.; Su, Z.; Li, Y.; Luan, P.; Wang, S.; Zhang, H.; Xiao, F.; Guo, H.; Cao, Z.; Li, H. Estimation of the Genetic Parameters of Traits Relevant to Feed Efficiency: Result from Broiler Lines Divergent for High or Low Abdominal Fat Content. Poult. Sci. 2021, 100, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, B.; Blum, J.C.; Boyer, J.P. Selecting Broilers for Low or High Abdominal Fat: Initial Observations. Br. Poult. Sci. 1980, 21, 107–113. [Google Scholar] [CrossRef]
- Whitehead, C.C.; Griffin, H.D. Development of Divergent Lines of Lean and Fat Broilers Using Plasma Very Low Density Lipoprotein Concentration as Selection Criterion: The First Three Generations. Br. Poult. Sci. 1984, 25, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Baéza, E.; Le Bihan-Duval, E. Chicken Lines Divergent for Low or High Abdominal Fat Deposition: A Relevant Model to Study the Regulation of Energy Metabolism. Animal 2013, 7, 965–973. [Google Scholar] [CrossRef]
- Resnyk, C.W.; Carré, W.; Wang, X.; Porter, T.E.; Simon, J.; Le Bihan-Duval, E.; Duclos, M.J.; Aggrey, S.E.; Cogburn, L.A. Transcriptional Analysis of Abdominal Fat in Genetically Fat and Lean Chickens Reveals Adipokines, Lipogenic Genes and a Link between Hemostasis and Leanness. BMC Genom. 2013, 14, 557. [Google Scholar] [CrossRef]
- Cryer, A. Tissue Lipoprotein Lipase Activity and Its Action in Lipoprotein Metabolism. Int. J. Biochem. 1981, 13, 525–541. [Google Scholar] [CrossRef]
- Alvarenga, R.R.; Zangeronimo, M.G.; Pereira, L.J.; Rodrigues, P.B.; Gomide, E.M. Lipoprotein Metabolism in Poultry. Worlds Poult. Sci. J. 2011, 67, 431–440. [Google Scholar] [CrossRef]
- Mansilla, W.D.; Moreno-Rubio, J.; Sevillano-Quintero, F.; Saraswathy, S.; García-Ruiz, A.I. The Effect of Gradually Decreasing the Dietary Energy Content, at Constant or Increased Lysine: Energy Ratio on Broiler Performance, Carcass Yield, and Body Composition. Poult. Sci. 2022, 101, 102132. [Google Scholar] [CrossRef]
- Swatson, H.K.; Gous, R.; Iji, P.A.; Zarrinkalam, R. Effect of Dietary Protein Level, Amino Acid Balance and Feeding Level on Growth, Gastrointestinal Tract, and Mucosal Structure of the Small Intestine in Broiler Chickens. Anim. Res. 2002, 51, 501–515. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Sell, J.L. Influence of Age on Lipase, Amylase, and Protease Activities in Pancreatic Tissue and Intestinal Contents of Young Turkeys. Poult. Sci. 1989, 68, 1561–1568. [Google Scholar] [CrossRef]
- Nitsan, Z.; Ben-Avraham, G.; Zoref, Z.; Nir, I. Growth and Development of the Digestive Organs and Some Enzymes in Broiler Chicks after Hatching. Br. Poult. Sci. 1991, 32, 515–523. [Google Scholar] [CrossRef]
- Noy, Y.; Sklan, D. Posthatch Development in Poultry. J. Appl. Poult. Res. 1997, 6, 344–354. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Sheikhahmadi, A.; Li, X.; Buyse, J.; Lin, H.; Song, Z. Effects of Dietary Energy Level on Appetite and Central Adenosine Monophosphate-Activated Protein Kinase (AMPK) in Broilers. J. Anim. Sci. 2019, 97, 4488–4495. [Google Scholar] [CrossRef] [PubMed]
