1. Introduction
Laying poultry farming is a sector that has remained prominent within agribusiness, mainly due to its productive performance linked to the thermal environment in tropical and subtropical regions. On many farms, the birds are raised in conventional cage systems from the rearing phase, which may compromise the welfare and future production of the batch [
1].
The dynamic use of different limestone granulometries and vitamin D3 supplementation are efficient nutritional strategies to mitigate the harmful effects of heat stress on the performance and welfare of laying hens. Heat-stressed birds tend to abruptly reduce their feed intake as a way of reducing the body heat production [
2]. However, the sudden reduction in the FI can lead to a nutritional deficiency, in cases of less nutritionally dense diets, or increase the requirements for minerals and vitamins, due to the greater excretion of these nutrients under heat stress conditions [
3]. The appropriate limestone granulometry allows the gradual release of calcium, keeping its blood concentration stable and optimizing mineral metabolism, which reduces bone mobilization and can reduce metabolic heat generation. Meanwhile, vitamin D3 is essential for efficient calcium absorption and bone regulation, preventing losses and possibly reducing metabolic stress. Together, these strategies improve metabolic efficiency, reducing the internal energy expenditure and favoring both the production and thermoregulation of birds.
The use of vitamin D3 has been decisive for researchers, as it has the ability to regulate, especially at the beginning of the hen’s sexual maturation, medullary bone development and the remodeling of structural bone tissue, acting to prevent bone diseases that affect laying hens in the production phase, such as osteoporosis, cage fatigue and keel bone deformities [
4]. Considering that young birds close to peak production are more susceptible to developing osteoporosis, nutritional strategies such as the dynamics of using different granulometries of limestone and vitamin D3 act to minimize possible economic losses [
5].
Meeting the nutritional requirements of Ca becomes a crucial factor in the period preceding the sexual maturity of birds, as it is a critical time for the development of medullary bone, a highly labile component responsible for bone remodeling during the laying period [
6]. To avoid bone problems such as osteoporosis, in modern lines of laying hens, it is recommended to use calcium sources of different granulometries, ranging from a 25% inclusion of fine limestone, characterized by particles of 0.0 mm to 0.5 mm, to a 75% inclusion of coarse limestone, characterized by particles of 1.5 mm to 4.5 mm [
7], as well as an adequate supplementation of vitamin D3, so that there is the correct absorption of calcium, ensuring the supply of minerals necessary for bone development capable of supporting the laying and longevity of the future laying hen [
8].
Due to the limited information on the influence of the use of limestone granulometry associated with different vitamin levels on the thermoregulation and behavior of pullets reared under conditions of thermal stress, it is necessary to evaluate the effect of different limestone granulometries and levels of vitamin D3 on the physiological and behavioral responses of pullets.
2. Material and Methods
2.1. Project Regulation
This project was submitted to the Committee of Ethics on the Use of Animals (CEUA)/UVA Ceará, Brazil, and all proposed procedures were approved under protocol N°. 007.11.021.UVA.504.03 (approved on 1 December 2021).
2.2. Study Location and Weather Conditions
The experiment lasted 56 consecutive days, divided into two periods of 28 days each, from December 2021 to January 2022 (transition season—dry), in Sobral, Ceará, Brazil (3°36′ N, 40°18′ E, 56 m above sea level). The climate of the region is classified as BShw, with summer rains and dry winter (hot semiarid with average monthly temperatures above 18 °C), according to the Koppen climate classification [
9]. The average temperature is 27 °C, and the annual rainfall is 808 mm year
−1, concentrated in the first five months of the year [
10]. The lighting management provided 16 h of light and 8 h of darkness.
2.3. Facilities and Equipment
The experimental unit consisted of nine birds, housed in galvanized wire cages measuring 90 cm in length × 45 cm in width × 45 cm in height, with three 30 cm subdivisions and a population density of 450 cm2 bird−1, containing a front feeder in a metal trough (Zatti, Santa Catarina, Brazil) and one drinker (Nipple, Holandesa, Brazil) per cage in an open-sided masonry shed, in a conventional breeding system with east–west orientation, measuring 12 m long by 8 m wide and with a ceiling height of 2.60 m, with wire mesh, concrete floor and ceramic tile ceiling.
2.4. Animals, Experimental Design and Diets
The animals were acquired from Granja Planalto, located in Uberlândia, Brazil. Three days after hatching, the chicks were transported to the Poultry Research Center at the Experimental Farm of the Vale do Acaraú State University (UVA). The birds were subjected to a strict vaccination program recommended for commercial laying hens. Initially, they were housed in a masonry shed divided into individual pens. After 8 weeks, they were transferred to conventional cages used for pullet rearing, where they remained until 17 weeks of age, according to the experimental design.
