Poultry Mites Contributing to Human Dermatitis: A Retrospective Study in Italy (2010–2024)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Processing
2.2. Data Collection
3. Results
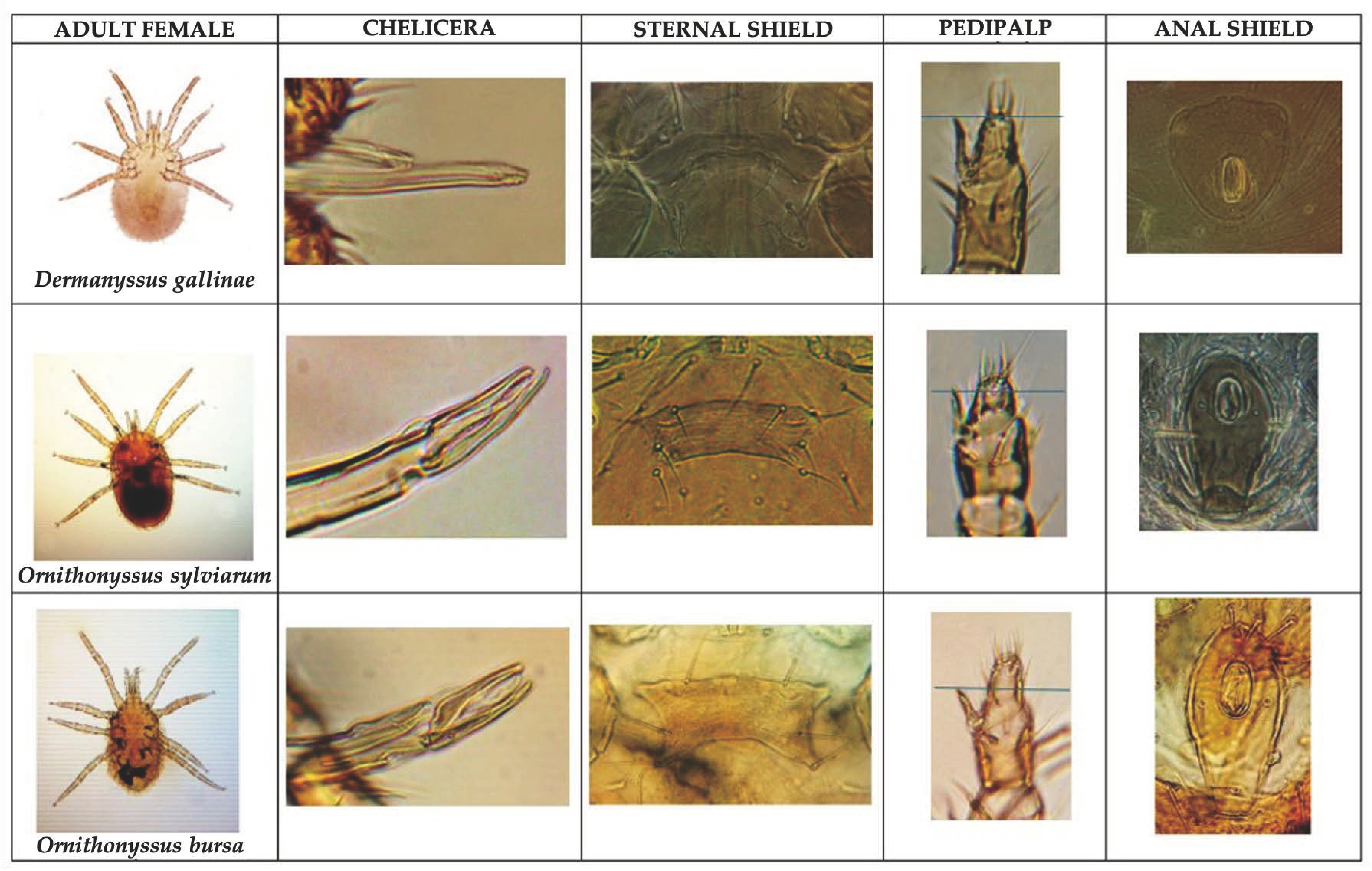
3.1. Morphological Characteristics
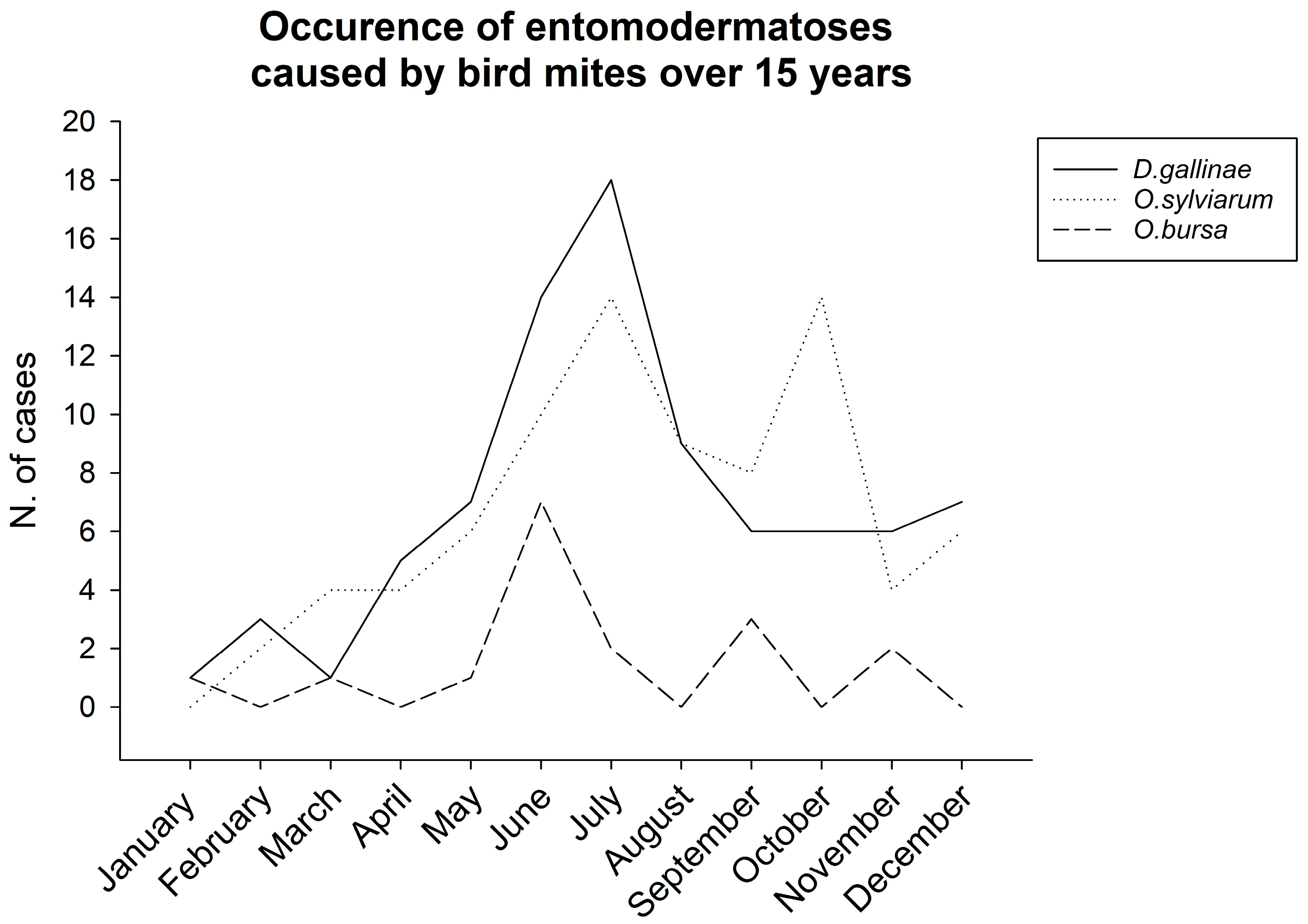
3.2. Timing of Mite Infestation (by Season)
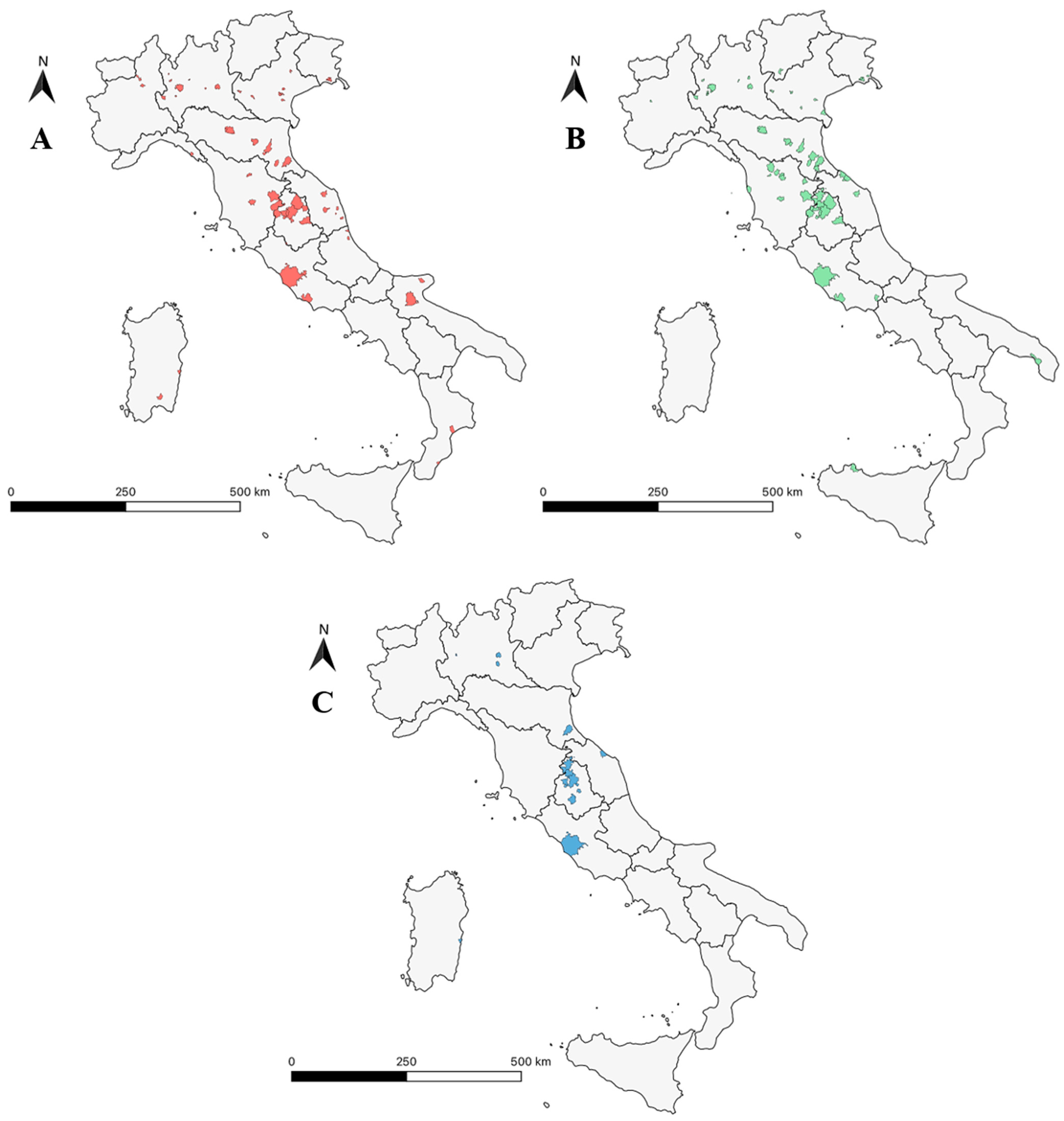
3.3. Geographical Distribution of the Mites
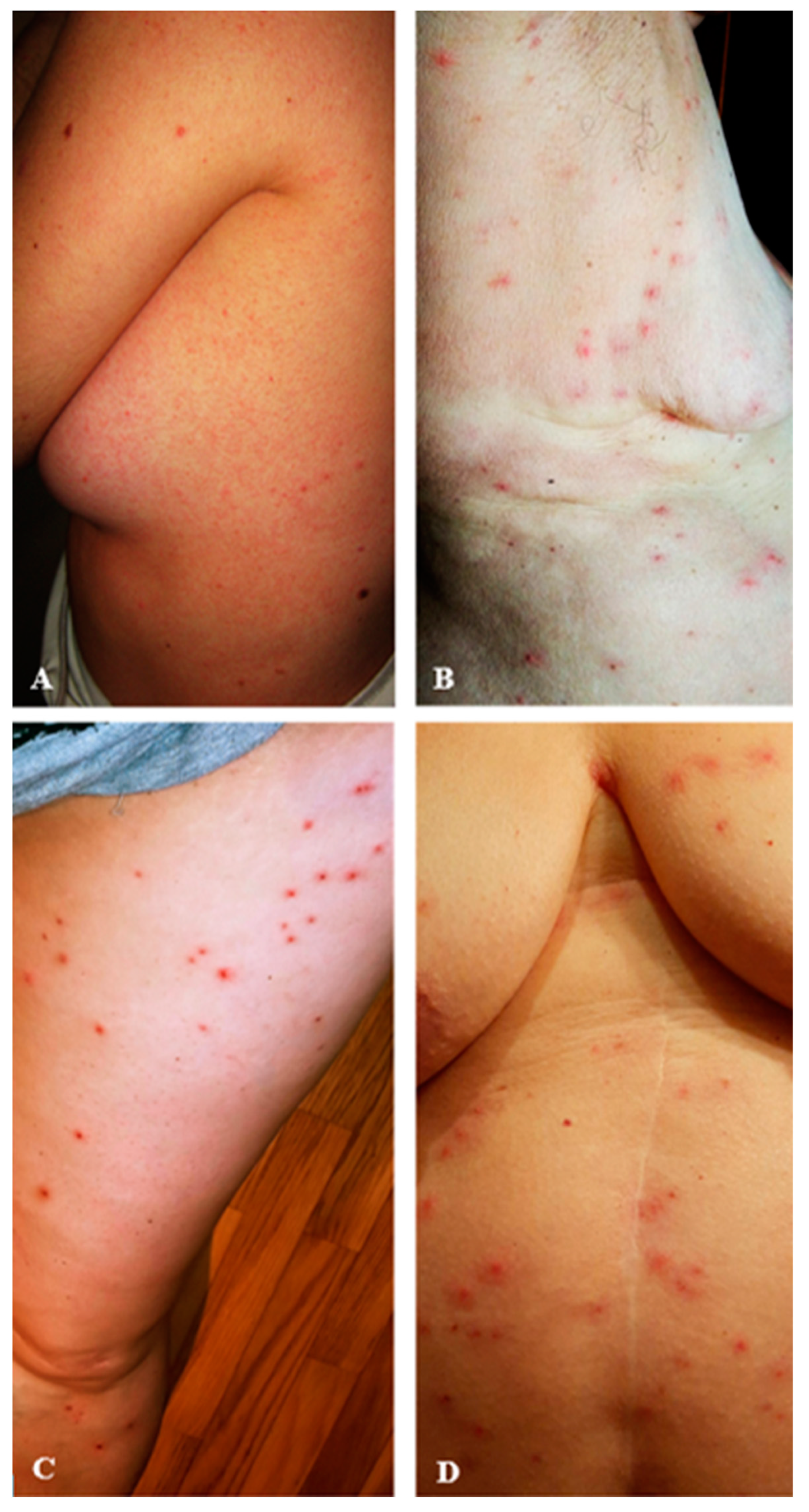
3.4. Characteristics of the Skin Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sparagano, O.A.E.; George, D.R.; Finn, R.D.; Giangaspero, A.; Bartley, K.; Ho, J. Dermanyssus gallinae and chicken egg production: Impact, management, and a predicted compatibility matrix for integrated approaches. Exp. Appl. Acarol. 2020, 82, 441–453. [Google Scholar] [CrossRef]
- Waap, H.; Aguin-Pombo, D.; Maia, M. Case Report: Human Dermatitis Linked to Ornithonyssus bursa (Dermanyssoidea: Macronyssidae) Infestation in Portugal. Front. Vet. Sci. 2020, 7, 567902. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, O. How to obtain a bloodmeal without being eaten by a host: The case of poultry red mite, Dermanyssus gallinae. Physiol. Entomol. 2005, 30, 232–240. [Google Scholar] [CrossRef]
