Abstract
Laying hens usually have 16 h of light and 8 h of darkness during egg laying, with eggshell formation primarily occurring during darkness when dietary calcium is lacking, leading to bone calcium resorption and osteoporosis. This study examined how interrupting the dark phase affects bone health in 396 Hy-line W36 hens assigned to control (C) or treatment groups (W1 and W2). All hens received 16 h of light and 8 h of darkness daily in different variations of scotophase interruption. Blood samples were taken at weeks 20, 30, 50, and 70, serum calcium was measured during darkness at two timepoints (SRT and END), and bone demineralization markers were examined using enzyme concentrations (TRACP-5b and CTX-I). Across weeks, tibias were CT-scanned for density (mg/cm3) and area (mm2), then used for breakage strength analysis (N) and ash%. No SRT Ca level differences emerged, but C hens had lower END Ca levels compared to W1 and W2 hens across all weeks, while W1 and W2 hens showed no significant differences. C hens displayed higher TRACP-5b and CTX-I concentrations across all weeks compared to W1 and W2 (all p ≤ 0.05). At week 70, C hens had the lowest cortical bone cross-sectional area and mineral density compared to W1 and W2 (all p ≤ 0.05). Tibiotarsi bone breakage strength was lower in C hens compared to W1 and W2. C hens had significantly lower ash% than treatment birds. Interrupting the scotophase period improved overall bone health in Hy-line W36 laying hens.
1. Introduction
Traditional management systems for egg production in the United States are the product of research on housing systems and intensive genetic selection. As such, expected behavioral and physiological changes have taken place in modern laying hens compared to that of their ancestors [1,2,3]. Such changes are associated with welfare concerns, including the development of osteoporosis (a progressive decrease in structural bone mineral density and bone mass), as it continues to take precedence in laying hens, fueled by societal and production pressures [4,5].
Most eggshell formation occurs during nighttime hours (scotophase)—a time in which the hens are not consuming feed. An empty gastrointestinal tract (GIT) could result in the deprivation of key nutrients, such as calcium, during eggshell formation. Interference of adequate serum calcium concentrations through exogenous (dietary) sources during eggshell synthesis and calcification significantly impacts the bird [6,7]. Considerable changes in bone biology occur due to estrogen at the peak of sexual maturity, simultaneously with substantial increases in medullary bone absorption and osteoclast activity [8,9]. Osteoblast cell activity switches from forming cortical bone to the production of woven medullary bone [10,11]. On the other hand, osteoclast cell activity, which is responsible for bone resorption, remains vigorous [5]. Increased estrogen promotes rapid buildup of medullary bone during the early lay cycle, which is intended for use as a source of calcium for eggshell formation [12,13]. However, as osteoclastic resorption of structural bone continues with no continuation of structural bone osteoblastic activity, the hens see a significant decrease in bone mineralization [14,15]. Osteoclasts are responsible for resorbing calcium from both medullary and structural bone during eggshell calcification, but due to insufficient serum calcium concentrations during a time of high demand, bone becomes the main source of calcium for eggshell formation [16,17]. Progressive resorption of medullary and structural bone occurs daily as the lay cycle continues, with no opportunity for rebuilding calcium stores [5]. This mechanism is generally believed to cause osteoporosis in laying hens due to a persistently negative calcium balance combined with an extended period of high egg production, impacting the structural integrity of the skeletal structure [5,18,19]. Osteoporosis causes an increase in bone fragility, resulting in increased susceptibility to bone fractures, especially after 35 weeks of age [5,9,20]. As a result, production pressures for high performance and osteoporosis have raised animal welfare concerns within the industry [21].
Understanding the mechanism behind the laying cycle is critical for meeting the needs of hens in a highly productive state. Not only is calcium of utmost importance, but light is also essential in the regulation of physiological functions in birds [22]. Reproduction, thermoregulation, behavioral responses, and immune system responses are influenced by light perception, which is one reason artificial lighting systems are widely utilized as the modern production standard. In relation to the anatomy of the avian eye, two types of photoreceptor cells are found within the retina: cones and rods. The rods are necessary for night vision, as they are more sensitive to lower-intensity illumination. Four types of cones are found within the retina of a chicken, which possesses nerve connections between the photoreceptors and the brain, allowing the bird to perceive visible and ultraviolet light spectra and polarized light [23,24,25,26]. Pineal gland activity is regulated by hypothalamic photoreceptor activation following light perception [27,28,29]. The pineal gland then translates these environmental cues, resulting in the excretion of melatonin, a regulatory hormone for daily biological function [22,30,31].
Melatonin is a natural hormone secreted from the pineal gland (epiphysis cerebri) from the precursor serotonin and has been associated with neuroendocrine regulation of metabolic and physiological mechanisms, such as sexual maturation, reproductive performance, thermoregulation, and nutrient utilization, in hens that are synchronized with daily changes in light [32]. Melatonin is also associated with the regulation of circadian rhythm through secretion from the pineal gland in no-light environments and is considered a “regulator of regulators” in biological functions [33,34]. Biological and chemical events, such as metabolism and hormone production, occur from the regulation of these circadian oscillators in the epiphysis [35]. Any changes in melatonin production in hens can cause interruption of systems dependent on circadian rhythmical function [36].
Approximately 80% of melatonin secreted into the bloodstream is produced in the epiphysis. However, it is also secreted from the skin, testes, bone marrow, and gastrointestinal tract [37]. Melatonin secretion from the epiphysis is a response to environmental cues; however, melatonin secretion in the gastrointestinal tract is seen in enterochromaffin cells as a postprandial response [37,38,39]. As for welfare, regulation of biological rhythm from melatonin secretion is important in preventing sudden death syndrome, skeletal impurities, and ascites, which can occur due to the disruption of daily rhythmic activity [38].
Prospective interventions have been explored to reduce negative calcium balance and osteoporosis in commercial laying hens [5]. Strategies such as increasing dietary calcium have been the most obvious answer among producers for increasing bone mineral content and strength; however, the threshold for calcium absorption in commercially formulated diets has already been met [39]. Therefore, calcium absorption cannot be increased in the diet to accommodate amounts greater than those currently used in standard commercial formulations, showing that simply supplying additional calcium sources to the bird is not a feasible solution [39,40,41]. Thus, targeting the delivery of calcium during the scotophase period, when calcium demand is the highest, might be a potential approach to decreasing the amount of endogenous (skeletal) calcium entering the blood. Allotting the birds an interruption in the scotophase period when food consumption is halted and calcium demands are the highest could be a potential solution to providing the birds with an exogenous source of calcium (dietary) during eggshell calcification and, therefore, improving bone health and hen welfare by decreasing the rate of calcium withdrawn from the bone.
We hypothesized that interrupting the scotophase period with one or two hours of photophase would increase serum calcium levels during nighttime hours and reduce the rate of calcium withdrawal from the bone, therefore increasing overall bone health. The objective of the current study was to investigate the influence of interrupting the scotophase period on the reduction of calcium withdrawal from structural bone during midnight hours, with the potential to improve hen bone health and welfare.
2. Materials and Methods
2.1. Ethics
This experiment was approved by Clemson University’s Institutional Animal Care and Use Committee (protocol # AUP2021-0068). All procedures followed IACUC guidelines for animal use in this experiment.