A total of 270 8-week-old replacement pullets of the Lonhman brown lineage, clinically healthy, were distributed in a completely randomized design, in a 2 × 2 × 2 + 1 factorial scheme, receiving diets with two limestone granulometries (100% fine limestone (FL) or 50% fine limestone +50% coarse limestone (CL)), two vitamin D3 levels of supplementations (1380 IU (12.5 g/kg) or 2760 IU (25 g/kg)) and a control diet (with fine limestone and no vitamin D3 supplementation) totaling 5 dietary treatments, assessed in 2 shifts in the day (morning and afternoon), with 6 replications and 9 birds each. The vitamin D3 metabolite supplemented in the diets was 25-hydroxycholecalciferol (25-OHD3). Experimental vitamin D3 levels were obtained using a commercial product with 11,040,000 IU of vitamin D3/kg (DSM NUTRITIONAL PRODUCTS BRAZIL S.A). The vitamin and mineral premix contained 2000 IU of vitamin D3 per kg of feed. The limestone particle size was classified by mean geometric diameter (MGD) as fine MGD (0.568 mm) and coarse MGD (1.943 mm). The diets were iso-nutrient and iso-energetic (
Table 1 and
Table 2) and were formulated according to the nutritional recommendations suggested by the lineage manual [
7]. The nutritional composition of the ingredients used in the diets followed the recommendations of Rostagno et al. [
11]. The effects of morning and afternoon shifts were also considered in the factorial arrangement. Drinking water was provided ad libitum.
2.5. Data Collection
Meteorological variables were recorded on the last two days of each 28-day period in both shifts, morning (8:00 a.m. to 11:30 a.m.) and afternoon (2:00 p.m. to 5:00 p.m.), along with thermoregulatory responses, with behavioral observations recorded from 7:00 a.m. to 5:00 p.m., in which one bird was randomly selected per experimental unit (totaling 6 birds per treatment), and identified in the posterior region with non-toxic paint, according to Farias et al. [
12] and Sena et al. [
13]. The handling and data collection protocols were performed by experienced professionals and with the least possible degree of invasiveness.
2.6. Meteorological Variables and Thermal Comfort Indices
The black globe (BGT, °C, INSTRUTHERM, mod TGD-200, Sao Paulo, Brazil), dry bulb (DBT, °C, Incoterm, 5195.03.0.00, Porto Alegre, Brazil) and wet bulb (WBT, °C, INCOTERM, model 5195.03.0.00, Porto Alegre, Brazil) temperature variables were collected at consecutive intervals of 30 min, using thermometers positioned in the center of the storage shed at 1 m high from the ground and at the height of the animals’ center of mass. The wind speed (m·s
−1) was assessed through the database of the Cearense Foundation of Meteorology and Water Resources—FUNCEME. Subsequently, from the meteorological variables, the Relative Humidity (RH, %) and black globe temperature and humidity index (BGHI) were calculated according to Buffintgon et al. [
14] (Equation (1)), and the Radiant Heat Load (RHL) (Equation (2)) and mean radiant temperature (MRT) (Equation (3)) were calculated as described below:
where
BTG—Black globe temperature, °C;
DPT—Dew point temperature, °C.
ơ—Stefan–Boltzman constant (5.67 × 10−8 W m−2 K−4);
MRT—Mean radiant temperature (K).
where
WS—Wind speeds m/s;
BTG—Black globe temperature, K;
DBT—Dry bulb temperature, K.
2.7. Thermoregulatory Responses
The respiratory rate (RR, mov min
−1) was observed for 30 s, and this value was multiplied by two to obtain movements per minute. Cloaca temperature (CT, °C) was measured with the aid of a digital thermometer (accuracy of ±0.1 °C, G-Tech, Rio de Janeiro, Brazil) being introduced at a depth of three centimeters until the thermometer stabilized. The thermal gradient (TG, °C) was calculated according to Equation (4).
