1. Introduction
Eggs are the result of efficient biological transformation by laying hens, which are able to transform food of lower biological value into products of high nutritional quality for human consumption. However, eggs are perishable products that lose their quality in a short time if not properly handled and stored.
Shelf life is defined as the storage time during which eggs remain suitable for consumption under certain conditions of temperature, relative humidity, and handling [
1,
2]. The egg, being a nutrient-rich product, becomes an ideal medium for the growth of microorganisms, including pathogens [
3]. Therefore, establishing the shelf life of eggs is essential to secure safety for the consumer and food quality. The diets provided for the laying hens are among many factors that can influence egg quality. Feeding can influence the internal and external characteristics of the eggs and cause physicochemical changes in the albumen and yolk, which may modify the taste, freshness, and flavor [
4]. Therefore, some feeding practices can be used as alternatives to improve shelf life and egg quality [
5]. Regarding this context, the effect of many ingredients and dietary nutritional levels were already studied. However, results on the antinutritive effects of some compounds on egg quality are still limited, and little scientific knowledge is available on the tools that can help producers deal with this problem.
The β-mannans are non-starch polysaccharides that exhibit anti-nutritive activity when present in poultry diets [
6]. These components are found in plant cell walls and are present in many ingredients used mainly in animal feed, such as soybeans and products made from them [
7]. β-mannans can also be found on the surface of microorganisms. For this reason, the animal’s innate immune system is activated by feeds containing β-mannans and responds with the proliferation of monocytes, macrophages, and dendritic cells, as well as increased production of cytokines. Those factors can cause an increase in inflammatory responses and unnecessary energy expenditure [
8]. By hydrolyzing the β-mannans, the β-mannanase enzyme can avoid the anti-nutritional effects, improving immunity, allowing better digestion and nutrient absorption, in addition to limiting the growth of pathogenic bacteria [
6].
Previous studies have reported improvements in egg quality when birds are fed β-mannanase [
9,
10]. However, to our knowledge, there are no studies on the effects on egg quality during the storage period. In this study, the effects of β-mannanase supplementation in the diet of laying hens were tested to evaluate egg quality during different storage periods.
2. Materials and Methods
2.1. Animal, Housing, and Experimental Design
The Institutional Ethics Committee on the Use of Animals (CEUA/UFRGS) under protocol number 39,783, approved this experimental protocol. One hundred cages were randomly selected in a commercial farm (Salvador do Sul, Rio Grande do Sul, Brazil) with about 14 thousand laying hens (light weight; 36 weeks old). In a complete randomized design, the replicates were allocated to two treatments: control treatment (CON; basal diet with no additives), and β-mannanase treatment (BMA; control diet supplemented with 300 g/ton of β-mannanase). The exogenous enzyme used (β-mannanase; Hemicell HT™, Elanco Animal Health, So Paulo, Brazil) is produced during the fermentation of the bacteria Paenibacillus lentus.
A corn-soybean meal diet was formulated to meet Hyline W 36 nutrient requirements [
11]. In order to replace β-mannanase, kaolin was included in the basal feed. Water was provided by nipple drinkers and food by gutter feeders, and during the whole experiment, they were supplied ad libitum.
The animals remained kept in conventional sheds, that were east-west oriented, with concrete flooring masonry walls, and wire mesh ceilings. Side curtains were installed in the shed, which could be opened or closed to provide thermal comfort. The average lowest and maximum temperatures were 18 °C and 36 °C, respectively, as well as 35.8% and 94.7% relative humidity. There were 16 h of light and 8 h of darkness each day under the light regime. Throughout the course of the trial, birds were housed in galvanized wire cages that were 100 cm long by 40 cm broad by 45 cm high, with four birds per cage for a total floor surface of 500 cm2/hen.
The supplementation period lasted 84 days. This time frame was split into three distinct phases for evaluation (36–40 weeks, phase 1; 41–44 weeks, phase 2; and 45–48 weeks, phase 3). On the final day of each phase, 120 eggs from each treatment were randomly chosen and collected (240 in total).