- Langhout, D.J.; Schutte, J.B.; Van Leeuwen, P.; Wiebenga, J.; Tamminga, S. Effect of Dietary High-and Low-Methylated Citrus Pectin on the Activity of the Ileal Microflora and Morphology of the Small Intestinal Wall of Broiler Chicks. Br. Poult. Sci. 1999, 40, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Uni, Z.; Smirnov, A.; Sklan, D. Pre- and Posthatch Development of Goblet Cells in the Broiler Small Intestine: Effect of Delayed Access to Feed. Poult. Sci. 2003, 82, 320–327. [Google Scholar] [CrossRef]
- Liu, K.; Jia, M.; Wong, E.A. Delayed Access to Feed Affects Broiler Small Intestinal Morphology and Goblet Cell Ontogeny. Poult. Sci. 2020, 99, 5275–5285. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.L.; Cloft, S.E.; Wong, E.A. Changes with Age in Density of Goblet Cells in the Small Intestine of Broiler Chicks. Poult. Sci. 2020, 99, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Batal, A.B.; Parsons, C.M. Effects of Age on Development of Digestive Organs and Performance of Chicks Fed a Corn-Soybean Meal versus a Crystalline Amino Acid Diet. Poult. Sci. 2002, 81, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Batal, A.B.; Parsons, C.M. Utilization of Different Soy Products as Affected by Age in Chicks. Poult. Sci. 2003, 82, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Sacranie, A.; Svihus, B.; Denstadli, V.; Moen, B.; Iji, P.A.; Choct, M. The Effect of Insoluble Fiber and Intermittent Feeding on Gizzard Development, Gut Motility, and Performance of Broiler Chickens. Poult. Sci. 2012, 91, 693–700. [Google Scholar] [CrossRef]
| Dietary Fat/AME | F− | F− | F+ | F+ |
|---|---|---|---|---|
| Dietary Amino Acids | AA− | AA+ | AA− | AA+ |
| Ingredient composition (g/kg) | ||||
| Corn | 334.2 | 364.3 | 338.5 | 329.6 |
| Wheat | 250.0 | 250.0 | 250.0 | 250.0 |
| Soybean meal > 48%CP | 186.5 | 245.0 | 186.5 | 245.0 |
| Wheat middlings | 60.0 | 20.0 | 80.0 | 30.0 |
| Peas | 50.0 | 50.0 | 20.0 | 20.0 |
| Potato protein | 10.0 | 10.0 | 10.0 | 10.0 |
| Corngluten meal 60%CP | 5.0 | 5.0 | 7.5 | 7.5 |
| Maize starch | 5.0 | 5.0 | 0.0 | 0.0 |
| Soybean oil | 13.0 | 13.0 | 60.0 | 60.0 |
| Limestone | 17.7 | 17.7 | 17.7 | 17.7 |
| Monocalciumphosphate | 7.7 | 7.7 | 7.7 | 7.7 |
| Salt | 1.4 | 1.4 | 1.4 | 1.4 |
| Sodiumbicarbonate | 3.3 | 3.3 | 3.3 | 3.3 |
| L-lysine HCl | 2.7 | 3.2 | 2.7 | 2.5 |
| DL-methionine | 2.4 | 3.1 | 2.6 | 3.1 |
| L-threonine | 1.0 | 1.2 | 1.0 | 1.1 |
| L-valine | 0.1 | 0.1 | 0.1 | 0.1 |
| L-arginine | 0.1 | 0.1 | 0.1 | 0.1 |
| L-isoleucine | 0.1 | 0.1 | 0.1 | 0.1 |
| Premix starter | 5.0 | 5.0 | 5.0 | 5.0 |
| Phytase | 3.3 | 3.3 | 3.3 | 3.3 |
| Glucanase-Xylanase | 2.5 | 2.5 | 2.5 | 2.5 |
| Total | 1000.0 | 1000.0 | 1000.0 | 1000.0 |
| Calculated chemical composition (g/kg) | ||||
| Crude protein | 185.0 | 215.0 | 185.0 | 215.0 |
| Crude fat | 34.1 | 34.9 | 82.6 | 79.4 |
| Crude ash | 56.5 | 56.9 | 56.2 | 56.7 |
| Crude fiber | 28.2 | 25.7 | 28.5 | 25.8 |
| Dry matter | 877.1 | 876.7 | 882.5 | 882.6 |
| Starch (Ewers) | 398.5 | 391.2 | 376.5 | 352.6 |