where
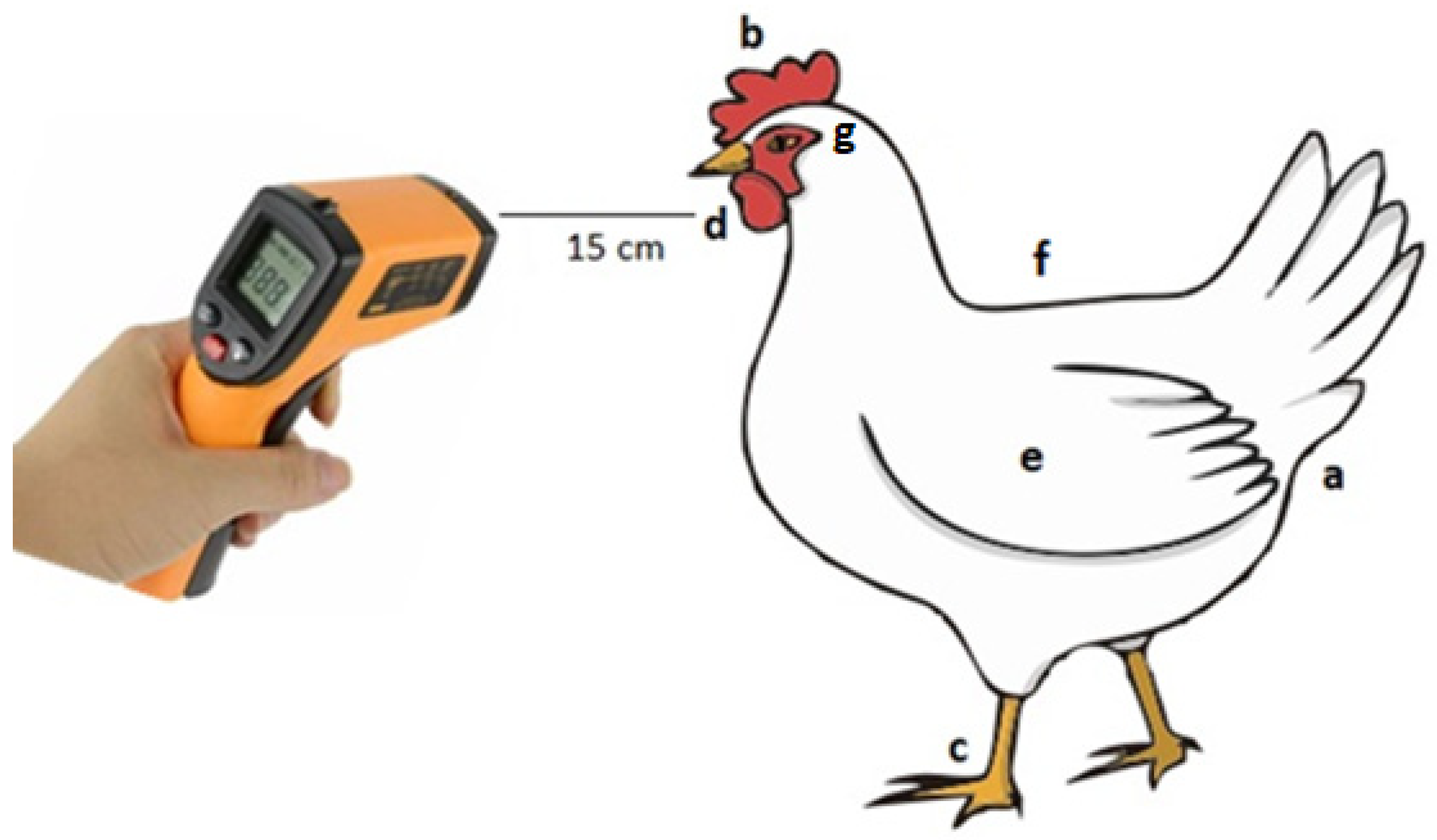
An infrared thermometer (precision ± 0.2 °C, STHT 77365, Stanley, New York, NY, USA) was used to measure the surface temperature of the different anatomical parts of the pullets’ body. To measure these temperatures, the equipment was positioned at a distance of 15 cm from the pullets’ body, as recommended in the user manual (
Figure 1). To calculate the body featherless surface temperature (FLST, °C), the average values of the following temperatures were used: cloaca (a), crest (b), upper part of the foot (c) and dewlap (d) temperatures. Regarding the surface temperature of the region with feathers (FST, °C), the average temperature of the wing (e), back (f) and head (g) was used. Wing surface temperature measurements were taken on the upper side of the wing.