2.2. Egg Quality Assessment
Fresh eggs’ quality was evaluated on the first day, and after each storage interval, the other eggs were maintained at 25 °C (room temperature) and randomized for quality evaluation (7, 14, 21, 28, 35, and 42 days). Weekly evaluations of 15 eggs per treatment were conducted, with the exception of the measurements of total solids content, shell characteristics, and thiobarbituric acid reactive substances (TBARS).
During the storage period, each egg was identified and weighed separately once a week. The following equation was used to determine the weight loss (%) of eggs during storage, according to Caner and Cansiz’s description [
4].
Using a digital caliper (TMX PD—150, China), the albumen height was calculated as the average of three measurements made at different positions on the albumen at a distance of 10 mm from the yolk. The equation suggested by Haugh was used to determine the Haugh unit [
12].
A digital caliper (TMX PD—150, China) was used to measure yolk width and height (mm). The Funk equation [
13] was used to determine the yolk index.
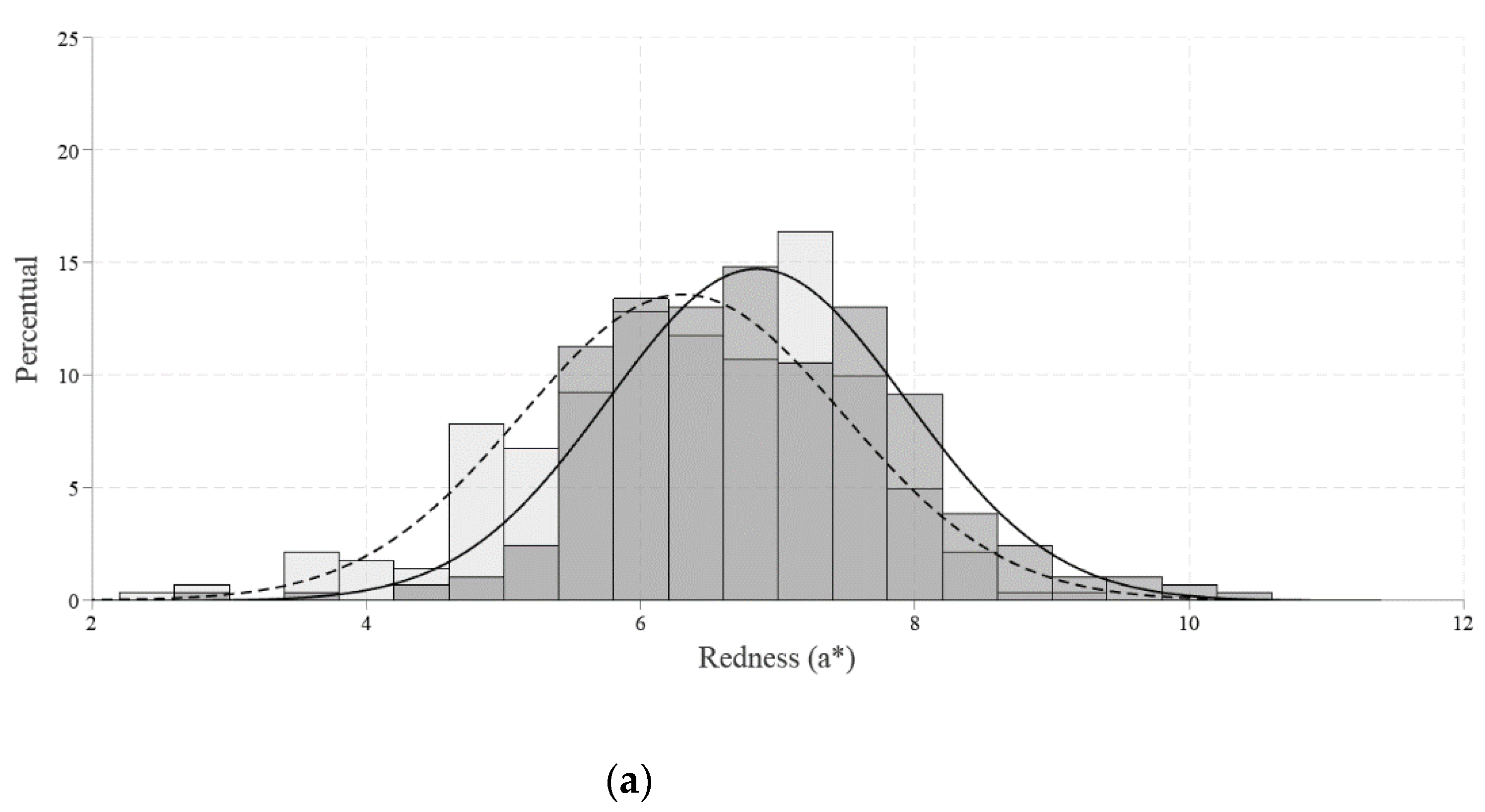
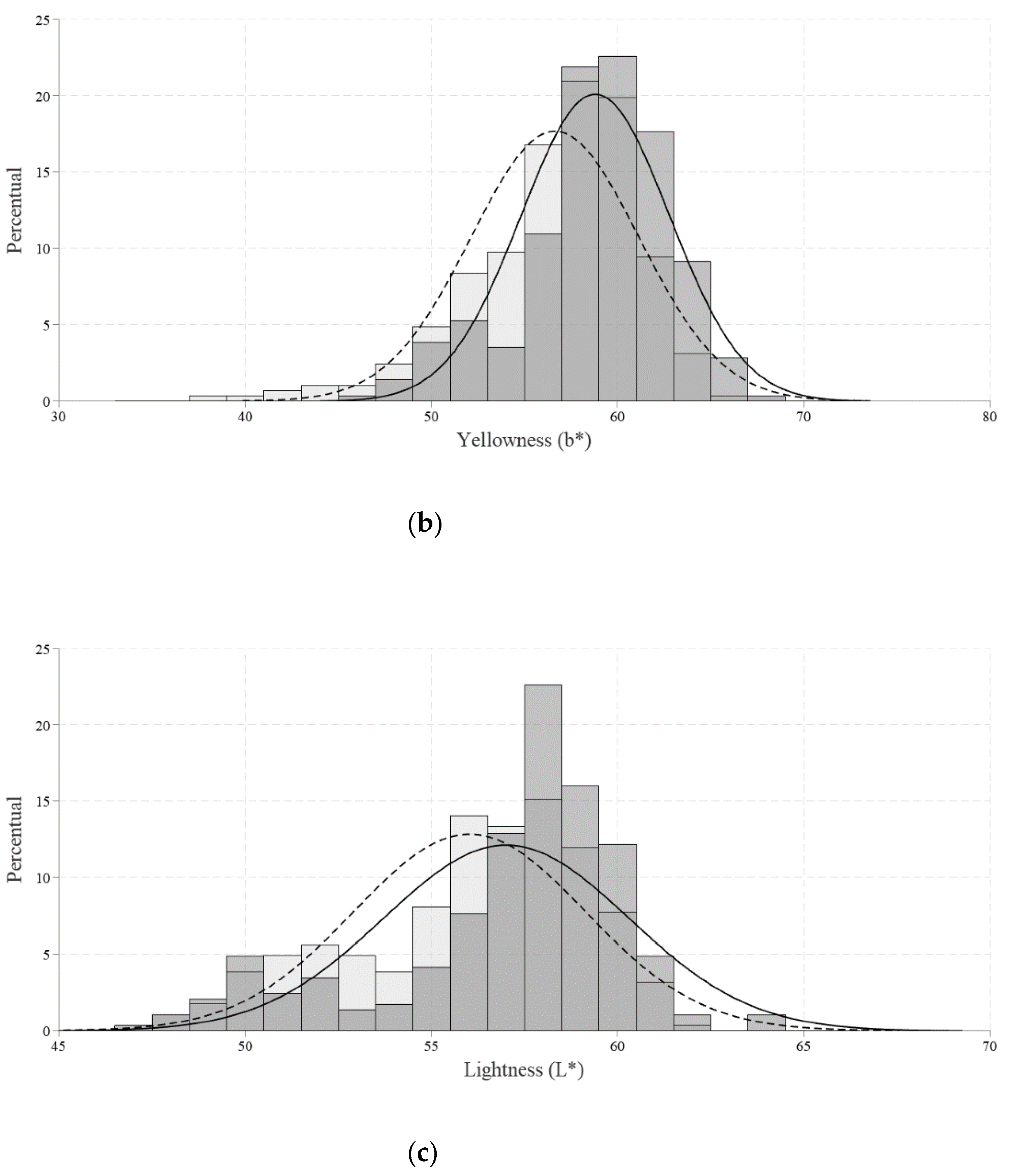
The Roche colorimetric fan (DSM Animal Nutrition & Health, Sao Paulo, Brazil) was used to measure the color of the yolk on a scale from 1 (light yellow) to 15 (red-dish orange). Additionally, each color space was assessed separately. The luminosity (L*), red intensity (a*), and yellow intensity (b*) were determined in this study using a portable spectrophotometer (Delta Vista model 450 G, Novo Hamburgo, Brazil).
