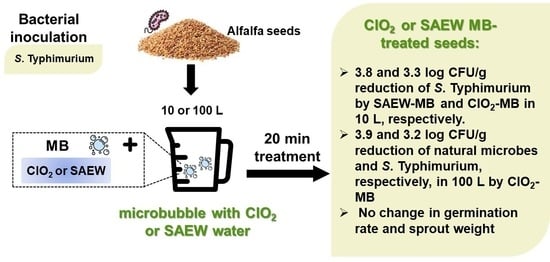
Application of Microbubbles Combining with Disinfectants to Inactivate Salmonella Typhimurium on Alfalfa Seeds and the Effects on Sprouting
Abstract
1. Introduction
2. Materials and Methods
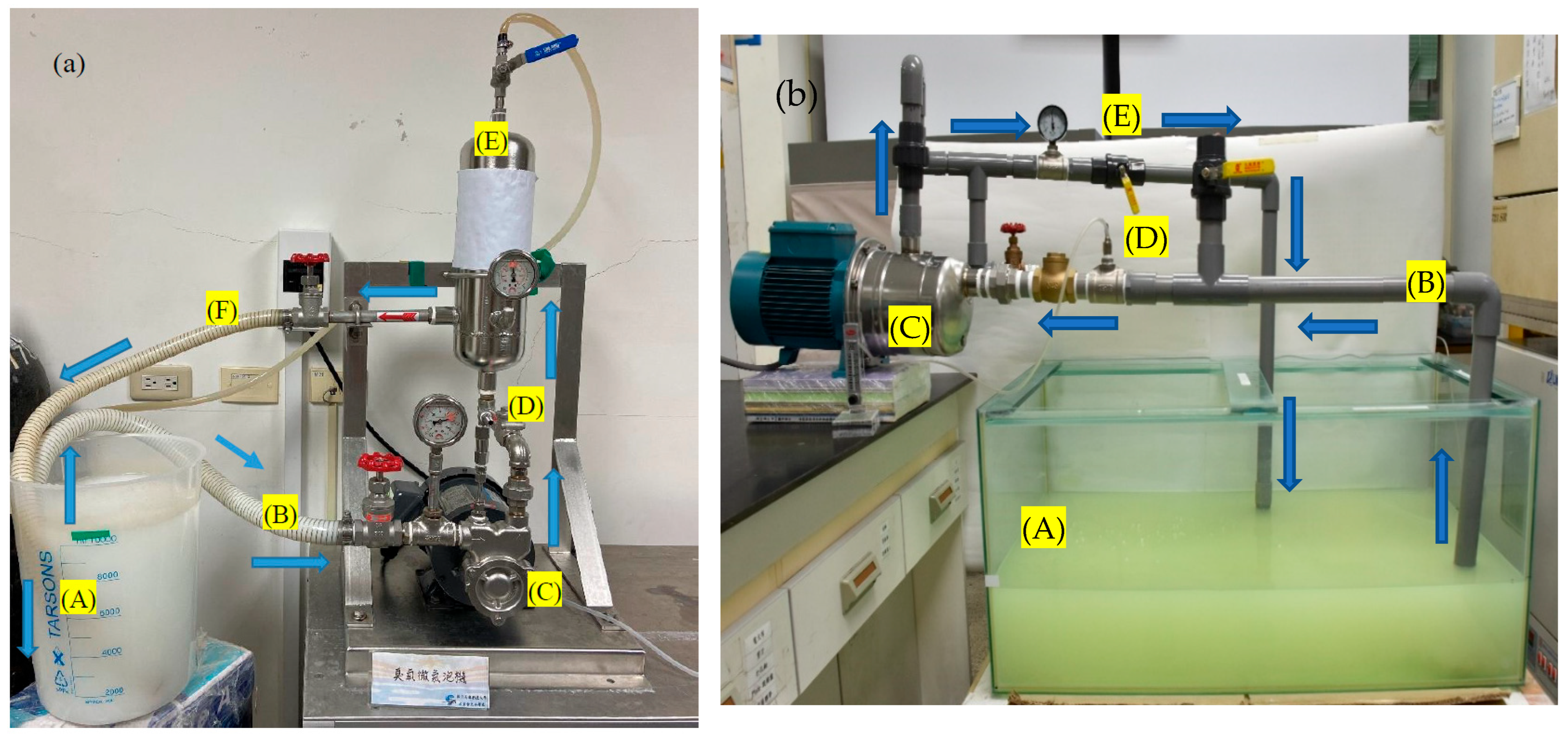
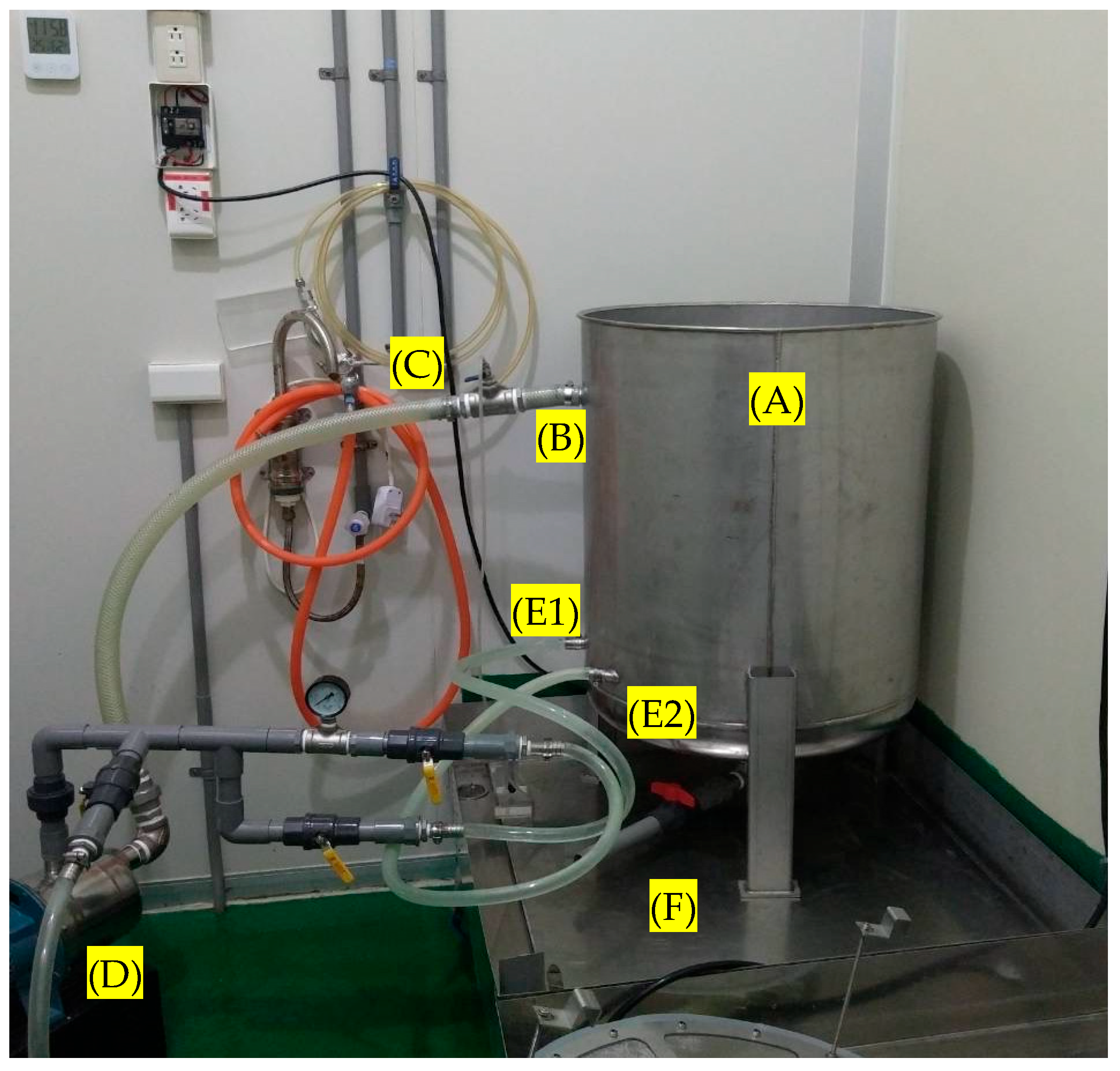
2.1. Microbubble Device
2.2. The Bacterial Culture and Inoculation of Alfalfa Seeds
2.3. Preparation of Disinfectants
2.4. Testing of Microbubbles Combined with Disinfectants on Alfalfa Seeds
2.5. Measurement of Oxidation-Reduction Potential (ORP), pH, and Electrical Conductivity (EC) of Treated Water
2.6. Germinating Rate of Alfalfa Seeds and Weight Yield of Sprouts
2.7. Scanning Electron Microscope (SEM) Observation
2.8. Statistical Analyses
3. Results
3.1. Bactericidal Effects of MBs Combined with Disinfectants
3.2. The Physicochemical Properties of ORP, pH, and EC of the Treated Water
3.3. Germinating Rate of Seeds and Weight Yield of Sprouts
3.4. Observation of SEM
4. Discussion
4.1. Bactericidal Effects of Treatments
4.2. The Values of ORP, pH, and EC of the Treated Water