2.8. Behavioral Responses
An ethogram was used to assess the behavioral responses, (adapted from Catalan et al. [
15]) using instantaneous sampling to evaluate behaviors (Sitting: Behavior in which the bird remained at rest, with its body resting on the cage; Eating: Behavior observed when the bird had its head positioned inside the feeder, ingesting feed; Drinking: Behavior characterized when the bird was seen pecking the nipple drinker to ingest water; Exploring feathers: Non-aggressive behavior, identified when the bird used its beak to examine its own feathers or the feathers of other birds; Pecking: Behavior associated with establishing dominance or responding to stressful situations, characterized by quick and intense pecks directed at the crest or other regions of the head of another bird) according to the methodology suggested by Barbosa Filho et al. [
16]. During the analysis, the evaluators recorded which behavior the bird was performing during 5 min of observation with intervals of 10 min for the next observation, from 7:00 a.m. to 5:00 p.m. From these observations, the frequencies of behavioral responses were calculated. All analyses were performed by the same evaluators in all periods of the experiment.
To minimize behavioral interference, 15 days before the beginning of behavioral data collection, observers remained in the experimental shed daily for a determined period, with the aim of promoting the habituation of the birds to the human presence and minimizing interference during observations. And at the moment of behavioral assessment, the observer remained approximately two meters from the cages, out of the birds’ field of vision.
2.9. Statistical Methods
Statistical analysis was performed in two stages. In the first, the thermoregulatory responses were submitted to analysis of variance (ANOVA) with repeated measures over time and, for comparison between the averages of the control treatment and the others, Dunnett’s test was applied (p < 0.05). Subsequently, an analysis was carried out, considering the factorial model in which the effects of limestone granulometry, two levels of supplementation of Vitamin D3 and day shifts were included, as well as the interaction between these factors. For the meteorological variables and the thermal comfort indices, the day shift (morning and afternoon) was considered as a fixed effect. These data were submitted to ANOVA, and the averages were compared evaluating the fixed effects for each group of variables. A significance level of 5% was adopted.
In the second stage, multivariate statistical techniques were used. Principal component analysis (PCA) and canonical discriminant analysis (CDA) were performed to identify, understand and integrate the effect of dietary supplementation of different limestone granulometries and vitamin D3 levels in both shifts on thermoregulatory and behavioral responses.
Exploratory factor analysis (EFA) was performed to identify the behavior of the variables through the effect of vitamin D3 level and the dynamics between thermoregulatory and behavioral responses according to granulometry and vitamin D3 level. The factor analysis model is expressed by Equation (5):
where
is the p-th score of the standardized variable (p = 1, 2, …, m),
is the extracted factor,
is the factor loading and
is the error.
Factor scores for each group were estimated by multiplying standardized variables by the coefficient of the corresponding factor score, as follows in Equation (6):
where
is the j-th factor extracted,
is the factor score coefficient and p is the number of variables [
17]. The first two components were extracted by orthogonal rotation and the varimax method, for better interpretability.
The CDA was carried out to demonstrate, through the centroids of the groups, the effect of the day shift on the thermoregulation and behavior of the birds according to the limestone granulometry and vitamin D3 levels. CDA was also used to evaluate the thermoregulatory and behavioral responses in the treatments (limestone granulometry) and in the two vitamin D3 levels. The general model of the CDA is described in Equation (7):
where
is the dependent variable (adaptive profile),
is the intercept,
is the explanatory variable and
is the discriminant coefficient for each explanatory variable. This evaluation was based on the number and percentages (%) of classification of birds in their group of origin.
The decision tree is a supervised machine learning method and was used to identify the main factors that differentiated the treatments under study. The CART algorithm was used in this study [
18]. While the effects of shifts, limestone sources and vitamin D3 levels were used as dependent variables, while thermoregulatory and behavioral responses were used as independent variables.
All procedures and univariate analyses were performed using SAS, version 10, 2000 (SAS Institute Inc., Cary, NC, USA), and multivariate analyses using SPPS, version 20, 2010 (SPSS Inc., Chicago, IL, USA).
3. Results
The environmental variables and the thermal comfort indices are presented in
Table 3. Only the RHL was lower (
p < 0.001) in the morning. The other environmental variables were not different. In addition, the BGHI had a greater tendency (
p = 0.06) in the afternoon.
Thermoregulatory responses were higher (
p < 0.001) in the afternoon, except for the TG, which was higher (
p < 0.001) in the morning (
Table 4). The TG also varied when using different limestone granulometries, being higher (
p < 0.001) when the fine limestone was added to the diet. The FST was higher (
p < 0.001) when using FL + CL, as well as when using 25 g of vitamin D3.
With the unfolding of the interaction for the evaluation of the thermal gradient (TG) (
Table 5), a reduction (
p < 0.05) was observed in the TG in the afternoon shift. In turn, the granulometry of the limestone (F + C limestone) reduced the TG in the morning shift. Regarding the effect of the interaction between the granulometry and the shift on the FST variable (
Table 6), an increase in this variable was observed in the late shift, and birds that received the fine limestone were smaller in the morning shift.