| AME (kcal/kg) 1 | 2750 | 2750 | 3050 | 3050 |
| Digestible lysine 1 | 10.0 | 12.0 | 10.0 | 12.0 |
| Digestible methionine 1 | 4.8 | 5.9 | 4.8 | 5.9 |
| Digestible methionine and cysteine 1 | 7.3 | 8.8 | 7.3 | 8.8 |
| Digestible threonine 1 | 6.5 | 7.8 | 6.5 | 7.8 |
| Digestible tryptophan 1 | 1.9 | 2.3 | 1.9 | 2.3 |
| Digestible valine 1 | 8.0 | 9.6 | 8.0 | 9.6 |
| Digestible isoleucine 1 | 6.6 | 8.0 | 6.6 | 8.0 |
| Digestible arginine 1 | 10.5 | 12.6 | 10.5 | 12.6 |
| Digestible glycine and serine 1 | 14.0 | 16.7 | 14.0 | 16.7 |
| Digestible leucine 1 | 12.7 | 15.3 | 12.7 | 15.3 |
| Calcium | 9.0 | 9.0 | 9.0 | 9.0 |
| Phosphorus | 5.7 | 5.3 | 5.1 | 5.1 |
| Analyzed chemical composition (g/kg) | ||||
| Crude protein | 184 | 213 | 182 | 216 |
| Crude fat | 34 | 35 | 83 | 80 |
| Dry matter | 883 | 881 | 885 | 887 |
| Starch | 378 | 383 | 372 | 348 |
| Calcium | 8.8 | 9.1 | 8.8 | 8.7 |
| Phosphorus | 5.5 | 5.4 | 4.8 | 4.8 |
| Sodium | 1.4 | 1.5 | 1.4 | 1.4 |
| Manganese (mg) | 91 | 118 | 111 | 98 |
| F | AA | F × AA | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F− | F+ | AA− | AA+ | F−AA− | F−AA+ | F+AA− | F+AA+ | SEM | F | AA | F × AA | |
| n | 20 | 20 | 20 | 20 | 10 | 10 | 10 | 10 | ||||
| BW (g/bird) | ||||||||||||
| 0 d | 45.85 | 45.9 | 45.75 | 46.0 | 45.7 | 46.0 | 45.8 | 46.0 | 0.1 | 0.521 | 0.214 | 0.787 |
| 4 d | 107.6 | 108.7 | 107.2 | 108.9 | 105.3 c | 109.6 a | 109.5 a | 108.2 b | 0.2 | 0.364 | 0.011 | 0.027 |
| 11 d | 347.3 | 356.7 | 339.5 | 364.6 | 329.5 c | 365.1 a | 349.4 b | 364.0 a | 3.0 | 0.030 | <0.001 | 0.010 |
| 28 d | 1839.3 | 1856.6 | 1813.5 | 1882.4 | 1801.5 | 1876.1 | 1823.5 | 1888.6 | 11.3 | 0.291 | <0.001 | 0.178 |
| 35 d | 2659.2 | 2653.4 | 2616.3 | 2695.2 | 2616.1 | 2701.2 | 2616.7 | 2689.0 | 17.1 | 0.998 | 0.032 | 0.395 |
| BW gain (g/bird) | ||||||||||||
| 0–4 d | 61.6 | 62.9 | 61.6 | 62.9 | 59.6 c | 63.6 a | 63.7 a | 62.2 b | 0.2 | 0.442 | 0.437 | 0.027 |
| 4–11 d | 240 | 248 | 232 | 256 | 224 c | 255 a | 240 b | 256 a | 2.5 | 0.031 | <0.001 | 0.010 |
| 0–11 d | 301 | 311 | 293 | 319 | 284 c | 319 a | 304 b | 318 a | 2.7 | 0.036 | <0.001 | 0.042 |
| 11–28 d | 1512 | 1499 | 1473 | 1538 | 1472 | 1551 | 1474 | 1525 | 9.2 | 0.293 | <0.001 | 0.579 |
| 28–35 d | 820 | 796 | 804 | 813 | 814 | 825 | 793 | 800 | 7.2 | 0.093 | 0.112 | 0.396 |
| 0–35 d | 2613 | 2607 | 2571 | 2649 | 2570 | 2655 | 2571 | 2643 | 16.8 | 0.442 | 0.020 | 0.437 |
| FI (g/bird) | ||||||||||||
| 0–4 d | 57.4 | 55.5 | 56.7 | 56.2 | 56.7 b | 58.2 a | 56.8 b | 54.2 c | 0.2 | 0.163 | 0.930 | 0.042 |
| 4–11 d | 288 | 281 | 285 | 284 | 286 ab | 291 a | 284 b | 278 c | 1.8 | 0.020 | 0.656 | 0.049 |
| 0–11 d | 346 | 337 | 342 | 341 | 342 b | 349 a | 341 b | 332 c | 2.1 | 0.009 | 0.747 | 0.048 |
| 11–28 d | 2086 | 2088 | 2062 | 2112 | 2053 | 2119 | 2071 | 2104 | 10.8 | 0.702 | 0.012 | 0.185 |
| 28–35 d | 1286 | 1267 | 1255 | 1298 | 1282 | 1290 | 1227 | 1307 | 9.6 | 0.636 | 0.023 | 0.147 |
| 0–35 d | 3718 | 3692 | 3659 | 3751 | 3677 | 3758 | 3639 | 3744 | 18.2 | 0.163 | 0.043 | 0.052 |
| F:G (g:g/bird) | ||||||||||||