4.3. Observation of Seed Surfaces Under SEM
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Millan-Sango, D.; Sammut, E.; Van Impe, J.F.; Valdramidis, V.P. Decontamination of alfalfa and mung bean sprouts by ultrasound and aqueous chlorine dioxide. LWT 2017, 78, 90–96. [Google Scholar] [CrossRef]
- Matos, A.; Garland, J.L.; Fett, W.F. Composition and physiological profiling of sprout-associated microbial communities. J. Food Prot. 2002, 65, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- CDC. Multistate Outbreak of Salmonella Infections Linked to Alfalfa Sprouts from One Contaminated Seed Lot (Final Update); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016. Available online: https://www.cdc.gov/salmonella/muenchen-02-16/index.html#print (accessed on 22 October 2025).
- Van Beneden, C.A.; Keene, W.E.; Strang, R.A.; Werker, D.H.; King, A.S.; Mahon, B.; Hedberg, K.; Bell, A.; Kelly, M.T.; Balan, V.K.; et al. Multinational outbreak of Salmonella enterica serotype Newport infections due to contaminated alfalfa sprouts. JAMA 1999, 281, 158–162. [Google Scholar] [CrossRef]
- Mahon, B.E.; Pönkä, A.; Hall, W.N.; Komatsu, K.; Dietrich, S.E.; Siitonen, A.; Cage, G.; Hayes, P.S.; Lambert-Fair, M.A.; Bean, N.H.; et al. An international outbreak of Salmonella infections caused by alfalfa sprouts grown from contaminated seeds. J. Infect. Dis. 1997, 175, 876–882. [Google Scholar] [CrossRef]
- Whitworth, J. Alfalfa Sprouts Linked to Salmonella Outbreak in New Zealand. 2019. Available online: https://www.foodsafetynews.com/2019/05/alfalfa-sprouts-linked-to-salmonella-outbreak-in-new-zealand/ (accessed on 22 October 2025).
- Harfield, S.; Beazley, R.; Denehy, E.; Centofanti, A.; Dowsett, P.; Housen, T.; Flood, L. An outbreak and case-control study of Salmonella Havana linked to alfalfa sprouts in South Australia, 2018. Commun. Dis. Intell. 2019, 43, 1–10. [Google Scholar] [CrossRef]
- CDC. Multistate Outbreak of Shiga Toxin-Producing Escherichia coli O157 Infections Linked to Alfalfa Sprouts Produced by Jack & The Green Sprouts (Final Update); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016. Available online: https://www.cdc.gov/ecoli/2016/o157-02-16/index.html#print (accessed on 22 October 2025).