With the unfolding of the interaction between the vitamin D3 level and shift (
Table 7), a reduction in the TG was observed in the afternoon shift. Birds supplemented with 12.5 g of vitamin D3 showed a higher TG in the afternoon. Observing the effect of the interaction between the vitamin D3 level and the evaluation shift on the FST (
Table 8), the lowest value was observed in the afternoon, regardless of the level of vitamin D3, while birds that consumed feed with 12.5 g/kg of vit. D3 showed lower FSTs in the afternoon.
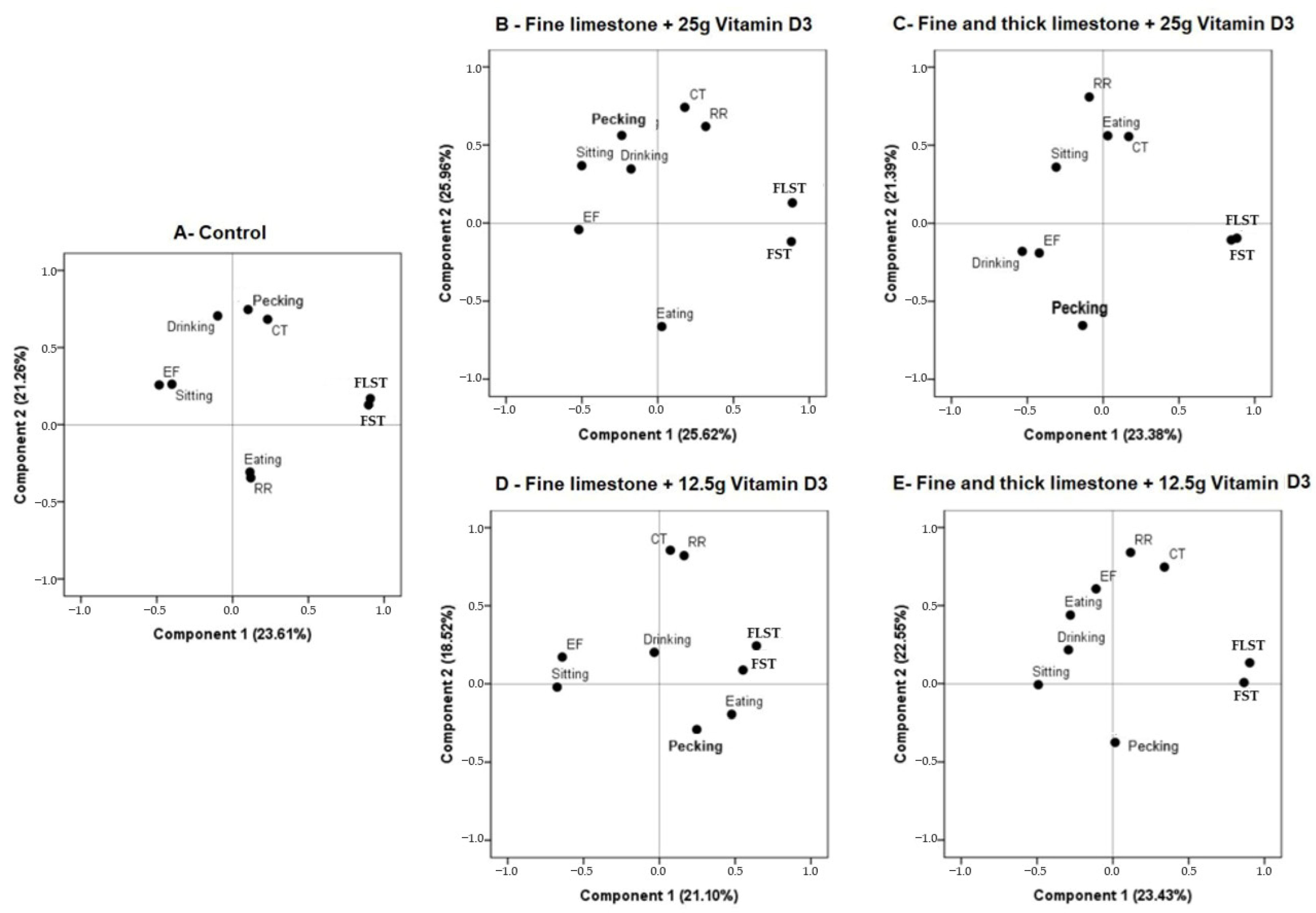
The effect of adding calcium and vitamin D3 in both levels on the thermoregulatory and behavioral responses is shown in
Figure 2. The first two components are the most important and explain more than 40% of the dynamics of the physiological and behavioral responses of birds in the control group and with different levels of vitamin D3 and fine limestone. For the control treatment, the main variables that explain the thermoregulatory and behavioral responses of the birds in order of importance were the FLST, FST, CT and pecking (
Figure 2A); fine limestone + 25 g D3 (
Figure 2B): the RR, CT, FLST and FST; fine and coarse limestone + 25 g D3 (
Figure 2C): eating, exploring feathers, the FLST, the FST and the RR; fine limestone + 12.5 g D3 (
Figure 2D): the CT, eating, pecking, the FLST and the FST; and fine and coarse limestone + 12.5 g D3 (
Figure 2E): the RR, CT, FLST and FST.