- Cafiero, M.A.; Viviano, E.; Lomuto, M.; Raele, D.A.; Galante, D.; Castelli, E. Dermatitis due to Mesostigmatic mites (Dermanyssus gallinae, Ornithonyssus [O.] bacoti, O. bursa, O. sylviarum) in residential settings. J. Dtsch. Dermatol. Ges. 2018, 16, 904–906. [Google Scholar] [CrossRef]
- Sárkány, P.; Bagi, Z.; Süli, Á.; Kusza, S. Challenges of Dermanyssus gallinae in Poultry: Biological Insights, Economic Impact and Management Strategies. Insects 2025, 16, 89. [Google Scholar] [CrossRef] [PubMed]
- Mullens, B.A.; Owen, J.P.; Kuney, D.R.; Szijj, C.E.; Klingler, K.A. Temporal changes in distribution, prevalence and intensity of northern fowl mite (Ornithonyssus sylviarum) parasitism in commercial caged laying hens, with a comprehensive economic analysis of parasite impact. Vet. Parasitol. 2009, 160, 116–133. [Google Scholar] [CrossRef]
- Murillo, A.C.; Mullens, B.A. A review of the biology, ecology, and control of the northern fowl mite, Ornithonyssus sylviarum (Acari: Macronyssidae). Vet. Parasitol. 2017, 246, 30–37. [Google Scholar] [CrossRef]
- Roy, L.; Chauve, C.M.; Buronfosse, T. Contrasted ecological repartition of the Northern Fowl mite Ornithonyssus sylviarum (Mesostigmata: Macronyssidae) and the chicken red mite Dermanyssus gallinae (Mesostigmata: Dermanyssidae). Acarologia 2010, 50, 207–219. [Google Scholar] [CrossRef][Green Version]
- Banović, P.; Foucault-Simonin, A.; Papić, L.; Savić, S.; Potkonjak, A.; Jurišić, A.; Radenković, M.; Mijatović, D.; Simin, V.; Bogdan, I.; et al. One health approach to study human health risks associated with Dermanyssus gallinae mites. Heliyon 2024, 10, e30539. [Google Scholar] [CrossRef]