- Ferguson, D.D.; Scheftel, J.; Cronquist, A.; Smith, K.; Woo-Ming, A.; Anderson, E.; Knutsen, J.; De, A.K.; Gershman, K. Temporally distinct Escherichia coli O157 outbreaks associated with alfalfa sprouts linked to a common seed source—Colorado and Minnesota, 2003. Epid. Infect. 2005, 133, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.J.; Farber, J.N.; Beuchat, L.R.; Parish, M.E.; Suslow, T.V.; Garrett, E.H.; Busta, F.F. Outbreaks associated with fresh produce: Incidence, growth, and survival of pathogens in fresh and fresh-cut produce. Compr. Rev. Food Sci. Food Saf. 2003, 2, 78–141. [Google Scholar] [CrossRef]
- Neetoo, H.; Chen, H. Individual and combined application of dry heat with high hydrostatic pressure to inactivate Salmonella and Escherichia coli O157:H7 on alfalfa seeds. Food Microbiol. 2011, 28, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial contamination of fresh produce: What, where, and how? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750. [Google Scholar] [CrossRef]
- Feng, G.; Churey, J.J.; Worobo, R.W. Thermal Inactivation of Salmonella and Escherichia coli O157:H7 on alfalfa seeds. J. Food Prot. 2007, 70, 1698–1703. [Google Scholar] [CrossRef]
- Stewart, D.S.; Reineke, K.F.; Ulaszek, J.M.; Tortorello, M.L. Growth of Salmonella during sprouting of alfalfa seeds associated with salmonellosis outbreaks. J. Food Prot. 2001, 64, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Taormina, P.J.; Beuchat, L.R.; Slutsker, L. Infections associated with eating seed sprouts: An international concern. Emerg. Infect. Dis. 1999, 5, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Charkowski, A.O.; Sarreal, C.Z.; Mandrell, R.E. Wrinkled alfalfa seeds harbor more aerobic bacteria and are more difficult to sanitize than smooth seeds. J. Food Prot. 2001, 64, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- FDA. Reducing Microbial Food Safety Hazards in the Production of Seed for Sprouting: Guidance for Industry; Food and Drug Administration: Silver Spring, MD, USA, 2022. Available online: https://www.fda.gov/media/127972/download (accessed on 20 May 2023).
- Singh, N.; Singh, R.K.; Bhunia, A.K. Sequential disinfection of Escherichia coli O157:H7 inoculated alfalfa seeds before and during sprouting using aqueous chlorine dioxide, ozonated water, and thyme essential oil. LWT 2003, 36, 235–243. [Google Scholar] [CrossRef]
- Kim, C.; Hung, Y.C.; Brackett, R.E.; Lin, C.S. Efficacy of electrolyzed oxidizing water in inactivating Salmonella on alfalfa seeds and sprouts. J. Food Prot. 2003, 66, 208–214. [Google Scholar] [CrossRef]
- Zhang, H.; Tikekar, R.V. Air microbubble assisted washing of fresh produce: Effect on microbial detachment and inactivation. Postharvest Biol. Technol. 2021, 181, 111687. [Google Scholar] [CrossRef]
- Jaquette, C.B.; Beuchat, L.R.; Mahon, B.E. Efficacy of chlorine and heat treatment in killing Salmonella Stanley inoculated onto alfalfa seeds and growth and survival of the pathogen during sprouting and storage. Appl. Environ. Microbiol. 1996, 62, 2212–2215. [Google Scholar] [CrossRef]
- Yao, S.; LiBrizzi, B.R.; Chen, H. Heating temperature and water activity of alfalfa seeds affect thermal inactivation of Salmonella and maintaining seed viability. Intl. J. Food Microbiol. 2023, 384, 109975. [Google Scholar] [CrossRef]
- Mohammad, Z.; Kalbasi-Ashtari, A.; Riskowski, G.; Juneja, V.; Castillo, A. Inactivation of Salmonella and Shiga toxin-producing Escherichia coli (STEC) from the surface of alfalfa seeds and sprouts by combined antimicrobial treatments using ozone and electrolyzed water. Food Res. Int. 2020, 136, 109488. [Google Scholar] [CrossRef]
- Hong, E.J.; Park, S.H.; Kang, D.H. Sequential treatment of hydrogen peroxide, vacuum packaging, and dry heat for inactivating Salmonella Typhimurium on alfalfa seeds without detrimental effect on seeds viability. Food Microbiol. 2019, 77, 130–136. [Google Scholar] [CrossRef]
- ISO 20480-1; Fine Bubble Technology—General Principles for Usage and Measurement of Fine Bubbles. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/68187.html (accessed on 22 October 2025).