It was also observed that the activity of “exploring feathers” was important to describe the behavior of the birds that consumed feed containing fine and coarse limestone at different levels of vitamin D3 (
Figure 2C,E), which was different from that observed with the fine limestone plus different levels of vitamin D (
Figure 2B,D).
The levels of vitamin D3 (12.5 g and 25 g) and its use with fine or coarse limestone, used in this study, had no effect on thermoregulatory and behavioral variables, since they were similar in both treatments (
Figure 2).
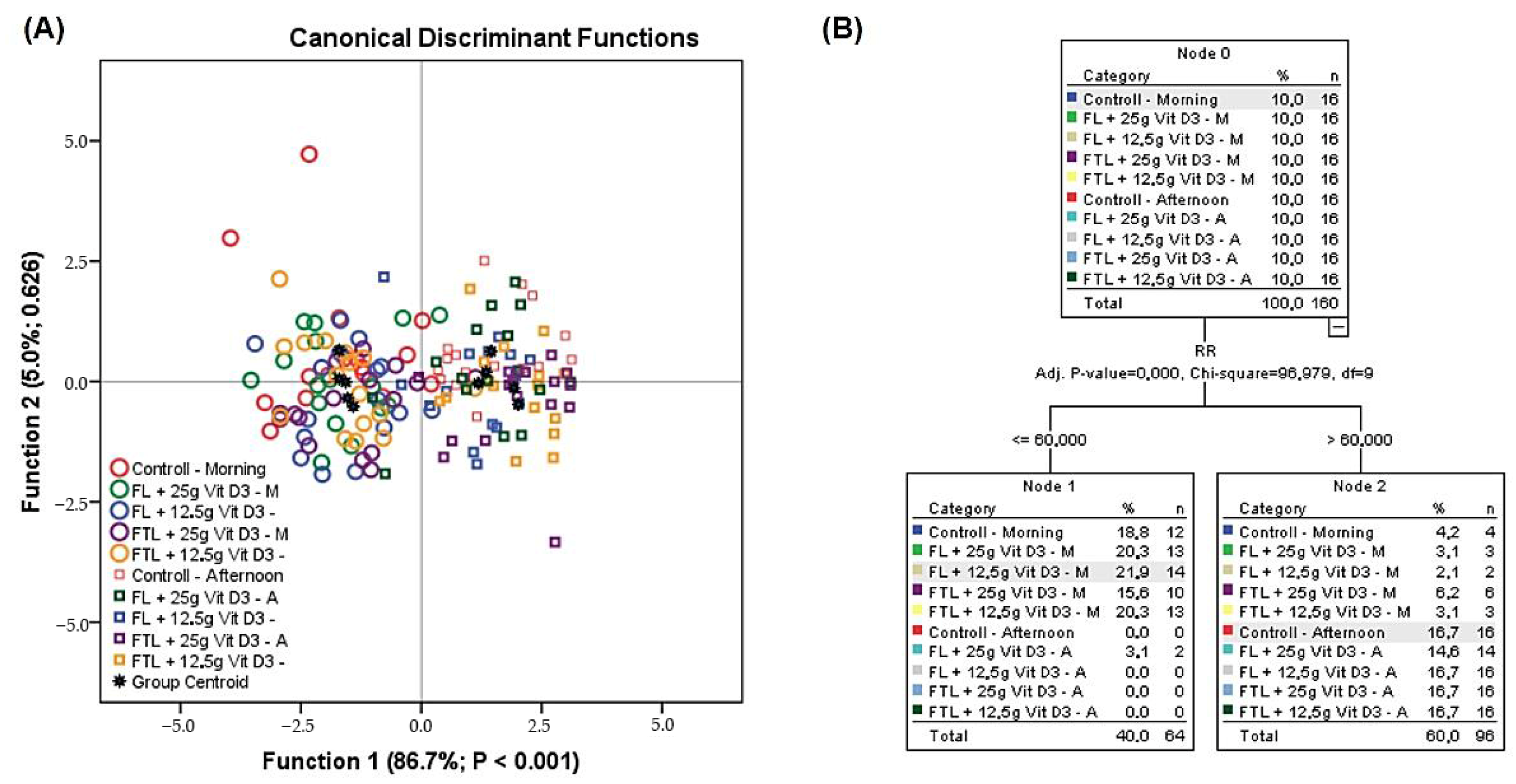
The dynamics of the thermoregulatory and behavioral responses of replacement pullets supplemented or not with different types of limestone granulometry and vitamin D3 levels in a semiarid environment are shown in
Figure 3. It was found that 88.50% of the birds were correctly classified in their group of origin, although no treatment was 100% correct in classifying the birds. The first two canonical functions discriminated 93.60%, making it possible to observe that in the first function there was discriminatory power (
p = 0.001), indicating a difference between treatments when analyzed jointly.