The dense and liquid albumen were homogenized after the separation of the yolk and albumen, and the pH was then assessed using a digital pH meter (Kasvi model k39-2014B, Paraná, Brazil) that had been calibrated with buffer solutions of pH 4, 7, and 10. The same equipment (Kasvi model k39-2014B, Paraná, Brazil) was used to measure the pH of the yolk by inserting the electrode into two randomly chosen sites from the yolk.
The specific gravity was calculated according to Wells [
14], where the specific gravity was determined. To determine lipid oxidation, Giampietro et al. [
15] method was utilized. Three yolks per treatment and three storage durations were used to assess TBARS values (0, 21, and 42 days). A spectrophotometer (532 nm) was used to quantify the amount of lipid peroxide decomposition. The TBARS standard utilized was 1,1,3,3-tetramethoxypropane (TMP), and findings were represented as mg TMP/kg egg yolk.
Pomeranz and Meloan’s [
16] technique for measuring the total solids content in albumen and yolk was utilized. Albumen and yolk were weighed individually into previously dried porcelain crucibles at a rate of five grams. After being kept in an oven at 60 °C for 12 h, the samples were weighed and then kept at 105 °C for 12 h before being weighed once again. At two-week intervals, seven eggs from each treatment were inspected to measure the total solids content. After they were separated, cleaned, dried, and weighed on days 0, 21, and 42, the percentage of shells was calculated.
2.3. Statistical Analyses