- Swayne, D.E. Diseases of Poultry, 14th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 1138–1140. [Google Scholar]
- Orton, D.I.; Warren, L.J.; Wilkinson, J.D. Avian mite dermatitis. Clin. Exp. Dermatol. 2000, 25, 129–131. [Google Scholar] [CrossRef]
- Mullen, G.R.; Durden, L.A. Medical and Veterinary Entomology, 3rd ed.; Academic Press: Cambridge, MA, USA; Academic Press, Elsevier: London, UK, 2018; pp. 533–595. [Google Scholar]
- Moroni, B.; Barlaam, A.; Misia, A.L.; Peano, A.; Rossi, L.; Giangaspero, A. Dermanyssus gallinae in non-avian hosts: A case report in a dog and review of the literature. Parasitol. Int. 2021, 84, 102378. [Google Scholar] [CrossRef]
- Cafiero, M.A.; Barlaam, A.; Camarda, A.; Radeski, M.; Mul, M.; Sparagano, O.; Giangaspero, A. Dermanysuss gallinae attacks humans. Mind the gap! Avian Pathol. 2019, 48, S22–S34. [Google Scholar] [CrossRef] [PubMed]
- Collgros, H.; Iglesias-Sancho, M.; Aldunce, M.J.; Expósito-Serrano, V.; Fischer, C.; Lamas, N.; Umbert-Millet, P. Dermanyssus gallinae (chicken mite): An underdiagnosed environmental infestation. Clin. Exp. Dermatol. 2013, 38, 374–377. [Google Scholar] [CrossRef]