Through machine learning algorithms the tree analysis revealed that the main effect under study is the day shift, in which the RR is the main variable that divides the animals according to morning and afternoon shifts.
4. Discussion
The present study is the first to evaluate the thermoregulatory and behavioral responses of commercial replacement pullets fed different types of limestone, vitamin D3 levels and day shifts. To better understand this relationship, it employed a machine learning approach.
The AT was above the range indicated for birds cited by the Lohmann Brown Lite Manual [
6], which is 18 °C to 20 °C from the fifth week of life, characterizing it as an unfavorable environment for heat dissipation processes during the experimental period. As for the BGHI, high values were found for both shifts for laying hens, which ranged from 82.55 to 84.45 (
Table 8). Barbosa Filho [
19] studied 21-week-old Hy-Line Brown laying hens, using an image analysis, and reported a BGHI between 75 °C and 84 °C, characterized as a danger situation, and 84 °C to 87 °C, characterized as an emergency. By adopting these reference values, it is possible to state that the birds were outside the thermoneutrality zone, which may have hindered the thermal exchanges between the birds and the rearing environment.
The RHL is an important indicator of thermal comfort that expresses the total solar radiation inside the installations. The limit considered critical for commercial laying hens is 500.00 W/m
−2 [
20]. In this research, higher values were observed in both shifts (
Table 3), triggering thermoregulation mechanisms.
The RH during the experimental phase was within the range considered ideal as recommended by the Lohmann Brown Lite Manual [
7], which is between 60 and 70%. In this study, despite the AT being above adequate levels, the RH between 62.18% and 66.22% favors the process of heat exchange between the bird and the environment. Moura [
21] mentions that five-month-old birds are capable of withstanding temperatures above 27 °C if they are inserted in an environment with adequate levels of Relative Humidity (less than 75%). The high values of the AT, BGHI, RHL and RH recorded in this study are justified because in tropical regions high temperatures prevail throughout the year as they are positioned close to the tropics, consequently receiving higher solar radiation.
The highest RR recorded in the afternoon is justified by the highest RHL in the afternoon shift. However, in both shifts they are within the reference values for commercial laying hens, which according to Garcia et al. [
20] can range from 23 mov/min in a thermoneutral environment (20 °C) to 273 mov/min at high temperatures (35 °C). The respiratory rate was the main thermoregulatory mechanism, as indicated by the decision tree. We believe that the birds triggered the latent heat exchange mechanism by increasing the RR as a means of trying to maintain homeothermy, as the increase in the RR is an important thermoregulatory response of heat loss when the bird is under heat stress [
22].
The CT was within the reference standards according to Abreu and Abreu [
23], who recommend values around 41.7 °C, demonstrating that despite the stress condition, endogenous thermoregulation mechanisms were efficient in heat dissipation.
It was found in the interpretation of all results that the different limestone particle sizes and vitamin D3 supplementation levels did not expressively affect the thermoregulatory responses of the birds. However, when the factors are assessed separately, the effect of dietary treatments on some specific parameters can be noted.
It was observed that for both levels of limestone granulometry and vitamin D3, the TG remained constant. In this study, TG values close to 6 °C regardless of the birds’ diets show that when the AT is high, the body core temperature, represented by the CT, tends to increase towards the more superficial regions of the bird, which confers a greater ease of heat loss by sensitive means. However, it was noticed that when the birds had access to the fine limestone granulometry, the TG had less energy expenditure to be metabolized by the birds’ organism. It was hypothesized that this occurred due to the coarse granulometry of the limestone increasing the caloric increment produced, as it requires a greater energy input for the digestion and absorption process.