The study’s design was entirely randomized. Every egg was treated as a separate experimental unit. SAS statistical software was used to carry out the statistical procedures (9.4, SAS Inst. Inc., Cary, NC, USA). The data were examined for normality before being submitted to analysis of variance with PROC MIXED. The effects of treatment, experimental phases, storage days, and interactions were all taken into consideration by the statistical models. The overall averages and probabilities for all the responses evaluated in the study are given in a table to simplify the presentation of the results. If an impact (p < 0.10) is pertinent to the project’s goal (i.e., the effect of the treatment or its interaction with phase and/or day), the means are further stated (separately per phase and day of assessment). Each storage day of each experimental phase was used to determine the probability of treatment effect. The probability was then evaluated at significance levels of 5% and 10%.
4. Discussion
Eggshell consists mainly of calcium carbonate, but also magnesium carbonate, calcium phosphate, and others. The balance between calcium and phosphorus ions is essential for the formation of the shell [
4]. The specific gravity indicates the amount of shell in relation to the other components of the egg and is closely related to the shell thickness and consequently to the deposition of calcium carbonate. Shell weight can also be used to confirm specific gravity findings and assess calcium metabolism. In this study, no significant differences in specific gravity were noticed, but higher values in terms of shell weight were found in eggs from supplemented birds. This is probably related to the greater preservation of albumen and yolk in eggs from β-mannanase treatment.