- Sharma, P.K.; Gibcus, M.J.; van der Mei, H.C.; Busscher, H.J. Influence of fluid shear and microbubbles on bacterial detachment from a surface. Appl. Environ. Microbiol. 2005, 71, 3668–3673. [Google Scholar] [CrossRef]
- Parmar, R.; Majumder, S.K. Microbubble generation and microbubble-aided transport process intensification—A state-of-the-art report. Chem. Eng. Process. Process Intensif. 2013, 64, 79–97. [Google Scholar] [CrossRef]
- Chuajedton, A.; Aoyagi, H.; Uthaibutra, J.; Pengphol, S.; Whangchai, K. Inactivation of Escherichia coli O157: H7 by treatment with different temperatures of micro-bubbles ozone containing water. Intl. Food Res. J. 2017, 24, 1006–1010. [Google Scholar]
- Hou, C.Y.; Chen, Y.R.; Wu, J.S.; Chen, H.L.; Hsiao, C.P.; Liu, C.T.; Lin, C.M. Antibacterial efficacy and physiochemical effects of ozone microbubble water on tomato. Sustainability 2022, 14, 6549. [Google Scholar] [CrossRef]
- Ikeura, H.; Hamasaki, S.; Tamaki, M. Effects of ozone microbubble treatment on removal of residual pesticides and quality of persimmon leaves. Food Chem. 2013, 138, 366–371. [Google Scholar] [CrossRef]
- Klintham, P.; Tongchitpakdee, S.; Chinsirikul, W.; Mahakarnchanakul, W. Combination of microbubbles with oxidizing sanitizers to eliminate Escherichia coli and Salmonella Typhimurium on Thai leafy vegetables. Food Control 2017, 77, 260–269. [Google Scholar] [CrossRef]
- Kwack, Y.; Kim, K.K.; Hwang, H.; Chun, C. An ozone micro-bubble technique for seed sterilization in alfalfa sprouts. Korean J. Hortic. Sci. Technol. 2014, 32, 901–905. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Kaavya, R.; Jayanath, Y.; Veenuttranon, K.; Lueprasitsakul, P.; Divya, V.; Kothakota, A.; Ramesh, S.V. Ozone as a novel emerging technology for the dissipation of pesticide residues in foods–a review. Trends Food Sci. Technol. 2020, 97, 38–54. [Google Scholar] [CrossRef]
- Phaephiphat, A.; Mahakarnchanakul, W. Surface decontamination of Salmonella Typhimurium and Escherichia coli on sweet basil by ozone microbubbles. Cogent Food Agric. 2018, 4, 1558496. [Google Scholar] [CrossRef]
- Chen, H.-L.; Arcega, R.D.; Liao, P.-Y.; Hou, C.-Y.; Liu, W.-C.; Chen, Y.-R.; Wu, J.-S.; Wang, W.-R.; Lin, C.-M. Reduction of pesticides and bacteria on Napa cabbage by ozone microbubble water. Postharvest Biol. Technol. 2023, 204, 112444. [Google Scholar] [CrossRef]