2.2. Animals and Housing
This trial was conducted at the Morgan Poultry Center, located at Clemson University in South Carolina, USA. A total of 396 17-week-old Hy-Line W36 chicks were randomly allocated across 18-floor pens (22 birds/pen) and utilized in a trial from 20 to 70 weeks of age following a 3-week acclimation period. Identical experimental pens (4.3 m2) were utilized, with clean wood shavings (5 cm) used as bedding in a fully enclosed, climate-controlled poultry house kept at an average of 25 °C. Nest boxes (0.2 m2 of nest space) and wooden perches (15 cm perch space/bird) were included in each pen. Pens were distributed across the house, and a black, heavy-duty fabric was placed on the front and sides between pens to eliminate light interference across treatments without significantly impacting ventilation. Favorable ventilation was ensured with tunnel ventilation and sidewall inlets. Cool-cell pads and additional floor fans were utilized in the summer months, and industrial heaters were utilized during the winter months to maintain optimal ambient temperatures. The birds had ad libitum access to water and feed through an automatic cup drinker and free-standing gravity feeder. All birds were fed the same commercially formulated diet across all treatments after receiving a phase-feeding regimen from 1 to 20 weeks of age. Each bird received a unique identification number via neck tag. The lights and light schedules were provided through the NatureDynamics Dome lighting system (ONCE by Signify, Plymouth, MN, USA) and the Interact Agriculture mobile application (Signify Netherlands B.V., Dordrecht, The Netherlands) from 20 to 70 weeks of age.
2.3. Treatments
From 20 to 70 weeks of age, all the pens were provided with varying light schedules to investigate the impact of interrupting the scotophase period on bone health in laying hens (Figure 1). After an acclimation period, the birds were exposed to different photophase (P):scotophase (S) lighting treatments at 20 weeks of age (6 pens/treatment and 22 birds/pen). The control group (C) received 16 h of continuous light and 8 h of continuous darkness, as recommended by the breeder and United Egg Producers guidelines [42,43]. Treatment one (W1) received 1 h of scotophase interruption (15P:4S:1P:4S). Treatment two (W2) received 2 h of scotophase interruption (14P:4S:2P:4S). Further information on the light spectrum utilized in the study can be found in the Supplementary Materials (Figure S1).

Figure 1.
Light schedules applied during the experiment. C: birds received 16 h of continuous light (photophase) and 8 h of continuous darkness (scotophase). W1: birds received 15 h of continuous photophase, 4 h of scotophase, 1 h of photophase, then 4 h of scotophase; W2: birds received 14 h of continuous photophase, 4 h of scotophase, 2 h of photophase, then 4 h of scotophase.
2.4. Blood Analysis
During weeks 20, 30, 50, and 70 of age, blood samples were collected from the brachial wing vein of 3 randomly selected birds/pen (n = 54/week) at 2 timepoints during dark hours (SRT: immediately after lights off, and END: immediately before lights on). SRT time for C hens was 22:00, while SRT time for W1 and W2 hens was 21:00 and 20:00, respectively. END time for all treatment groups was performed at 05:00. Whole blood samples (1 mL) were transferred from EDTA vacutainers into Eppendorf tubes and centrifuged for 10 min at 6000 RPM at 4 °C. The serum was separated and frozen at −20 °C for quantification of systematic bone markers. Total serum calcium was analyzed using an automatic blood chemical analyzer (Film DRI CHEM 7000i, Fuji film, Tokyo, Japan). Serum concentrations of bone resorption markers, including tartrate-resistant acid phosphatase 5b (TRACP-5b) and C-terminal telopeptide of type I collagen (CTX-I), were also assessed using commercially available ELISA kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing, China; MyBioSource, San Diego, CA, USA) to monitor calcium withdrawal from bone reserves according to the manufacturer’s instructions [44,45,46]. These ELIZA kits utilize a quantitative sandwich enzyme immunoassay technique for the antibodies specific to TRACP-5b and CTX-I, followed by a colorimetric analysis approach to determine the proportions of TRACP-5b and CTX-I bound to the antibodies in the initial binding process.
2.5. In Vivo Computed Tomography (CT) Image Procurement and Analysis
At 20, 30, 50, and 70 weeks of age, 3 randomly selected birds per pen (n = 54/week) were transported to Clemson University’s Godley–Snell Research Center for image acquisition. The birds were placed in transfer crates and transferred from the farm to an exterior climate-controlled room at Godley–Snell with dim lighting to mediate stress levels. Color-coded restraint devices were developed for utilization specific to CT scans. Three birds were scanned at once, where the hens’ legs were gently folded inside a cape and safely fastened around the bird with Velcro. Dark mesh fabric was sewn to the top of the restraint device for adequate ventilation and concurrent darkness during the scanning process. Dimmed lights were utilized to promote stillness in the imaging room. The birds were placed in a right lateral recumbency, headfirst, in a single-file line between taped sponge wedges, ensuring no skeletal superimposition, above a hydroxyapatite calibration phantom (QRM Quality Assurance in Radiology and Medicine, Möhrendorf, Germany). CT images were captured with a helical acquisition, bone-sharp 0–11 kg protocol, 0.5 mm slice thickness, and a 200 mA “FC30” algorithm. Following the capture of the images, reconstruction occurred using a 0.5 mm “standard” algorithm. Individual identification numbers were logged in the scan description for identification. CT images were captured using a Toshiba Aquilion TSX-101A 16-slice scanner. Following the capture of the CT images, the hens were returned to their respective pens via transfer crates and monitored for stress indicators. CT image analysis was then performed to determine the in vivo cross-sectional area and bone mineral density (BMD) using an image analysis protocol adapted from the methods of Harrison et al. [44] (Figure 2). At week 70, the hens were euthanized on-farm by CO2 inhalation following the completion of the scans. The euthanized birds were immediately frozen post-mortem at −20 °C for further analysis.
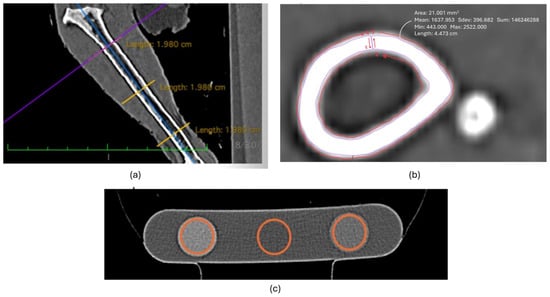
Figure 2.
Image examples of CT analysis of the tibiotarsal region. (a) Determination of proximal, middle, and distal points of interest of the tibiotarsus, (b) orientation of tracing regions of interest for analysis, and (c) orientation of tracing regions of interest for the hydroxyapatite rods in the phantom.
2.6. Measures of Bone Health Ex Vivo
2.6.1. Tibia Breaking Strength
Following CT scanning at 70 weeks of age, the birds (n = 54) were prepped for dissection of the tibiotarsi for further analysis. Mechanical properties of the right tibiotarsi were analyzed using a three-point bending test following recommendations specified by the American National Standards Institute (ANSI), using an Instron Dynamic and Static Material Test system (Model 5944, Instron Corp., Canton, MA, USA) equipped with a 500 N load cell and Automated Material Test System software (8800 MT Controller, Instron Corp., Canton, MA, USA), as described by Anderson et al. and Harrison et al. [47,48]. Data for load and displacement were collected and used to calculate the bone-breaking strength (N) and bone stiffness (N/mm).