- Kavallari, A.; Küster, T.; Papadopoulos, E.; Hondema, L.S.; Øines, Ø.; Skov, J.; Sparagano, O.; Tiligada, E. Avian mite dermatitis: Diagnostic challenges and unmet needs. Parasite Immunol. 2018, 40, e12539. [Google Scholar] [CrossRef]
- George, D.R.; Finn, R.D.; Graham, K.M.; Mul, M.F.; Maurer, V.; Moro, C.V.; Sparagano, O.A. Should the poultry red mite Dermanyssus gallinae be of wider concern for veterinary and medical science? Parasit. Vectors 2015, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Stingeni, L.; Bianchi, L.; Hansel, K.; Neve, D.; Foti, C.; Corazza, M.; Bini, V.; Moretta, I.; Principato, M. Dermatitis caused by arthropods in domestic environment: An Italian multicentre study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Principato, S.; Principato, M.; Stingeni, L.; Mannucci, G.; Moretta, I. Indoor Dust Direct Examination (E.D.P.A.®) and biotic pollution in confined environments. ProScience 2018, 5, 83–89. [Google Scholar]
- Castelli, E.; Viviano, E.; Torina, A.; Caputo, V.; Bongiorno, M.R. Avian mite dermatitis: An Italian case indicating the establishment and spread of Ornithonyssus bursa (Acari: Gamasida: Macronyssidae) (Berlese, 1888) in Europe. Int. J. Dermatol. 2015, 54, 795–799. [Google Scholar] [CrossRef]
- Cafiero, M.A.; Camarda, A.; Circella, E.; Santagada, G.; Schino, G.; Lomuto, M. Pseudoscabies caused by Dermanyssus gallinae in Italian city dwellers: A new setting for an old dermatitis. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1382–1383. [Google Scholar] [CrossRef]