| 0–4 d | 0.932 | 0.880 | 0.918 | 0.893 | 0.945 c | 0.918 b | 0.889 ab | 0.870 a | 0.008 | <0.001 | <0.001 | <0.001 |
| 4–11 d | 1.206 | 1.136 | 1.230 | 1.113 | 1.274 d | 1.138 b | 1.185 c | 1.087 a | 0.009 | <0.001 | <0.001 | <0.001 |
| 0–11 d | 1.148 | 1.083 | 1.168 | 1.068 | 1.203 d | 1.093 b | 1.122 c | 1.044 a | 0.006 | <0.001 | <0.001 | <0.001 |
| 11–28 d | 1.398 | 1.392 | 1.400 | 1.390 | 1.394 ab | 1.403 b | 1.404 b | 1.378 a | 0.004 | 0.064 | 0.051 | <0.001 |
| 28–35 d | 1.568 | 1.580 | 1.561 | 1.587 | 1.574 ab | 1.562 ab | 1.548 a | 1.612 b | 0.005 | 0.429 | 0.237 | 0.037 |
| 0–35 d | 1.424 | 1.415 | 1.424 | 1.414 | 1.431 a | 1.414 b | 1.415 b | 1.416 b | 0.003 | 0.051 | 0.051 | <0.001 |
| F | AA | F × AA | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F− | F+ | AA− | AA+ | F−AA− | F−AA+ | F+AA− | F+AA+ | SEM | F | AA | F × AA | |
| n | 20 | 20 | 20 | 20 | 10 | 10 | 10 | 10 | ||||
| Nutrient intake | ||||||||||||
| AME (kcal/bird) | ||||||||||||
| 0–4 d | 165.4 | 169.5 | 167.8 | 167.1 | 162.2 c | 168.5 ab | 173.4 a | 165.6 bc | 2.2 | 0.083 | 0.873 | 0.040 |
| 4–11 d | 827.1 | 893.4 | 865.6 | 854.8 | 823.0 c | 831.1 c | 908.2 a | 878.5 b | 2.9 | <0.001 | <0.001 | <0.001 |
| Fat (g/bird) | ||||||||||||
| 0–4 d | 1.98 | 4.75 | 3.47 | 3.26 | 1.94 d | 2.03 c | 5.00 a | 4.50 b | 0.01 | <0.001 | <0.001 | 0.031 |
| 4–11 d | 9.91 | 22.70 | 16.45 | 16.16 | 9.67 d | 10.15 c | 23.23 a | 22.17 b | 0.08 | <0.001 | 0.643 | 0.048 |
| Lys (g) | ||||||||||||
| 0–4 d | 0.64 | 0.61 | 0.57 | 0.68 | 0.57 c | 0.71 a | 0.57 c | 0.65 b | 0.02 | 0.147 | <0.001 | 0.022 |
| 4–11 d | 3.18 | 3.10 | 2.86 | 3.41 | 2.87 c | 3.48 a | 2.85 c | 3.33 b | 0.05 | 0.315 | <0.001 | 0.001 |
| Starch (g/bird) | ||||||||||||
| 0–4 d | 22.7 | 18.7 | 21.1 | 22.7 | 22.6 a | 22.8 a | 19.6 b | 17.9 c | 1.3 | <0.001 | 0.245 | 0.044 |
| 4–11 d | 113.8 | 105.2 | 112.0 | 113.8 | 113.8 a | 113.8 a | 110.1 ab | 100.4 b | 1.5 | <0.001 | 0.167 | <0.001 |
| Nutrient-to-gain ratio | ||||||||||||
| AME:g (Kcal:g/bird) | ||||||||||||
| 0–4 d | 2.70 | 2.76 | 2.77 | 2.69 | 2.73 | 2.68 | 2.79 | 2.71 | 0.03 | 0.394 | 0.069 | 0.061 |
| 4–11 d | 3.40 | 3.58 | 3.70 | 3.25 | 3.62 | 3.16 | 3.84 | 3.32 | 0.05 | 0.061 | 0.023 | 0.056 |
| Fat:g (g:g/bird) | ||||||||||||
| 0–4 d | 0.044 | 0.076 | 0.064 | 0.055 | 0.049 b | 0.037 c | 0.078 a | 0.073 a | 0.001 | <0.001 | <0.001 | <0.001 |
| 4–11 d | 0.056 | 0.092 | 0.082 | 0.066 | 0.066 c | 0.045 d | 0.097 a | 0.087 b | 0.001 | <0.001 | <0.001 | <0.001 |
| Lys:g (mg:g/bird) | ||||||||||||
| 0–4 d | 1.024 | 0.975 | 0.929 | 1.085 | 0.938 c | 1.112 a | 0.923 d | 1.057 b | 0.004 | 0.057 | <0.001 | 0.003 |
| 4–11 d | 1.295 | 1.252 | 1.215 | 1.334 | 1.236 c | 1.355 a | 1.193 d | 1.311 b | 0.005 | <0.001 | <0.001 | <0.001 |
| Starch:g (g:g/bird) | ||||||||||||
| 0–4 d | 0.36 | 0.30 | 0.33 | 0.32 | 0.36 a | 0.35 ab | 0.31 b | 0.29 b | 0.01 | 0.017 | 0.201 | 0.048 |
| 4–11 d | 0.45 | 0.42 | 0.48 | 0.41 | 0.49 a | 0.43 bc | 0.46 ab | 0.39 c | 0.01 | 0.053 | <0.001 | 0.001 |