2.6.2. Tibia Ash Percentage
Following the last series of CT scans at 70 weeks of age, the euthanized birds (n = 54) were dissected for analysis of the tibiotarsi. Prior to testing, formerly frozen whole legs were slowly thawed at refrigerator temperature for 24 h. The muscles surrounding the left tibiotarsi regions and other soft tissues were dissected, and the fibula was removed from the region. Following dissection, the dry crucible weight was recorded. The left tibiotarsi were cut into small pieces, placed into ceramic crucibles, and then weighed. Left tibiotarsi were air-dried at 100 °C for one hour, transferred into a desiccator for an additional hour, and weights were recorded. The tibiotarsi were placed into crucibles and ashed for 6 h at 600 °C. The oven utilized in this experiment was the Thermolyne 30400 (Barnstead International, Dubuque, IA, USA). Following the ashing process, the crucible was placed in a desiccator for one hour, and the ash weight was recorded. To calculate the percentage of tibia ash, we divided the tibia ash weight by the tibia dry weight and multiplied by 100.
2.7. Statistical Analysis
A power analysis was performed to determine the minimum sample size for the study, indicating that our sample size was adequate to fulfill the requirements to show statistical significance per parameter. The data were analyzed using R software (version 3.3.1) along with the “stats” package (R Core Team, 4.3.2, 2013). Generalized linear mixed-effects models (GLMMs) were employed to examine the main effects of treatment (C, W1, and W2) and bird age (weeks 20, 30, 50, and 70) on each variable. These models were constructed using the “lme4” package [49]. In each GLMM, the interaction term between main effects was also evaluated as a fixed effect, while pen and individual birds were considered as random effects. The “Quasibinomial” family was chosen for proportion data (ash %), and the “Poisson” family was used for other data types. Post hoc comparisons were conducted using Tukey’s HSD multiple comparison procedure with the “multcomp” package [50]. For proportion data (ash %), the “DHARMa” package was utilized to assess residual distribution and GLMM assumptions, while the Shapiro–Wilk test was applied for normality analysis of the model residuals (i.e., breaking strength (N) and stiffness (N/mm)). Statistical significance was established at p < 0.05. Descriptive statistics were computed using the “psych” package, and data were presented as mean ± standard error of the mean (SEM).
3. Results
3.1. Serum Calcium Concentration
Treatment had a significant effect on serum calcium concentrations immediately before lights on (END) at 20, 30, 50, and 70 weeks of age (Table 1). C hens showed the lowest concentration of END serum calcium across all weeks compared to the W1 (weeks 20: p = 0.019 and 0.022; 30: p = 0.012 and 0.026; 50: p = 0.023 and 0.014; 70: p = 0.012 and 0.011, respectively) and W2 hens (weeks 20: p = 0.016 and 0.018; 30: p = 0.018 and 0.017; 50: p = 0.019 and 0.021; 70: p = 0.011 and 0.013, respectively). SRT serum calcium concentrations were not significantly different across treatments within each week.

Table 1.
Mean serum calcium concentrations ± SEM immediately following lights off (SRT) and immediately before lights on (END).
Across weeks and within the same treatment, C hens showed the highest SRT concentration at week 20 compared to weeks 50 and 70 (p = 0.036 and 0.041, respectively; Table 1), while week 30 SRT serum calcium concentrations were significantly higher than week 70 (p = 0.043) but not significantly different from week 20 or 50. W1 hens did not show a significant difference in SRT serum calcium concentrations at weeks 20, 30, and 50; however, week 70 was significantly lower than weeks 20 and 30 (p = 0.036 and 0.042, respectively; Table 1). W2 hens were not significantly different at weeks 20, 30, and 50. However, the SRT serum calcium level at week 70 was significantly lower than at weeks 20 and 30 (p = 0.035 and 0.039, respectively; Table 1).
C hens’ END serum calcium concentrations were highest at weeks 20 (p = 0.024 and 0.036) and 30 (p = 0.034 and 0.039) compared to weeks 50 and 70. However, there were no significant differences between END serum calcium concentrations at weeks 20 and 30 or weeks 50 and 70. W1 and W2 hens showed no significant differences in END serum calcium concentrations across all weeks (Table 1).
3.2. Bone Demineralization
There was a significant effect of treatment on TRACP-5b concentrations at weeks 20, 30, 50, and 70 (p = 0.026). C hens showed higher concentrations of TRACP-5b compared to W1 and W2 hens at weeks 30 (p = 0.019 and 0.026), 50 (p = 0.034 and 0.029), and 70 (p = 0.025 and 0.036; Figure 3), while there were no significant differences between treatments at week 20.
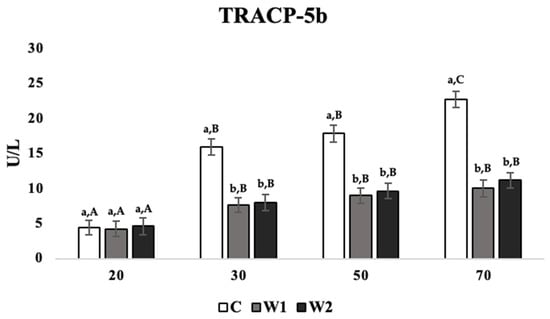
Figure 3.
Mean serum concentrations of tartrate-resistant acid phosphatase 5b (TRACP-5b; U/L) in laying hens at 20, 30, 50, and 70 weeks of age (n = 54/week), with treatments consisting of no scotophase interruption (C), 1 h of scotophase interruption (W1), and 2 h of scotophase interruption (W2). a,b Means with differing superscripts indicate statistically significant differences across treatments of the same week at p < 0.05. A–C Means with differing superscripts indicate statistically significant differences within treatments across weeks at p < 0.05.
Across weeks and within treatments, C hens showed the highest TRACP-5b concentration at week 70 compared to all other weeks during the trial (weeks 20: p = 0.012; 30: p = 0.036; 50: p = 0.042; Figure 3), while TRACP-5b concentrations at weeks 30 and 50 were significantly higher than week 20 (p = 0.026 and 0.032, respectively). For treatments W1 and W2, hens showed significantly lower TRACP-5b levels at week 20 compared to weeks 30 (p = 0.039 and 0.043), 50 (p = 0.032 and 0.039), and 70 (p = 0.038 and 0.036), while TRACP-5b concentrations at weeks 30, 50, and 70 were not significantly different (Figure 3).
For the CTX-I concentration, the treatment had a significant effect across all weeks in the trial (p = 0.027). At 70 weeks of age, C hens showed the highest concentration of CTX-I compared to W1 and W2 hens across weeks 30 (p = 0.023 and 0.031), 50 (p = 0.021 and 0.026), and 70 (p = 0.019 and 0.022; Figure 4), while there were no significant differences between treatments at week 20.
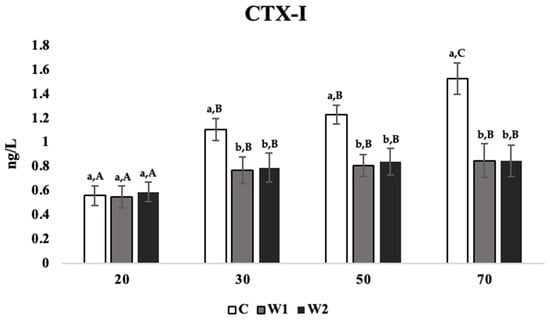
Figure 4.