Still related to the shell is the albumen, which can suffer changes due to the porosity of the shell. Regarding albumen weight, higher values were observed in the birds fed with β-mannanase compared to the control group. The weight of the yolk and albumen has a positive relationship with the weight of the egg [
17], as their masses are greater in eggs of greater weight when compared to those of lower weight. Egg weight can be correlated with several factors, such as heritability, age, and bird weight. Egg weight also has a strong influence on the nutritional level of the diet [
18]. Furthermore, mannans are known to decrease viscosity and hinder the action of enzymes [
19] β-mannanase, by breaking down β-mannans, may facilitate the action of enzymes and increase the amount of protein absorbed, which may explain the higher yolk and albumen weight observed in this study.
During storage, changes in albumen and yolk are detected, and the rate of these changes is influenced by temperature [
4] and other factors. Egg freshness can be evaluated through parameters such as pH [
20]. The changes that occur in the egg during storage affect the functional properties of the yolk. These changes include the thinning of the albumen, the increase in pH, the weakening and stretching of the yolk membrane (which separates the albumen from the yolk), and the increase in the water content of the yolk [
21,
22]. In the present study, β-mannanase decreased yolk pH at all periods, improving its quality.
Regarding the yolk color, luminosity values (L* color) from the β-mannanase group were higher than the control group. Such findings indicate lower luminosity, that is, they were opaquer because they transmit less light. Higher values were also found for the intensity of red (a* color) and the intensity of yellow (b* color) compared to the control group. The use of the palette also showed an increase in the yolk color in the β-mannanase group when compared to the control. According to Narinc [
23] the L* a* b* method is more reliable than the palette since it demonstrates numerical values. A higher color intensity of the yolk increases the acceptance of eggs by consumers [
24] and is seen as something positive. Pigmentation occurs through the absorption of carotenoid pigments present in diets [
25]. The major carotenoids in corn are xanthophylls, lutein, and zeaxanthin (Perry et al., 2009). Such components are unsaturated and lipophilic [
26], that is, they accumulate in the yolk that has the highest concentration of fat in the egg. Furthermore, carotenoids have many double bonds in their molecules and can be oxidized depending on storage time, lighting, and ambient temperature, which reduces yolk pigmentation [
21,
27]. Therefore, β-mannanase can decrease the effects of storage and, consequently, slow down the deleterious effects of yolk pigmentation. Furthermore, by improving the absorption of nutrients and/or increasing the production of micelles, which transport carotenoids, β-mannanase can provide more carotenes to the yolk and generate a yellowish or reddish color.
To clarify the observed TBARS results, it is important to understand that lipid oxidation (peroxidation) is one of the most important reactions in food chemistry, consisting of a series of chemical and biochemical reactions that cause changes in the type and concentration of molecules present in foods, which can alter taste and nutritional quality and produce toxic compounds. Like other lipid molecules, cholesterol is susceptible to oxidation, producing cholesterol oxidation products (COPs) or oxysterols. Oxysterols are found in many foods, especially cholesterol-rich foods such as eggs. TBARS is the most commonly used method for quantifying malondialdehyde (MDA) in foods, which is one of the end products formed through the decomposition of certain lipid peroxidation products [
28]. Giampietro et al. [
29] observed that TBARS values of egg yolks increased over storage periods. In the present study, we observed that β-mannanase was able to decrease TBARS values, which may be related to greater production of micelles and consequently a greater amount of carotenoids deposited in yolk, which act as antioxidants [
30]. Another factor that may be related is the lower viscosity generated by β-mannanase [
19]. The viscosity impairs the absorption of nutrients and can lead to a greater amount of free radicals, the enzyme may reduce this production.
Few studies link gut health to egg quality. Regarding shelf life, our group did not find studies that relate the use of β-mannanase to this topic. The results found in this study can help and serve as an alternative in promoting the maintenance of intestinal health in laying hens, in addition to the possible decrease in the deterioration of egg quality by improving the use of nutrients by the bird.