- Abdigoudarzi, M.; Mirafzali, M.S.; Belgheiszadeh, H. Human Infestation with Dermanyssus gallinae (Acari: Dermanyssidae) in a Family Referred with Pruritus and Skin Lesions. J. Arthropod Borne Dis. 2013, 8, 119–123. [Google Scholar]
- de Sousa, M.D.G.; Filho, F.B. Gamasoidosis (bird mite dermatitis): Dermanyssus gallinae in a young boy. Pan Afr. Med. J. 2018, 30, 144. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Dechent, C.; Uribe, P. A case of gamasoidosis caused by Dermanyssus gallinae, misdiagnosed as delusional parasitosis. Clin. Exp. Dermatol. 2018, 43, 950–952. [Google Scholar] [CrossRef]
- de Oliveira Alves, A.; Bernardes Filho, F. Gamasoidosis (bird mite dermatitis): A case series in a family. Pediatr. Neonatol. 2018, 59, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Cheikhrouhou, S.; Trabelsi, S.; Aloui, D.; Bouchekoua, M.; Khaled, S. Avian mite bites acquired from pigeons: Report of three cases and review of the literature. Tunis. Med. 2020, 98, 241–245. [Google Scholar] [PubMed]
- Prouteau, C.; Ameline, M.; Roy, L.; Delaunay, P.; Gangneux, J.P.; Dupuy, A. Prurigo à Dermanyssus gallinae [Gamasoidosis caused by Dermanyssus gallinae]. Ann. Dermatol. Venereol. 2020, 147, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Sioutas, G.; Minoudi, S.; Tiligada, K.; Chliva, C.; Triantafyllidis, A.; Papadopoulos, E. Case of Human Infestation with Dermanyssus gallinae (Poultry Red Mite) from Swallows (Hirundinidae). Pathogens 2021, 10, 299. [Google Scholar] [CrossRef]