| F | AA | F × AA | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F− | F+ | AA− | AA+ | F−AA− | F−AA+ | F+AA− | F+AA+ | SEM | F | AA | F × AA | |
| n | 20 | 20 | 20 | 20 | 10 | 10 | 10 | 10 | ||||
| BW (g) | 116.5 | 118.4 | 116.9 | 118.0 | 113.9 b | 119.2 a | 119.9 a | 116.9 ab | 1.8 | 0.352 | 0.516 | 0.035 |
| Supply organ weight d 4, % of BW | ||||||||||||
| Heart | 0.96 | 0.95 | 0.94 | 0.97 | 0.94 | 0.97 | 0.94 | 0.97 | 0.11 | 0.931 | 0.473 | 0.975 |
| Liver | 4.92 | 4.36 | 4.49 | 4.79 | 4.60 b | 5.24 a | 4.39 c | 4.34 c | 0.07 | 0.003 | 0.098 | 0.044 |
| Proventriculus | 1.14 | 1.11 | 1.11 | 1.14 | 1.13 | 1.14 | 1.08 | 1.15 | 0.01 | 0.761 | 0.411 | 0.527 |
| Gizzard | 4.35 | 4.54 | 4.47 | 4.42 | 4.56 | 4.14 | 4.39 | 4.69 | 0.17 | 0.441 | 0.947 | 0.084 |
| Pancreas | 0.42 | 0.45 | 0.41 | 0.45 | 0.40 | 0.43 | 0.43 | 0.47 | 0.02 | 0.161 | 0.099 | 0.118 |
| Duodenum | 1.79 | 1.96 | 1.86 | 1.89 | 1.80 | 1.77 | 1.91 | 2.02 | 0.08 | 0.120 | 0.562 | 0.627 |
| Jejunum | 3.47 | 3.76 | 3.63 | 3.61 | 3.40 | 3.56 | 3.86 | 3.66 | 0.12 | 0.123 | 0.964 | 0.244 |
| Ileum | 2.59 | 2.76 | 2.70 | 2.65 | 2.63 | 2.54 | 2.77 | 2.75 | 0.09 | 0.204 | 0.638 | 0.720 |
| Ceca | 0.75 | 0.85 | 0.75 | 0.83 | 0.72 | 0.77 | 0.78 | 0.90 | 0.07 | 0.433 | 0.106 | 0.956 |
| F | A | F × AA | p-Values | |||||||||
| F− | F+ | AA− | AA+ | F−AA− | F−AA+ | F+AA− | F+AA+ | SEM | F | AA | F × AA | |
| n | 20 | 20 | 20 | 20 | 10 | 10 | 10 | 10 | ||||
| BW (g) | 363.2 | 368.1 | 354.8 | 376.4 | 341.7 c | 384.7 a | 368.0 b | 368.1 b | 3.7 | 0.599 | 0.025 | 0.025 |
| Supply organ weight d 11, % of BW | ||||||||||||
| Heart | 0.75 | 0.76 | 0.77 | 0.74 | 0.77 | 0.73 | 0.78 | 0.76 | 0.09 | 0.624 | 0.495 | 0.700 |
| Liver | 4.08 | 3.95 | 3.98 | 4.05 | 4.11 | 4.06 | 3.85 | 4.05 | 0.12 | 0.213 | 0.517 | 0.059 |
| Proventriculus | 0.76 | 0.77 | 0.79 | 0.73 | 0.79 | 0.72 | 0.79 | 0.75 | 0.01 | 0.520 | 0.005 | 0.507 |
| Gizzard | 2.97 | 3.05 | 3.05 | 2.97 | 3.11 | 2.82 | 2.99 | 3.12 | 0.08 | 0.406 | 0.472 | 0.064 |
| Pancreas | 0.43 | 0.41 | 0.45 | 0.40 | 0.46 a | 0.41 bc | 0.42 ab | 0.39 c | 0.01 | 0.116 | 0.040 | 0.046 |
| Duodenum | 1.41 | 1.54 | 1.48 | 1.48 | 1.44 | 1.39 | 1.51 | 1.57 | 0.11 | 0.078 | 0.901 | 0.388 |
| Jejunum | 2.61 | 2.66 | 2.67 | 2.61 | 2.65 | 2.57 | 2.69 | 2.64 | 0.09 | 0.648 | 0.555 | 0.890 |
| Ileum | 1.95 | 1.95 | 2.00 | 1.91 | 2.03 | 1.88 | 1.98 | 1.93 | 0.06 | 0.959 | 0.223 | 0.551 |
| Ceca | 0.60 | 0.68 | 0.63 | 0.65 | 0.61 | 0.59 | 0.64 | 0.72 | 0.06 | 0.129 | 0.409 | 0.218 |
| F | AA | F × AA | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F− | F+ | AA− | AA+ | F−AA− | F−AA+ | F+AA− | F+AA+ | SEM | F | AA | F × AA | |
| n | 10 | 10 | 10 | 10 | ||||||||
| Day 4 | ||||||||||||
| Duodenum | ||||||||||||
| Length (cm) | 14.4 | 15.8 | 15.0 | 15.2 | 14.5 | 14.2 | 15.5 | 16.2 | 0.2 | <0.001 | 0.288 | 0.491 |
| Weight (g/cm) | 0.147 | 0.146 | 0.146 | 0.147 | 0.145 | 0.149 | 0.147 | 0.145 | 0.003 | 0.306 | 0.797 | 0.671 |
| Jejunum | ||||||||||||
| Length (cm) | 32.6 | 33.8 | 33.9 | 32.4 | 32.9 | 32.3 | 35.1 | 32.7 | 0.4 | 0.234 | 0.298 | 0.098 |