- Klintham, P.; Tongchitpakdee, S.; Chinsirikul, W.; Mahakarnchanakul, W. Two-step washing with commercial vegetable washing solutions, and electrolyzed oxidizing microbubbles water to decontaminate sweet basil and Thai mint: A case study. Food Control 2018, 94, 324–330. [Google Scholar] [CrossRef]
- Lin, C.M.; Chen, S.Y.; Lin, Y.T.; Hsiao, C.P.; Liu, C.T.; Hazeena, S.H.; Wu, J.S.; Hou, C.Y. Inactivating Salmonella Enteritidis on shell eggs by using ozone microbubble water. Intl. J. Food Microbiol. 2023, 398, 110213. [Google Scholar] [CrossRef] [PubMed]
- Rozman, U.; Pušnik, M.; Kmetec, S.; Duh, D.; Šostar Turk, S. Reduced susceptibility and increased resistance of bacteria against disinfectants: A systematic review. Microorganisms 2021, 9, 2550. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, B.; Zhu, W.; Tian, K.; Zhang, H. A Review on ultrasonic catalytic microbubbles ozonation processes: Properties, hydroxyl radicals generation pathway and potential in application. Catalysts 2019, 9, 10. [Google Scholar] [CrossRef]
- Inatsu, Y.; Kitagawa, T.; Nakamura, N.; Kawasaki, S.; Nei, D.; Bari, M.L.; Kawamoto, S. Effectiveness of stable ozone microbubble water on reducing bacteria on the surface of selected leafy vegetables. Food Sci. Technol. Res. 2011, 17, 479–485. [Google Scholar] [CrossRef]
- Fransisca, L.; Feng, H. Effect of surface roughness on inactivation of Escherichia coli O157:H7 by new organic acid-surfactant combinations on alfalfa, broccoli, and radish seeds. J. Food Prot. 2012, 75, 261–269. [Google Scholar] [CrossRef]
- Machado-Moreira, B.; Tiwari, B.K.; Richards, K.G.; Abram, F.; Burgess, C.M. Application of plasma activated water for decontamination of alfalfa and mung bean seeds. Food Microbiol. 2021, 96, 103708. [Google Scholar] [CrossRef] [PubMed]


| Treatments | CMB | SMB | ||
|---|---|---|---|---|
| Washing time | 10 min | 20 min | 30 min | 20 min |
| Untreated | 5.50 ± 0.09 a | 5.50 ± 0.09 a | 5.50 ± 0.09 a | 5.48 ± 0.18 a |
| Water soaking | 5.13 ± 0.15 b (0.37) | 5.13 ± 0.15 b (0.37) | 5.13 ± 0.15 a (0.37) | 5.13 ± 0.15 ab (0.37) |
| Water washing | 5.08 ± 0.17 Ab (0.42) | 4.93 ± 0.36 Aab (0.57) | 4.50 ± 0.71 Ab (1.05) | 5.01 ± 0.14 ab (0.47) |
| MB | 5.21 ± 0.67 Aab (0.29) | 5.48 ± 0.27 Aa (0.02) | 5.32 ± 0.15 Aa (0.18) | 5.46 ± 0.27 Ab (0.02) |
| ClO2 | 4.70 ± 0.23 Abc (0.80) | 4.37 ± 0.45 Ab (1.13) | 3.34 ± 0.14 Bc (2.16) | - |
| CMB | 4.22 ± 0.21 Ac (1.28) | 2.25 ± 0.27 Bc (3.25) | 2.05 ± 0.30 Bd (3.45) | - |
| SAEW | - | - | - | 4.60 ± 0.04 Ab (0.88) |
| SMB | - | - | - | 1.65 ± 0.77 Bc (3.83) |
| Treatments | Washing Time | pH | EC (μs/cm) | ORP (mV) | Temperature (°C) |
|---|---|---|---|---|---|