- Sonday, Z.; Todd, G.; Jeebhay, M.F. Occupational bird mite dermatitis (gamasoidosis) among workers in a seed house. Curr. Allergy Clin. Immunol. J. 2023, 36, 178–183. [Google Scholar]
- Minagawa, F.H.; Almeida, E.R.M.; Souza, R.C.; Santos, D.C.D.; Haddad Junior, V.; Miot, H.A. Gamasoidosis (avian mite dermatitis) outbreak in a student dormitory. Rev. Soc. Bras. Med. Trop. 2024, 57, e011012024. [Google Scholar] [CrossRef]
- Budria, A.; Candolin, U. How does human-induced environmental change influence host-parasite interactions? Parasitology 2014, 141, 462–474. [Google Scholar] [CrossRef]
- Masini, P.; Zampetti, S.; Rossetti, M.V.; Biancolini, F.; Miñón Llera, G.; Hansel, K.; Stingeni, L. Gamasoidosis from the tropical fowl mite Ornithonyssus bursa (Acari: Gamasida: Macronyssidae) (Berlese, 1888): Successful eradication of the domestic infestation with dry saturated steam. Int. J. Dermatol. 2022, 61, e230–e232. [Google Scholar] [CrossRef]
- Cafiero, M.A.; Galante, D.; Camarda, A.; Giangaspero, A.; Sparagano, O. Why dermanyssosis should be listed as an occupational hazard. Occup. Environ. Med. 2011, 68, 628. [Google Scholar] [CrossRef] [PubMed]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology, 3rd ed.; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009. [Google Scholar]