| Weight (g/cm) | 0.124 | 0.100 | 0.111 | 0.114 | 0.120 | 0.129 | 0.101 | 0.099 | 0.002 | <0.001 | 0.914 | 0.268 |
| Ileum | ||||||||||||
| Length (cm) | 33.0 | 32.5 | 32.6 | 33.0 | 32.6 | 33.4 | 32.5 | 32.6 | 0.5 | 0.602 | 0.450 | 0.486 |
| Weight (g/cm) | 0.091 | 0.133 | 0.112 | 0.111 | 0.093 | 0.088 | 0.132 | 0.133 | 0.003 | <0.001 | 0.845 | 0.657 |
| Day 11 | ||||||||||||
| Duodenum | ||||||||||||
| Length (cm) | 10.8 | 11.4 | 11.3 | 10.9 | 11.1 | 10.4 | 11.4 | 11.3 | 0.2 | 0.079 | 0.322 | 0.387 |
| Weight (g/cm) | 0.485 | 0.494 | 0.463 | 0.516 | 0.451 | 0.519 | 0.476 | 0.512 | 0.008 | 0.416 | <0.001 | 0.284 |
| Jejunum | ||||||||||||
| Length (cm) | 47.6 | 48.5 | 47.70 | 48.45 | 45.3 b | 50.0 a | 50.1 a | 46.9 b | 0.9 | 0.567 | 0.611 | 0.026 |
| Weight (g/cm) | 0.203 | 0.202 | 0.201 | 0.203 | 0.207 | 0.198 | 0.195 | 0.208 | 0.006 | 0.857 | 0.723 | 0.088 |
| Ileum | ||||||||||||
| Length (cm) | 48.3 | 48.1 | 48.9 | 47.4 | 48.7 | 47.8 | 49.2 | 47.0 | 0.4 | 0.996 | 0.522 | 0.716 |
| Weight (g/cm) | 0.151 | 0.152 | 0.149 | 0.154 | 0.149 | 0.153 | 0.150 | 0.154 | 0.004 | 0.714 | 0.101 | 0.285 |
| F | AA | F × AA | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F− | F+ | AA− | AA+ | F−AA− | F−AA+ | F+AA− | F+AA+ | SEM | F | AA | F × AA | |
| n | 20 | 20 | 20 | 20 | 10 | 10 | 10 | 10 | ||||
| Duodenum | ||||||||||||
| Villus length (µm) | 1053 | 999 | 1025 | 1027 | 1048 | 1058 | 1001 | 997 | 17 | <0.001 | 0.328 | 0.191 |
| Crypt depth (µm) | 166 | 181 | 169 | 177 | 169 b | 162 b | 169 b | 192 a | 5.7 | 0.196 | 0.487 | 0.047 |
| V:C ratio | 6.6 | 6.1 | 6.5 | 6.1 | 6.4 a | 6.7 a | 6.6 a | 5.5 b | 0.2 | 0.218 | 0.240 | 0.032 |
| Jejunum | ||||||||||||
| Villus length (µm) | 518 | 560 | 540 | 536 | 530 ab | 503 b | 549 ab | 570 a | 13.9 | <0.001 | 0.078 | <0.001 |
| Crypt depth (µm) | 138 | 157 | 146 | 148 | 144 bc | 132 c | 148 b | 165 a | 4.0 | 0.010 | 0.472 | 0.021 |
| V:C ratio | 3.9 | 3.6 | 3.8 | 3.7 | 3.8 a | 3.9 a | 3.8 a | 3.5 b | 0.1 | 0.174 | 0.458 | 0.002 |
| Ileum | ||||||||||||
| Villus length (µm) | 401 | 404 | 402 | 404 | 406 | 396 | 398 | 411 | 10 | 0.853 | 0.921 | 0.697 |
| Crypt depth (µm) | 127 | 140 | 125 | 149 | 123 b | 129 b | 128 b | 152 a | 4.3 | 0.103 | 0.070 | <0.001 |
| V:C ratio | 3.3 | 3.1 | 3.3 | 3.0 | 3.4 | 3.1 | 3.2 | 2.9 | 0.1 | 0.202 | 0.089 | 0.097 |
| F | AA | F × AA | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F− | F+ | AA− | AA+ | F−AA− | F−AA+ | F+AA− | F+AA+ | SEM | F | AA | F × AA | |
| n | 10 | 10 | 10 | 10 | ||||||||
| Duodenum | ||||||||||||
| Villus length (µm) | 1523.1 | 1600.6 | 1557.8 | 1565.9 | 1534.2 | 1512 | 1581.4 | 1619.7 | 27.9 | 0.080 | 0.888 | 0.097 |
| Crypt depth (µm) | 276.1 | 282.1 | 279.6 | 278.6 | 270.3 | 281.9 | 288.9 | 275.2 | 5.0 | 0.567 | 0.919 | 0.227 |
| V:C ratio | 5.6 | 5.8 | 5.7 | 5.8 | 5.8 | 5.5 | 5.6 | 6.0 | 0.1 | 0.547 | 0.878 | 0.109 |
| Jejunum | ||||||||||||
| Villus length (µm) | 875.5 | 907.3 | 871.7 | 911.1 | 861.6 | 889.4 | 881.8 | 932.8 | 22.5 | 0.493 | 0.398 | 0.802 |
| Crypt depth (µm) | 227.1 | 242 | 241.7 | 227.4 | 239.5 | 214.6 | 243.9 | 240.1 | 5.8 | 0.205 | 0.205 | 0.368 |