Mean serum concentrations of C-terminal telopeptide of type I collagen (CTX-I; ng/L) in laying hens at 20, 30, 50, and 70 weeks of age (n = 54/week), with treatments consisting of no scotophase interruption (C), 1 h of scotophase interruption (W1), and 2 h of scotophase interruption (W2). a,b Means with differing superscripts indicate statistically significant differences across treatments of the same week at p < 0.05. A–C Means with differing superscripts indicate statistically significant differences within treatments across weeks at p < 0.05.
Age negatively affected TRACP-5b concentrations within the same treatment and across weeks. C hens showed significantly higher concentrations of CTX-I at week 70 compared to weeks 50, 30, and 20 (p = 0.035, 0.032, and 0.018, respectively; Figure 4), while week 20 was significantly lower than weeks 30 and 50 (p = 0.026 and 0.032, respectively). W1 and W2 hens showed significantly lower CTX-I concentrations at week 20 compared to weeks 30, 50, and 70 (W1: p = 0.023, 0.036, and 0.025; W2: p = 0.022, 0.029, and 0.032, respectively; Figure 4), while there were no significant differences across these weeks.
3.3. Tibiotarsal CT Image Analysis
Following CT scan analysis, no differences between treatments and weeks for total bone cross-sectional area (TCSA) and no statistically significant differences in TBMD were observed at week 20 for any of the bone sections assessed.
There were statistically significant differences in total bone mineral density across weeks and treatment groups (p = 0.025; Table 2). C hens showed lower TBMD at all locations than other treatments at 30 (proximal: p = 0.018, 0.029; middle: p = 0.031, 0.024; distal: p = 0.031, 0.028), 50 (proximal: p = 0.016, 0.022; middle: p = 0.017, 0.015; distal: p = 0.020, 0.025), and 70 (proximal: p = 0.011, 0.013; middle: p = 0.015, 0.019; distal: p = 0.021, 0.019) weeks. Age negatively affected TBMD for all sections, independently of the treatment (p = 0.021). Across weeks, proximal measurements for TBMD in C hens at week 20 were significantly higher than weeks 30 (p = 0.032), 50 (p = 0.022), and 70 (p = 0.012), while week 70 was significantly lower than weeks 30 (p = 0.026) and 50 (p = 0.035). TBMD in the middle and distal locations for C hens were the lowest in week 70 compared to weeks 20 (middle: p = 0.012; distal: p = 0.013), 30 (middle: p = 0.021; distal: p = 0.022), and 50 (middle: p = 0.035; distal: p = 0.033). Proximal, middle, and distal TBMD measurements in W1 hens at week 20 were significantly higher than in weeks 30 (proximal: p = 0.039; middle: p = 0.036; distal: p = 0.032), 50 (proximal: p = 0.028; middle: p = 0.024; distal: p = 0.028), and 70 (proximal: p = 0.018; middle: p = 0.011; distal: p = 0.018). TBMD measurements in W2 hens were the lowest at week 70 compared to weeks 20 (proximal: p = 0.019; middle: p = 0.021; distal: p = 0.016), 30 (proximal: p = 0.032; middle: p = 0.024; distal: p = 0.026), and 50 (proximal: p = 0.036; middle: p = 0.031; distal: p = 0.028), while there were no significant differences at weeks 30 and 50 (Table 2).

Table 2.
Mean total area of tibiotarsi cross-section within regions of interest ± SEM and total bone mineral density of tibiotarsi cross-section within regions of interest ± SEM of hens at weeks 20, 30, 50, and 70 (n = 54/week). Mean area of tibiotarsi cross-section cortex within regions of interest ± SEM and cortex bone mineral density of tibiotarsi cross-section within regions of interest ± SEM of hens at weeks 20, 30, 50, and 70 (n = 54/week).
Similarly, there were no differences between treatments in the cortex cross-sectional area (CCSA) and bone mineral density (CBMD) at week 20 for any of the bone sections assessed. Effects of age and treatment were detected in the CCSA (p = 0.028; Table 2). C hens had significantly lower proximal, middle, and distal CCSA values in weeks 30 (proximal: p = 0.022, 0.024; middle: p = 0.015, 0.026; distal: p = 0.021, 0.032), 50 (proximal: p = 0.017, 0.022; middle: p = 0.021, 0.036; distal: p = 0.015, 0.034), and 70 (proximal: p = 0.028, 0.019; middle: p = 0.034, 0.021; distal: p = 0.034, 0.022) compared to W1 and W2 hens (Table 2).
Across weeks, C hens had the lowest CCSA in proximal, middle, and distal at week 70 compared to weeks 50 (middle: p = 0.036; distal: p = 0.029), 30 (proximal: p = 0.036; middle: p = 0.034; distal: p = 0.036), and 20 (proximal: p = 0.019; middle: p = 0.022; distal: p = 0.026). On the other hand, both W1 and W2 hens showed no significant differences across weeks in the CCSA at any location.
Statistically significant differences were detected in CBMD across treatments and weeks (p = 0.029; Table 2). C hens showed lower proximal, middle, and distal CBMD values compared to W1 and W2 hens at weeks 30 (proximal: p = 0.023, 0.031; middle: p = 0.020, 0.024; distal: p = 0.019, 0.025), 50 (proximal: p = 0.031, 0.019; middle: p = 0.022, 0.031; distal: p = 0.020, 0.028), and 70 (proximal: p = 0.017, 0.028; middle: p = 0.019, 0.025; distal: p = 0.031, 0.028), while there were no significant differences between W1 and W2 hens. Across weeks, significant differences for CBMD were detected in all treatment groups and bone sections, where week 70 showed the lowest CBMD values, and weeks 20, 30, 50, and 70 were all significantly different (Table 2).
3.4. Tibia Ash Percentage
Significant differences in the tibia ash percentage were detected across treatments (p = 0.021). The tibia ash percentage in C hens was significantly lower compared to W1 and W2 (p = 0.016 and 0.026, respectively; Figure 5).
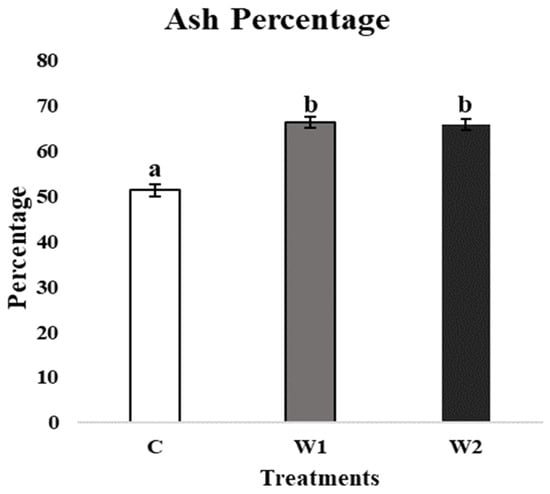
Figure 5.
Mean tibia ash percentage (%) of laying hens at 70 weeks of age (n = 54) with treatments consisting of no scotophase interruption (C), 1 h of scotophase interruption (W1), and 2 h of scotophase interruption (W2). a,b Means with differing superscripts indicate statistically significant differences between treatments at p < 0.05.