| Water washing | 10 min | 8.0 ± 0.0 a | 342.3 ± 5.5 c | 459.3 ± 14.8 b | 25.2 ± 0.1 b |
| MB | 8.2 ± 0.1 a | 375.0 ± 18.1 c | 433.3 ± 92.1 b | 32.6 ± 0.2 a | |
| ClO2 | 6.2 ± 0.0 c | 764.3 ± 16.9 b | 660.3 ± 4.6 a | 25.1 ± 0.4 b | |
| CMB | 7.2 ± 0.1 b | 848.7 ± 5.5 a | 645.7 ± 8.6 a | 34.7 ± 0.2 a | |
| Water washing | 20 min | 7.9 ± 0.0 a | 397.0 ± 76.7 c | 441.3 ± 39.8 b | 26.2 ± 1.3 b |
| MB | 8.2 ± 0.0 a | 384.3 ± 2.3 c | 251.3 ± 54.3 c | 37.2 ± 0.3 a | |
| ClO2 | 6.1 ± 0.3 c | 1175.0 ± 36.4 b | 668.0 ± 9.5 a | 26.4 ± 0.9 b | |
| CMB | 7.1 ± 0.2 b | 1321.7 ± 37.6 a | 651.8 ± 14.8 a | 39.0 ± 1.6 a | |
| Water washing | 30 min | 8.1 ± 0.0 a | 405.0 ± 35.2 c | 477.3 ± 29.7 c | 26.0 ± 2.5 b |
| MB | 8.4 ± 0.0 a | 418.7 ± 9.1 c | 272.3 ± 59.5 d | 40.4 ± 0.9 a | |
| ClO2 | 6.1 ± 0.1 c | 1421.0 ± 29.6 b | 664.7 ± 4.5 a | 26.8 ± 0.1 b | |
| CMB | 7.2 ± 0.2 b | 1697.5 ± 34.4 a | 634.0 ± 14.6 b | 43.5 ± 0.9 a | |
| Water washing | 20 min | 8.0 ± 0.1 a | 397.0 ± 76.7 b | 411.3 ± 39.8 b | 26.2 ± 1.3 b |
| MB | 8.3 ± 0.1 a | 410.7 ± 8.6 c | 275.3 ± 35.5 d | 36.4 ± 0.6 a | |
| SAEW | 6.1 ± 0.3 b | 4026.7 ± 922.0 a | 952.3 ± 23.0 a | 26.6 ± 0.2 b | |
| SMB | 6.5 ± 0.4 b | 4520.0 ± 739.0 a | 942.7 ± 32.6 a | 35.5 ± 2.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, C.-Y.; Chou, S.-K.; Wu, J.-S.; Chen, H.-L.; Zhang, P.-W.; Liu, C.-T.; Hsiao, C.-P.; Lin, C.-M. Application of Microbubbles Combining with Disinfectants to Inactivate Salmonella Typhimurium on Alfalfa Seeds and the Effects on Sprouting. Seeds 2025, 4, 51. https://doi.org/10.3390/seeds4040051
Hou C-Y, Chou S-K, Wu J-S, Chen H-L, Zhang P-W, Liu C-T, Hsiao C-P, Lin C-M. Application of Microbubbles Combining with Disinfectants to Inactivate Salmonella Typhimurium on Alfalfa Seeds and the Effects on Sprouting. Seeds. 2025; 4(4):51. https://doi.org/10.3390/seeds4040051
Chicago/Turabian StyleHou, Chih-Yao, Shih-Kao Chou, Jong-Shinn Wu, Hsiu-Ling Chen, Pei-Wen Zhang, Chih-Tung Liu, Chun-Ping Hsiao, and Chia-Min Lin. 2025. "Application of Microbubbles Combining with Disinfectants to Inactivate Salmonella Typhimurium on Alfalfa Seeds and the Effects on Sprouting" Seeds 4, no. 4: 51. https://doi.org/10.3390/seeds4040051
APA StyleHou, C.-Y., Chou, S.-K., Wu, J.-S., Chen, H.-L., Zhang, P.-W., Liu, C.-T., Hsiao, C.-P., & Lin, C.-M. (2025). Application of Microbubbles Combining with Disinfectants to Inactivate Salmonella Typhimurium on Alfalfa Seeds and the Effects on Sprouting. Seeds, 4(4), 51. https://doi.org/10.3390/seeds4040051