| V:C ratio | 4.0 | 3.9 | 3.8 | 4.1 | 3.8 | 4.2 | 3.7 | 4.0 | 0.1 | 0.379 | 0.075 | 0.662 |
| Ileum | ||||||||||||
| Villus length (µm) | 565.4 | 520.85 | 545.2 | 541.05 | 566.3 | 564.5 | 524.1 | 517.6 | 15.6 | 0.068 | 0.896 | 0.941 |
| Crypt depth (µm) | 189.0 | 183.1 | 178.2 | 193.9 | 178.3 | 199.7 | 178.1 | 188.1 | 4.1 | 0.073 | 0.040 | 0.483 |
| V:C ratio | 3.1 | 2.9 | 3.1 | 2.9 | 3.2 | 2.9 | 3.0 | 2.8 | 0.1 | 0.122 | 0.042 | 0.527 |
| Organ | Diet Treatment | a | a (95% CI) | b | b (95% CI) | Pseudo-R2 |
|---|---|---|---|---|---|---|
| Heart | F−AA− | 0.013 | 0.011–0.014 | 0.903 | 0.861–0.958 | 0.90 |
| F−AA+ | 0.014 | 0.012–0.016 | 0.896 | 0.868–0.912 | 0.91 | |
| F+AA− | 0.012 | 0.010–0.013 | 0.919 | 0.891–0.942 | 0.94 | |
| F+AA+ | 0.014 | 0.011–0.015 | 0.895 | 0.879–0.914 | 0.93 | |
| Liver | F−AA− | 0.031 | 0.027–0.036 | 1.048 | 1.007–1.102 | 0.89 |
| F−AA+ | 0.037 | 0.034–0.041 | 1.019 | 0.992–1.056 | 0.90 | |
| F+AA− | 0.033 | 0.030–0.038 | 1.025 | 1.001–1.048 | 0.93 | |
| F+AA+ | 0.027 | 0.026–0.029 | 1.069 | 1.038–1.103 | 0.97 | |
| Proventriculus | F−AA− | 0.074 | 0.067–0.077 | 0.601 | 0.581–0.624 | 0.90 |
| F−AA+ | 0.077 | 0.071–0.082 | 0.602 | 0.574–0.636 | 0.86 | |
| F+AA− | 0.064 | 0.051–0.079 | 0.644 | 0.592–0.721 | 0.76 | |
| F+AA+ | 0.074 | 0.068–0.076 | 0.612 | 0.595–0.631 | 0.94 | |
| Gizzard | F−AA− | 0.051 | 0.036–0.056 | 0.919 | 0.881–0.953 | 0.79 |
| F−AA+ | 0.059 | 0.052–0.063 | 0.879 | 0.853–0.907 | 0.87 | |
| F+AA− | 0.063 | 0.039–0.076 | 0.863 | 0.817–0.961 | 0.77 | |
| F+AA+ | 0.056 | 0.051–0.059 | 0.907 | 0.880–0.936 | 0.89 | |
| Pancreas | F−AA− | 0.002 | 0.002–0.002 | 1.122 | 1.008–1.168 | 0.88 |
| F−AA+ | 0.003 | 0.002–0.003 | 1.069 | 1.014–1.153 | 0.82 | |
| F+AA− | 0.003 | 0.003–0.004 | 1.051 | 1.009–1.114 | 0.89 | |
| F+AA+ | 0.005 | 0.005–0.005 | 0.971 | 0.939–0.994 | 0.92 | |
| Duodenum | F−AA− | 0.020 | 0.017–0.021 | 0.949 | 0.901–0.980 | 0.82 |
| F−AA+ | 0.020 | 0.017–0.021 | 0.941 | 0.913–0.991 | 0.86 | |
| F+AA− | 0.021 | 0.017–0.025 | 0.935 | 0.889–0.975 | 0.79 | |
| F+AA+ | 0.019 | 0.016–0.022 | 0.970 | 0.941–1.001 | 0.82 | |
| Jejunum | F−AA− | 0.027 | 0.021–0.035 | 1.007 | 0.967–1.089 | 0.75 |
| F−AA+ | 0.035 | 0.031–0.037 | 0.951 | 0.920–0.982 | 0.81 | |
| F+AA− | 0.039 | 0.032–0.044 | 0.937 | 0.883–0.967 | 0.80 | |
| F+AA+ | 0.034 | 0.026–0.039 | 0.962 | 0.878–0.993 | 0.79 | |
| Ileum | F−AA− | 0.033 | 0.027–0.036 | 0.927 | 0.869–0.997 | 0.76 |
| F−AA+ | 0.037 | 0.033–0.039 | 0.888 | 0.857–0.927 | 0.83 | |
| F+AA− | 0.039 | 0.035–0.042 | 0.889 | 0.889–0.928 | 0.88 | |
| F+AA+ | 0.042 | 0.039–0.044 | 0.869 | 0.853–0.901 | 0.93 | |
| Ceca | F−AA− | 0.005 | 0.004–0.006 | 1.054 | 1.002–1.188 | 0.77 |
| F−AA+ | 0.005 | 0.004–0.006 | 1.038 | 1.001–1.183 | 0.78 | |
| F+AA− | 0.004 | 0.004–0.005 | 1.059 | 0.997–1.123 | 0.75 | |
| F+AA+ | 0.005 | 0.004–0.006 | 1.065 | 1.003–1.145 | 0.75 |
| Day 0–4 | Day 0–11 | Day 4–11 | Day 0–4–11 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F−AA− | F−AA+ | F+AA− | F−AA− | F−AA+ | F+AA− | F−AA− | F−AA+ | F+AA− | F−AA− | F−AA+ | F+AA− | ||