3.5. Tibia Biomechanical Properties
There was a significant difference in tibiotarsi breaking strength (p = 0.021) and stiffness (p = 0.019) at 70 weeks of age. C hens showed the lowest breaking strength compared to W1 and W2 hens at week 70 (p = 0.013 and 0.025, respectively; Figure 6). C hens also showed the lowest stiffness compared to W1 and W2 hens at 70 weeks of age (p = 0.021 and 0.029, respectively; Figure 6).
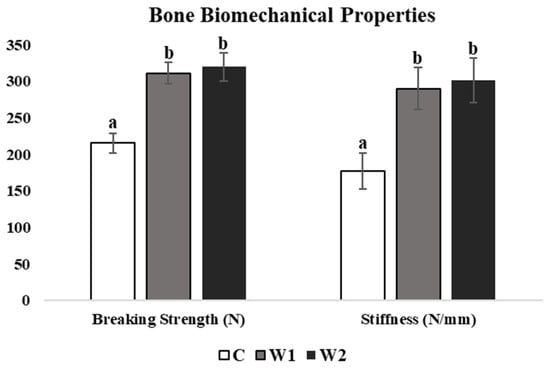
Figure 6.
Mean tibia breaking strength (N) and stiffness (N/mm) of laying hens at 70 weeks of age (n = 54), with treatments consisting of no scotophase interruption (C), 1 h of scotophase interruption (W1), and 2 h of scotophase interruption (W2). a,b Means with differing superscripts indicate statistically significant differences between treatments within a parameter at p < 0.05.
4. Discussion
Little research has been carried out to explore the increasing availability of dietary calcium in hens during nighttime hours when eggshell calcification occurs, which causes the highest demand for calcium withdrawal from bones in laying hens [5,9]. Therefore, the current study investigated the influence of interrupting the scotophase period on bone health parameters in Hy-line W36 laying hens. The results from this trial indicated that interrupting the scotophase period was beneficial for serum calcium concentrations, slowing bone demineralization, maintaining adequate bone mineral density and area, and improving bone-breaking strength and stiffness.
4.1. Serum Calcium
In our study, hens across all treatments and weeks showed higher SRT total serum calcium concentrations at the end of the photophase period (immediately after lights off) compared to END concentrations, at the end of the scotophase period (immediately before lights on). Such differences between the SRT and END blood calcium concentrations indicate the utilization of blood calcium as the primary source of shell calcification during night hours. These findings are consistent with a previous study that reported a fall in plasma calcium concentrations as blood passed through the shell gland during the eggshell calcification process [51].
The egg-laying cycle consists of an approximate 24 h process, where the formation of internal egg components, such as maturation of the yolk, albumen, and chalazae, occurs in an approximate 4 h period [52]. Afterward, the egg is passed into the shell gland of the uterus, where dietary calcium is absorbed from the gastrointestinal tract for supplementation of calcium carbonate during eggshell formation [52]. Eggshell formation occurs as a result of the precipitation of calcite (a crystalline form of calcium carbonate) from uterine fluid, where the blood-rich shell gland collects plasma calcium during shell calcification, which is proven by the fall of plasma calcium concentrations as blood passes through the shell gland [51].
The main source of calcium during this time is supplied through the blood from dietary calcium uptake [50,52]; however, in a time of dietary calcium depletion, such as the scotophase period, the body is forced to supplement the high demand for calcium through other available sources [5,16,17,51,53]. As a result, osteoclastic activity increases the calcium mobilization rate from medullary bone to supplement calcium, allowing the bird to increase the serum calcium content for the continuation of eggshell calcification [53]. However, osteoclastic activity is not discriminatory to only medullary bone, as it also increases calcium mobilization from structural bones [9]. In a previous study, total calcium absorption within the digestive tract was highest between the first four hours following the start of the scotophase period, reaching as high as 43% absorption. In the following 4 h period (4–8 h from the initialization of the scotophase period), total calcium absorption from the GIT reached only 15% [54]. In this previous study, laying hens were fed a commercially formulated diet with a 3.69% calcium content, which was removed at 8 pm. Researchers euthanized a series of birds at various timepoints following the removal of feed content. This study found that at the initial point of dissection, the hens’ digestive system contained 1.15 g of calcium, but by 6 am, calcium contents only amounted to 0.11 g, with 0.34 g excreted in the feces. These results indicate that hens absorbed 61% of total calcium needed for eggshell formation from the digestive tract [54].
In the current study, although SRT calcium concentrations were not significantly different across treatments at all weeks, END calcium levels in control hens were significantly lower than in hens who received a lights-on break during the scotophase period at all weeks of the trial. The observed variances between C and test hens may be ascribed to the dietary calcium intake during the scotophase period in test hens. Consequently, the END blood calcium levels were sustained, resulting in a lesser decline compared to the control hens. In the control group, where bone served as the primary calcium source for eggshell calcification during this phase, the drop in calcium levels was more pronounced. An increase in calcium mobilization from the bone can lead to weak and brittle bones and cause a degenerative disease known as osteoporosis, as the hens have no opportunity for rebuilding due to ceased osteoblast activity [5,9,16,17,19]. This aligns with the findings in the current study, where C hens exhibited progressively lower total SRT and END serum calcium concentrations across weeks compared to W1 and W2 hens, which received interruption of dark hours.
4.2. Bone Demineralization
In the current study, TRACP-5b and CTX-I enzyme concentrations within blood circulation were used as indicators of osteoclast-specific activity during bone resorption, increasing with an increased rate of resorption [44,45,46]. At week 20, no differences in TRACP-5b or CTX-I were detected, indicating that hens in all treatment groups started with similar levels of bone resorption; however, across weeks, C hens displayed the highest level of demineralization in TRACP-5b and CTX-I compared to W1 and W2 hens. Differences in bone resorption markers in C hens were detected starting at week 30 and continued to increase through weeks 50 and 70, linking negative effects of mobilization of calcium with increased age, unlike in W1 and W2, where levels changed at weeks 30 and then were consistent at weeks 50 and 70. These findings indicate a more aggressive mobilization of calcium from skeletal stores in C hens compared to W1 and W2 hens. This can likely be attributed to the midnight access to feed in W1 and W2 hens and the ability to maintain a higher blood calcium level throughout the night, when calcium demands are the highest for eggshell calcification, with low levels of calcium withdrawal bone reserve. These findings clarified the mechanism of calcium mobilization from bone to sustain eggshell formation, as described in previous studies [44,45,46], and aligned with the aforementioned findings in the current study (Section 4.1), attributed to the access to feed during scotophase hours for W1 and W2 hens.