For the birds to be able to properly activate the thermoregulation mechanisms and thus exchange heat with the environment, it is directly dependent on the TG, since the difference in the internal temperature between the bird and the environment favors thermal exchanges by sensible heat [
24]. The fact that all variables were influenced by the shift—with the RR, CT, FLST and FST being higher in the afternoon, while the TG was higher in the morning—was favorable for the heat exchange to occur. Results were similar to that found by Farias et al. [
12], who reported that when the TG showed higher values in the morning, the birds mainly used sensitive mechanisms, while in the afternoon, with a reduction in the thermal gradient, there was an increase in the cloaca temperature and respiratory rate, in order to maintain homeostasis.
There were no interaction effects on the FLST from the different granulometry and vitamin D3 levels (
Table 3), except between shifts, being higher in the afternoon. This was expected, since the hottest shift is the afternoon, with an average temperature of 30.37 °C, consequently causing the bird to trigger its thermoregulation mechanisms.
The FST of the bird’s body is an important variable to be analyzed, as they use them for the thermoregulation process. As they are highly vascularized regions without feathers, most of the heat exchange with the environment occurs through the feet, cloaca and crest [
23,
25]. The FST was affected by the shift, being higher in the afternoon.
When evaluating the effect of the granulometry and vitamin D3 levels, an effect on the dietary treatments on the FST was observed, where the use of the fine limestone granulometry and 12.5 g of vit. D3 reduced the FST. It was hypothesized that this reduction in the FST occurred due to the fine particle size and lower level of vitamin supplementation reducing the caloric increase produced, which represents a nutritional palliative, aiming to improve the conditions of birds reared in hot environments. It is also inferred that the birds used sensible heat exchange, directing the heat through the blood flow to the body’s featherless surface, mainly when using a level of 25 g of vitamin D3, as a strategy to maintain their adequate body temperature.
In a study carried out by Sena et al. [
13], it was observed that the temperatures of certain regions of the bird’s body, weather feathered or not (surface temperature of the cloaca, feet and wings), were higher during the afternoon shift, which suggests that the bird was likely adjusting its heat exchange in response to the environmental conditions, similarly to what was observed in this study.
In all treatments, the birds used sensible heat exchange mechanisms (FST and FLST) for heat dissipation (
Figure 2). The “sitting” behavioral activity was more evident, except when using the FL + 12.5 g of D3. This behavior is common in birds in a situation of caloric thermal discomfort [
26]. The fact that the birds remain seated for longer is a strategy used to minimize the increase in their body temperature with the decrease in movement, or seek heat dissipation by conduction, with the direct contact with a surface of a lower temperature than their body [
27], which may indicate that birds receiving FL + D3 had less difficulty in heat dissipation processes.
The behavioral activity “eating” is less evident among the behaviors due to the unfavorable environmental conditions, since at high temperatures birds reduce their feed intake and increase their water consumption, directing energy production towards thermoregulation. It was observed that the birds had high temperatures and respiratory rates, mechanisms used to dissipate heat.
The CT maintains a correlation pattern with the RR, which may infer that the bird activated the mechanisms of heat loss by conduction (sensible heat) and latent evaporative mechanisms (increase in respiratory rate) [
28]. These results are similar to what was found by Sena et al. [
13], who worked with bacterial phytase and concluded that the respiratory rate is positively related to the cloaca temperature.
The birds remained in thermal discomfort and were consequently stressed (
Figure 2), due to the fact that the behaviors that express welfare were positioned close to the line (0,0), that is, slightly or not performed by the bird. Barbosa Filho et al. [
16], in an experiment with Hy-Line Brown birds under heat stress or thermal comfort with cages and beds plus nests, stated that the birds that have a higher frequency of sitting, standing still and drinking water were the birds reared in cages and in conditions of thermal stress. Except for (2D), where it is observed that the birds started to eat more and had lower body temperatures and RRs than in the other treatments. Possibly, the vitamin and mineral support was better, which contributed positively to thermoregulation.
The CDA (
Figure 3A) presented the classification dynamics according to the shift in the day, not showing any effect of the different levels of vitamin D3 nor the different granulometries of the limestone, which is justified because the surface temperatures, RR and CT were activated in the afternoon due to higher RHL values. However, although all these thermoregulatory mechanisms are activated, the RR is the main thermoregulatory mechanism of dissipation in birds under conditions of a caloric challenge, which is common in semiarid regions (
Figure 3B).
Finally, it is recommended to carry out future studies evaluating the effect of supplementation with different limestone granulometries and vitamin D3 levels on the hematological profile and caloric increment for a better understanding and validation of the results of this research.