| Heart | F−AA+ | 0.960 | 0.547 | 0.311 | 0.398 | ||||||||
| F+AA− | 0.743 | 0.577 | 0.616 | 0.834 | 0.296 | 0.365 | 0.396 | 0.341 | |||||
| F+AA+ | 0.817 | 0.859 | 0.362 | 0.760 | 0.618 | 0.453 | 0.404 | 0.230 | 0.172 | 0.382 | 0.617 | 0.288 | |
| Liver | F−AA+ | 0.274 | 0.177 | 0.003 | 0.054 | ||||||||
| F+AA− | <0.001 | <0.001 | <0.001 | <0.001 | 0.653 | 0.007 | 0.104 | 0.347 | |||||
| F+AA+ | <0.001 | <0.001 | 0.486 | 0.159 | 0.171 | <0.001 | <0.001 | <0.001 | 0.011 | 0.096 | 0.051 | 0.110 | |
| Proventriculus | F−AA+ | 0.147 | 0.682 | 0.193 | 0.511 | ||||||||
| F+AA− | 0.763 | 0.188 | 0.713 | 0.836 | 0.198 | 0.058 | <0.001 | <0.001 | |||||
| F+AA+ | 0.134 | 0.692 | 0.234 | 0.698 | 0.765 | 0.814 | 0.395 | 0.237 | 0.066 | 0.337 | 0.418 | <0.001 | |
| Gizzard | F−AA+ | 0.674 | 0.059 | 0.633 | 0.487 | ||||||||
| F+AA− | 0.837 | 0.332 | 0.561 | 0.382 | 0.136 | 0.179 | 0.364 | 0.587 | |||||
| F+AA+ | 0.086 | 0.051 | 0.091 | 0.864 | 0.035 | 0.453 | 0.682 | 0.694 | 0.201 | 0.381 | 0.607 | 0.696 | |
| Pancreas | F−AA+ | 0.109 | 0.114 | <0.001 | <0.001 | ||||||||
| F+AA− | 0.428 | 0.790 | 0.067 | 0.340 | 0.108 | <0.001 | 0.264 | <0.001 | |||||
| F+AA+ | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | |
| Duodenum | F−AA+ | 0.989 | 0.886 | 0.745 | 0.637 | ||||||||
| F+AA− | <0.001 | 0.001 | <0.001 | 0.748 | 0.236 | 0.295 | 0.536 | 0.549 | |||||
| F+AA+ | <0.001 | <0.001 | 0.014 | <0.001 | <0.001 | 0.037 | 0.742 | 0.639 | 0.377 | <0.001 | <0.001 | <0.001 | |
| Jejunum | F−AA+ | 0.324 | 0.623 | <0.001 | <0.001 | ||||||||
| F+AA− | 0.067 | 0.072 | 0.617 | 0.708 | <0.001 | 0.082 | <0.001 | 0.496 | |||||
| F+AA+ | 0.071 | 0.069 | 0.142 | 0.529 | 0.606 | 0.731 | <0.001 | 0.851 | 0.095 | <0.001 | 0.374 | 0.467 | |
| Ileum | F−AA+ | <0.001 | <0.001 | 0.163 | <0.001 | ||||||||
| F+AA− | 0.143 | <0.001 | 0.094 | <0.001 | 0.006 | 0.042 | <0.001 | <0.001 | |||||
| F+AA+ | <0.001 | <0.001 | <0.001 | <0.001 | 0.164 | <0.001 | <0.001 | <0.001 | 0.087 | <0.001 | 0.432 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diehl, E.; van Eerden, E.; Duijster, M.; Kwakkel, R. Supply Organ Development in Young Broilers in Response to Changing Dietary Fat and Amino Acids in the Starter Period. Poultry 2025, 4, 56. https://doi.org/10.3390/poultry4040056
Diehl E, van Eerden E, Duijster M, Kwakkel R. Supply Organ Development in Young Broilers in Response to Changing Dietary Fat and Amino Acids in the Starter Period. Poultry. 2025; 4(4):56. https://doi.org/10.3390/poultry4040056
Chicago/Turabian StyleDiehl, Edward, Ellen van Eerden, Masja Duijster, and René Kwakkel. 2025. "Supply Organ Development in Young Broilers in Response to Changing Dietary Fat and Amino Acids in the Starter Period" Poultry 4, no. 4: 56. https://doi.org/10.3390/poultry4040056
APA StyleDiehl, E., van Eerden, E., Duijster, M., & Kwakkel, R. (2025). Supply Organ Development in Young Broilers in Response to Changing Dietary Fat and Amino Acids in the Starter Period. Poultry, 4(4), 56. https://doi.org/10.3390/poultry4040056