4.3. CT Image Analysis
Some studies have explored CT scans as a comparable source of calculation of BMD and cross-sectional area [47,55]. The interruption of scotophase influenced measures of TBMD, CA, and CBMD; however, no impact was observed in TA. Values at week 20 were similar across all treatment groups, indicating that all hens started at a similar state of bone health. Hens in C pens showed the lowest TBMD across all bone sections and across all weeks, while W1 and W2 tended to contain the highest TBMD; however, according to the results, W2 hens saw more variation in TBMD across weeks than W1 hens. Previous studies indicated that bone mineral density is affected by factors such as age, sex, production type, diet, and management practices [56]. Birds tend to deposit bone minerals before the beginning of laying, and bone mineral density decreases simultaneously with an increase in egg production. During the phase of growth prior to sexual maturity (rearing phase), two processes affect skeletal development: longitudinal growth of long bones by endochondral ossification and widening of long bones through intramembranous ossification [9]. Once the hens reach sexual maturity, drastic changes in bone biology occur, in which osteoblasts switch from forming mature lamellar cortical bone to producing woven medullary bone structures. Medullary bone structures are utilized as an alternate source of calcium for eggshell formation, especially in the leg bones in laying hens. According to previous studies, the osteoblast production switch to medullary bone compared to cortical bone is absolute and concurrently occurs with the continuation of osteoclastic activity across the lay cycle [5,9,57]. As calcium demands are increased during scotophase when dietary calcium availability is minimal, a surge in medullary bone osteoclast activity occurs. However, due to the ill-defined nature of osteoclasts, exposed cortical bone succumbs, leading to osteoporotic skeletal structures [9]. As osteoclastic activity continues to pull calcium from skeletal stores of medullary and structural bone, BMD and the area of the bone structures are expected to decrease [56]. The hens in C pens likely mobilized more cortical bone than hens in pens with interrupted scotophase due to a lack of restoration periods throughout the lay cycle. Hens in W1 and W2 seemed more successful in protecting calcium stores in structural bone formations, likely resulting from the availability of total dietary calcium sources during eggshell calcification, which relates to the higher CCSA values observed in W1 and W2 hens than in C hens.
In the current study, age and treatment influenced CCSA, where hens in C pens demonstrated the lowest CA values, and W2 demonstrated the highest values; however, W2 values were quite similar to W1. Previous studies reported that the loss of cortical bone can be replaced with medullary bone, hence the importance of measuring CCSA separately across weeks from TSCA, as TSCA is unlikely to change in the presence of medullary bone formation [9]. CBMD for each bone section in C hens was lower than W1 and W2 each week, indicating an increased rate of mobilization of bone structures. Values for CBMD were quite high compared to TBMD due to the lack of medullary cavities in the measurement regions, as these values focus on cortical bone only. Across weeks, a diminishing CBMD was observed for each treatment. This can likely be associated with the lay cycle phase and the age of birds. An association between age and percent of resorption was observed, indicated by the decrease in area and density across weeks and treatments.
4.4. Tibia Ash Percentage
Bone ash percentage is an indicator used to assess the mineral content of the bones [58]. In the current study, tibiae of hens provided with scotophase interruption showed higher ash percentages than control hens, approaching end-lay at 70 weeks of age. Bone ash% is a measure sensitive to changes in calcium intake [59], with a low ash% particularly late in the lay cycle, associated with lower structural calcium content [60]. Similarly, in our study, the lower ash% in the control hens indicated a higher incidence of mineral mobilization from bone to sustain the calcium needed for eggshell calcification, unlike in test hens, where the presence of dietary minerals may result in reducing the rate of mobilization from bones, which in turn resulted in a higher ash% upon testing [58,59,60,61].
4.5. Bone Biomechanical Properties
In the current study, interrupting the scotophase period impacted the tibia biomechanical properties of hens at week 70 of age, resulting in a higher breaking strength and stiffness in hens receiving 1 or 2 h of light in the midpoint of the scotophase period compared to control hens. Determining the breaking strength of the tibia involves applying monaxial force until it breaks to assess the force required to provoke structural failure [62]. Several studies indicated that bone-breaking strength is correlated with the amount of medullary bone in the skeletal structure due to its interconnected lattice-like formation [63], where the formation of the weak medullary bone usually occurs following the resorption of structural cortical bone [9]. The weakness associated with medullary bone can be associated with an irregular arrangement of collagen fibrils within the medullary cavity [9]. Similarly, bone stiffness characterizes how much the bone deforms when loaded under force [64], where higher stiffness rates indicate less deformity in the structure and are dependent on cortical bone morphology, such as thickness and density [63,64]. Therefore, higher bone stiffness would also indicate a higher cortical bone content within the structure, slower bone resorption, and, in turn, a decrease in medullary bone formation. A higher breaking strength and stiffness ratio would likely indicate the prevalence of cortical bone within the structure and a slower mobilization of calcium from bone stores. Therefore, our results suggested that interrupting the scotophase period resulted in slowing down the process of degrading cortical into medullary bone to sustain calcium supply for eggshell formation by increasing dietary calcium during the scotophase period, which in turn resulted in increasing the bone-breaking strength and stiffness. This can likely be associated with food storage in the crop during eggshell formation, limiting the amount of calcium uptake from the skeletal structures, resulting in higher breaking strength and stiffness, aligning with other findings in the current study. In the current study, findings of bone ash% and bone biomechanical properties were consistent with and supported the previous findings (Section 4.1, Section 4.2 and Section 4.3) and were consistent with the benefits observed by the interruption of the scotophase and the allowance of bone resorption depression, leading to a healthier bone in the bird [62,63,64].
5. Conclusions
The results from our investigation indicated that changes to industry light schedules may result in better bone health, potentially improving hen welfare and profitability for producers. Likewise, the availability of dietary calcium during a period of high demand induced by eggshell calcification exerted positive effects on serum calcium concentrations at the end of the daily scotophase period and decreased bone demineralization. In turn, these positive effects also increased the bone mineral density, the area of cortical bone structures, and the ash percentages and bone-breaking strength and stiffness. Nevertheless, exposure to light during midnight hours may not only affect skeletal health in laying hens. In the current study, it is important to note that treatment did not affect the average daily feed intake (ADFI) across all weeks, with ADFI values of 85.29, 119.09, 122.53, and 120.4 g of feed/day observed at 20, 30, 50, and 70 weeks of age, respectively. Similarly, the feed conversion ratio (FCR) remained unaffected by the treatment across the same timepoints (week 20: 1.88; week 30: 2.12; week 50: 2.16; week 70: 1.89). However, a treatment effect was detected on hens’ daily egg production (HDEP%) at 50 and 70 weeks of age, where C hens exhibited the lowest HDEP (week 50: 90.89%; week 70: 80.56%) compared to W1 (week 50: 96.88%; week 70: 87.58%) and W2 (week 50: 96.56%; week 70: 86.55%), with no significant differences between W1 and W2. Therefore, further research on the effects of interrupted scotophase on parameters of concern for producers, such as feed intake, egg quality, behavior, and welfare, is intended to be published in Parts II and III of this series.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/poultry3040028/s1. Figure S1: Illumination was set to 30 Lux (XX * 1.487 cLux), measured at the height of the hens’ eyes. The spectrum that was used was generated with 3000 K white LEDs plus 630 nm peak-wavelength red LEDs, resulting in the spectral density graph shown. For the daily photophase segment, sunrise transitions from dark to light were set to 30 min, as were sunset transitions from light to dark. For transitions to the mid-scotophase light pulses, transition times were set to 5 min.
Author Contributions
Conceptualization, A.J.C., M.A.-R. and A.A.; methodology, A.J.C., C.H. and A.A.; formal analysis, A.C, A.J.B. and A.A.; investigation, A.J.C., C.H., A.J.B., G.M.H., A.B.S., M.A.-R. and A.A.; data curation, A.J.C., C.H., A.J.B. and A.A.; writing—original draft preparation, A.J.C., M.A.-R. and A.A.; writing—review and editing, A.J.C., C.H., A.J.B., G.M.H., A.B.S., M.A.-R. and A.A.; project administration, A.J.C., A.J.B. and C.H.; funding acquisition, M.A.-R. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Egg Industry Center (project # RG-2015127). This material is based upon work supported by NIFA/USDA, under project number SC-1029, technical Contribution No. 7300 of the Clemson University Experiment Station. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by Clemson University’s Institutional Animal Care and Use Committee (protocol # AUP2021-0068).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank the employees and student workers at the Morgan Poultry Center for their help, care, and troubleshooting. We would also like to thank our undergraduate volunteers, especially Zoie Morrow and Shaunie Cruz.