- Sparagano, O.A.; George, D.R.; Harrington, D.W.; Giangaspero, A. Significance and control of the poultry red mite, Dermanyssus gallinae. Annu. Rev. Entomol. 2014, 59, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Valiente Moro, C.; Chauve, C.; Zenner, L. Vectorial role of some dermanyssoid mites (Acari, Mesostigmata, Dermanyssoidea). Parasite 2005, 12, 99–109. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretta, I.; Principato, S.; Brustenga, L.; Principato, M.A. Poultry Mites Contributing to Human Dermatitis: A Retrospective Study in Italy (2010–2024). Poultry 2025, 4, 21. https://doi.org/10.3390/poultry4020021
Moretta I, Principato S, Brustenga L, Principato MA. Poultry Mites Contributing to Human Dermatitis: A Retrospective Study in Italy (2010–2024). Poultry. 2025; 4(2):21. https://doi.org/10.3390/poultry4020021
Chicago/Turabian StyleMoretta, Iolanda, Simona Principato, Leonardo Brustenga, and Mario Antonello Principato. 2025. "Poultry Mites Contributing to Human Dermatitis: A Retrospective Study in Italy (2010–2024)" Poultry 4, no. 2: 21. https://doi.org/10.3390/poultry4020021
APA StyleMoretta, I., Principato, S., Brustenga, L., & Principato, M. A. (2025). Poultry Mites Contributing to Human Dermatitis: A Retrospective Study in Italy (2010–2024). Poultry, 4(2), 21. https://doi.org/10.3390/poultry4020021