Conflicts of Interest
Authors G.M.H. and A.B.S were employed by the company ONCE by Signify. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interestThe authors declare no conflicts of interest.
References
- Notter, D.R. The importance of genetic diversity in livestock populations of the future1. J. Anim. Sci. 1999, 77, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Romanov, M.N.; Weigend, S. Analysis of genetic relationships between various populations of domestic and jungle fowl using microsatellite markers. Poult. Sci. 2001, 80, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Wolc, A.; Arango, J.; Jankowski, T.; Dunn, I.; Settar, P.; Fulton, J.; O’Sullivan, N.; Preisinger, R.; Fernando, R.; Garrick, D.; et al. Genome-wide association study for egg production and quality in layer chickens. J. Anim. Breed. Genet. 2014, 131, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Murrell, J.; Wilkins, L.; Nicol, C. The effect of keel fractures on egg-production parameters, mobility and behavior in individual laying hens. Anim. Welf. 2012, 21, 127–135. [Google Scholar] [CrossRef]
- Whitehead, C.; Fleming, R. Osteoporosis in cage layers. Poult. Sci. 2000, 79, 1033–1041. [Google Scholar] [CrossRef]
- Rennie, J.S.; Fleming, R.; McCormack, H.A.; McCorquodale, C.C.; Whitehead, C.C. Studies on effects of nutritional factors on bone structure and osteoporosis in laying hens. Br. Poult. Sci. 1997, 38, 417–424. [Google Scholar] [CrossRef]
- Koutoulis, K.; Kyriazakis, I.; Perry, G.; Lewis, P. Effect of Different Calcium Sources and Calcium Intake on Shell Quality and Bone Characteristics of Laying Hens at Sexual Maturity and End of Lay. Int. J. Poult. Sci. 2009, 8, 342–348. [Google Scholar] [CrossRef]
- van de Velde, J.; Vermeiden, J.; Touw, J.; Veldhuijzen, J. Changes in activity of chicken medullary bone cell populations in relation to the egg-laying cycle. Metab. Bone Dis. Relat. Res. 1984, 5, 191–193. [Google Scholar] [CrossRef]
- Whitehead, C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004, 83, 193–199. [Google Scholar] [CrossRef]
- Wilson, S.; Duff SR, I.; Whitehead, C.C. Effects of age, sex and housing on the trabecular bone of laying strain domestic fowl. Res. Vet. Sci. 1992, 53, 52–58. [Google Scholar] [CrossRef]
- Jendral, M.; Korver, D.; Church, J.; Feddes, J. Bone Mineral Density and Breaking Strength of White Leghorns Housed in Conventional, Modified, and Commercially Available Colony Battery Cages. Poult. Sci. 2008, 87, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.M.; Hansen, K.K. Role of estrogen in avian osteoporosis. Poult. Sci. 2004, 83, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Dacke, C.G.; Arkle, S.; Cook, D.J.; Wormstone, I.M.; Jones, S.; Zaidi, M.; Bascal, Z.A. Medullary bone and avian calcium regulation. J. Exp. Biol. 1993, 184, 63–88. [Google Scholar] [CrossRef]
- Hans, D.; Krieg, M. The clinical use of quantitative ultrasound (QUS) in the detection and management of osteoporosis. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2008, 55, 1529–1538. [Google Scholar] [CrossRef]
- Johnson, M.L.; Lara, N.; A Kamel, M. How genomics has informed our understanding of the pathogenesis of osteoporosis. Genome Med. 2009, 1, 84. [Google Scholar] [CrossRef]
- Saunders-Blades, J.L.; MacIsaac, J.L.; Korver, D.R.; Anderson, D.M. The effect of calcium source and particle size on the production performance and bone quality of laying hens. Poult. Sci. 2009, 88, 338–353. [Google Scholar] [CrossRef]
- Araujo JA, D.; Silva JH, V.D.; Costa FG, P.; Sousa JM, B.D.; Givisiez PE, N.; Sakomura, N.K. Effect of the levels of calcium and particle size of limestone on laying hens. Rev. Bras. Zootec. 2011, 40, 997–1005. [Google Scholar] [CrossRef]
- Whitehead, C. Skeletal disorders in laying hens: The problem of osteoporosis and bone fractures. Poult. Sci. Symp. World’s Poult. Sci. Assoc. 2004, 27, 259–278. [Google Scholar]
- Rubin, C.-J.; Brändström, H.; Wright, D.; Kerje, S.; Gunnarsson, U.; Schutz, K.; Fredriksson, R.; Jensen, P.; Andersson, L.; Ohlsson, C.; et al. Quantitative trait loci for BMD and bone strength in an intercross between domestic and wildtype chickens. J. Bone Miner. Res. 2007, 22, 375–384. [Google Scholar] [CrossRef]
- Cransberg, P.H.; Parkinson, G.; Wilson, S.; Thorp, B. Sequential studies of skeletal calcium reserves and structural bone volume in a commercial layer flock. Br. Poult. Sci. 2001, 42, 260–265. [Google Scholar] [CrossRef]
- Silversides, F.G.; Korver, D.R.; Budgell, K.L. Effect of strain of layer and age at photostimulation on egg production, egg quality, and bone strength. Poult. Sci. 2006, 85, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.F.; Pang, C.S.; Poon AM, S.; Wan, Q.; Song, Y.; Brown, G.M. An overview of melatonin and melatonin receptors in birds. Poult. Avian Biol. Rev. 1996, 7, 217–228. [Google Scholar]
- Lewis, P.D.; Morris, T.R. Poultry and coloured light. Worlds Poult. Sci. J. 2000, 56, 189–207. [Google Scholar] [CrossRef]
- Govardovskiĭ, V.I.; Zueva, L.V. Visual pigments of chicken and pigeon. Vis. Res. 1977, 17, 537–543. [Google Scholar] [CrossRef]
- Kelber, A.; Vorobyev, M.; Osorio, D. Animal colour vision--behavioural tests and physiological concepts. Biol. Rev. Camb. Philos. Soc. 2003, 78, 81–118. [Google Scholar] [CrossRef]
- Prescott, N.B.; Wathes, C.M. Spectral sensitivity of the domestic fowl (Gallus g. domesticus). Br. Poult. Sci. 1999, 40, 332–339. [Google Scholar] [CrossRef]
- Li, T.; Howland, H.C. The effects of constant and diurnal illumination of the pineal gland and the eyes on ocular growth in chicks. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3692–3697. [Google Scholar] [CrossRef][Green Version]
- Imtd, J.; Rossi, L. Influence of Artificial Lighting on the Performance and Egg Quality of Commercial Layers: A Review. Braz. J. Poult. Sci. 2014, 16, 337–344. [Google Scholar]
- Thiele, H.H. Light stimulation of commercial layers. Lohmann Inf. 2009, 44, 28–37. [Google Scholar]
- Abbas, A.O.; Gehad, A.E.; Hendricks, G.L.; Gharib HB, A.; Mashaly, M.M. The Effect of Lighting Program and Melatonin on the Alleviation of the Negative Impact of Heat Stress on the Immune Response in Broiler Chickens. Int. J. Poult. Sci. 2007, 6, 651–660. [Google Scholar] [CrossRef][Green Version]
- Navara, K.J.; Nelson, R.J. The dark side of light at night: Physiological, epidemiological, and ecological consequences. J. Pineal Res. 2007, 43, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Lazăr, R.; Solcan, C.; Creţu, C.; Lazăr, M.; Muntean, C.; Boişteanu, P.C. Characterization of the relations between morphology and physiological status of the pineal gland in connection with the somatic development level in turkeys reared in Romania. Arq. Bras. Med. Veterinária Zootec. 2015, 67, 763–770. [Google Scholar] [CrossRef][Green Version]
- Stehle, J.H.; Von Gall, C.; Korf, H.W. Melatonin: A clock-output, a clock-input. J. Neuroendocrinol. 2003, 15, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.K.; Kumar, V. Melatonin: An internal signal for daily and seasonal timing. Indian. J. Exp. Biol. 2014, 52, 425–437. [Google Scholar]
- Csernus, V.J. The avian pineal gland. Chronobiol. Int. 2006, 23, 329–339. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, B.P.; Rani, S. The Bird Clock: A Complex, Multi-Oscillatory and Highly Diversified System. Biol. Rhythm Res. 2004, 35, 121–144. [Google Scholar] [CrossRef]
- Bubenik, G.A. Gastrointestinal melatonin: Localization, function, and clinical relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef]
- Calislar, S.; Yeter, B.; Şahin, A. Importance of melatonin on poultry. J. Agric. Nat. 2018, 21, 987–997. [Google Scholar] [CrossRef]
- Newman, S.; Leeson, S. Skeletal integrity in layers at the completion of egg production. Worlds Poult. Sci. J. 1997, 53, 265–277. [Google Scholar] [CrossRef]
- Whitehead, C. Nutrition and poultry welfare. World’s Poult. Sci. J. 2002, 58, 349–356. [Google Scholar] [CrossRef]
- Harms, R.H.; Douglas, C.R.; Sloan, D.R. Midnight Feeding of Commercial Laying Hens Can Improve Eggshell Quality. J. Appl. Poult. Res. 1996, 5, 1–5. [Google Scholar] [CrossRef]
- Hy-Line International. Hy-Line Management Guide. Available online: https://www.hyline.com/filesimages/Hy-Line-Products/Hy-Line-Product-PDFs/W-36/36%20COM%20ENG.pdf (accessed on 4 April 2024).
- Animal Husbandry and Guidelines for U.S. Egg Laying Flocks. United-Egg-Producers. 2017. Available online: https://uepcertified.com/wp-content/uploads/2021/08/CF-UEP-Guidelines_17-3.pdf (accessed on 4 April 2024).
- Zhao, D.; Wang, J.; Liu, Y.; Liu, X. Expressions and clinical significance of serum bone Gla-protein, bone alkaline phosphatase and C-terminal telopeptide of type I collagen in bone metabolism of patients with osteoporosis. Pak. J. Med. Sci. 2015, 31, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Vasikaran, S.; Eastell, R.; Bruyere, O.; Foldes, A.J.; Garnero, P.; Griesmacher, A.; McClung, M.; Morris, H.A.; Silverman, S.; Trenti, T.; et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos. Int. 2011, 22, 391–420. [Google Scholar] [CrossRef] [PubMed]
- Chubb, S.A.P. Measurement of C-terminal telopeptide of type I collagen (CTX) in serum. Clin. Biochem. 2012, 45, 928–935. [Google Scholar] [CrossRef]
- Harrison, C.; Jones, J.; Bridges, W.; Anderson, G.; Ali, A.; Mercuri, J. Associations among computed tomographic measures of bone and muscle quality and biomechanical measures of tibiotarsal bone quality in laying hens. Am. J. Vet. Res. 2023, 84, ajvr.23.05.0109. [Google Scholar] [CrossRef]
- Anderson, M.G.; Johnson, A.M.; Jacobs, L.; Ali, A.B.A. Influence of Perch-Provision Timing on Anxiety and Fearfulness in Laying Hens. Animals 2023, 13, 3003. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Soft. 2015, 67, 1–10. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Taylor, T.G. How an Eggshell Is Made. Sci. Am. 1970, 222, 88–97. [Google Scholar] [CrossRef]
- Hrabia, A. Chapter 35-Reproduction in the female. In Sturkie’s Avian Physiology, 7th ed.; Scanes, C.G., Dridi, S., Eds.; Academic Press: San Diego, CA, USA, 2022; pp. 941–986. [Google Scholar]
- Kermanshahi, H.; Hadavi, A. Effect of Added Extra Calcium Carbonate into the Diets, One Hour Before Starting Dark Period on Performance and Egg Quality of Laying Hens. Int. J. Poult. Sci. 2006, 5, 946–948. [Google Scholar]
- Roland, D.; Sloan, D.R.; Harms, R.H. Calcium Metabolism in the Laying Hen: 4. The Calcium Status of the Hen at Night1. Poult. Sci. 1973, 52, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Robison, C.I.; Karcher, D.M. Analytical bone calcium and bone ash from mature laying hens correlates to bone mineral content calculated from quantitative computed tomography scans. Poult. Sci. 2019, 98, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Almeida Paz, I.; Bruno, L. Bone mineral density: Review. Braz. J. Poult. Sci. 2006, 8, 69–73. [Google Scholar] [CrossRef]
- Hudson, H.; Britton, W.; Rowland, G.; Buhr, R. Histomorphometric bone properties of sexually immature and mature White Leghorn hens with evaluation of fluorochrome injection on egg production traits. Poult. Sci. 1993, 72, 1537–1547. [Google Scholar] [CrossRef]
- Field, R.A. Ash and calcium as measures of bone in meat and bone mixtures. Meat Sci. 2000, 55, 255–264. [Google Scholar] [CrossRef]
- Cheng, T.K.; Coon, C.N. Sensitivity of Various Bone Parameters of Laying Hens to Different Daily Calcium Intakes1. Poult. Sci. 1990, 69, 2209–2213. [Google Scholar] [CrossRef]
- Al-Batshan, H.A.; Scheideler, S.E.; Black, B.L.; Garlich, J.D.; Anderson, K.E. Duodenal calcium uptake, femur ash, and eggshell quality decline with age and increase following molt. Poult. Sci. 1994, 73, 1590–1596. [Google Scholar] [CrossRef]
- Petruk, A. The Timing of Calcium Intake and its Effect on the Calcium Metabolism of Broiler Breeder and Laying Hens. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2004. [Google Scholar]
- Harrison, C. Evaluation of Bone and Muscle Quality in Laying Hens using Quantitative, Radiographic, Computed Tomographic, Biomechanical, and Tissue Level Measures. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2023. [Google Scholar]
- Fleming, R.; McCormack, H.; McTeir, L.; Whitehead, C. Medullary bone and humeral breaking strength in laying hens. Res. Vet. Sci. 1998, 64, 63–67. [Google Scholar] [CrossRef]
- Jepsen, K.J.; Silva, M.J.; Vashishth, D.; Guo, X.E.; van der Meulen, M.C. Establishing biomechanical mechanisms in mouse models: Practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. J. Bone Miner. Res. 2015, 30, 951–966. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).