Dormancy Breaking of Teramnus labialis (L.f.) Spreng Seeds Is Affected by the Extent of Liquid Nitrogen Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Material
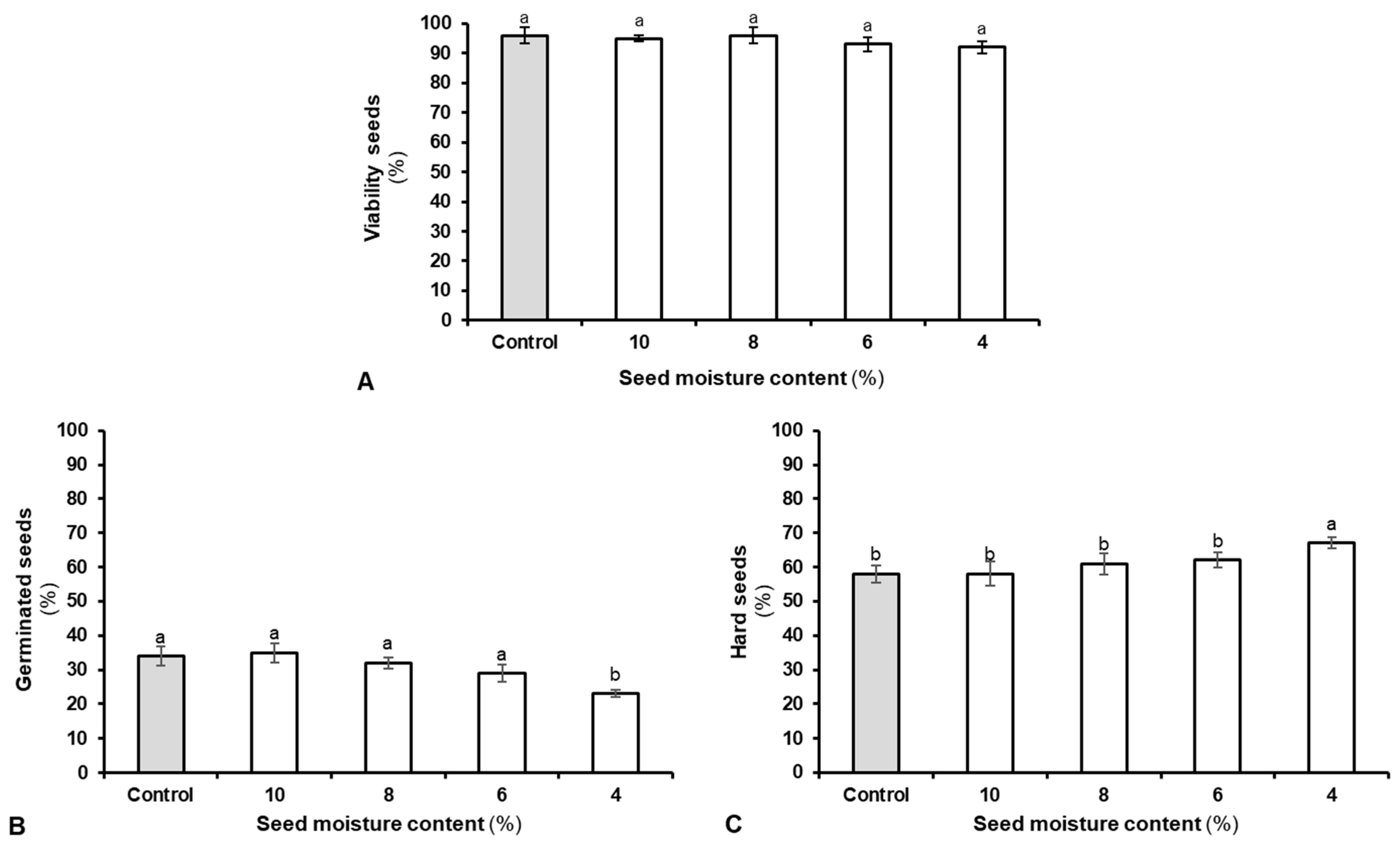
2.2. Seed Physiological Evaluations
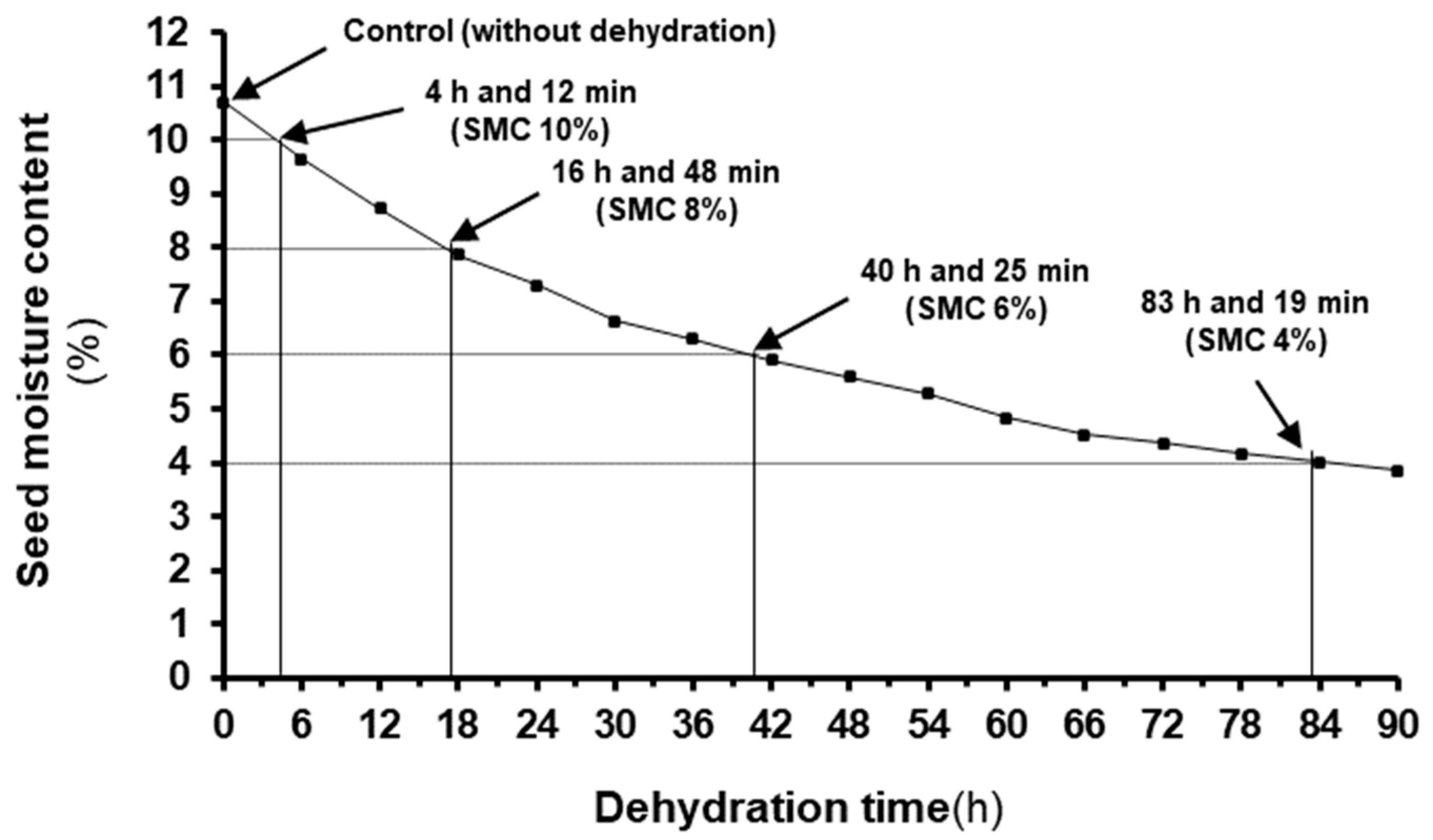
2.3. Seed Drying Experiments
2.4. LN Scarification Experiments
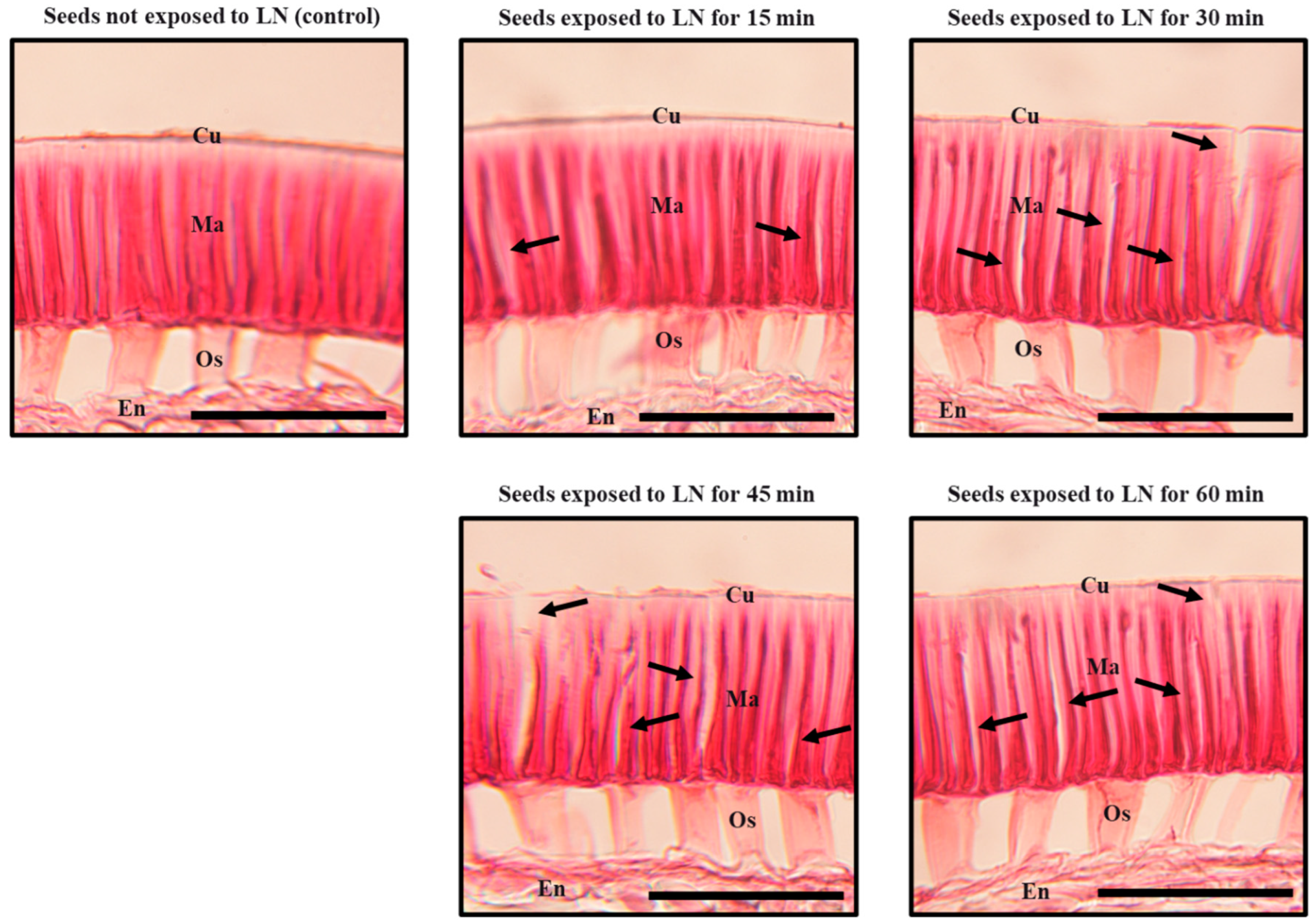
2.5. Histological Evaluation of Seed Coat
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutta, A.; Trivedi, A.; Nath, C.P.; Gupta, D.S.; Hazra, K.K. A comprehensive review on grain legumes as climate-smart crops: Challenges and prospects. Environ. Chall. 2022, 7, 100479. [Google Scholar] [CrossRef]
- Bermúdez-Torres, K.; Ferval, M.; Hernández-Sánchez, A.M.; Tei, A.; Gers, C.; Wink, M.; Legal, L. Molecular and chemical markers to illustrate the complex diversity of the genus lupinus (Fabaceae). Diversity 2021, 13, 263. [Google Scholar] [CrossRef]
- Díaz, A.; Martín, P.C.; Castillo, E.; Hernández, J.L. Supplementation of yearlings Cuban charolais grazing multiple associations of herbaceous legumes and tropical grasses. Cuba. J. Agric. Sci. 2012, 46, 249–252. [Google Scholar]
- Alagumanivasagam, G.; Muthu, A.K.; Kumar, D.S.; Suresh, K.; Manavalan, R. In vivo antioxidant and lipid peroxidation effect of methanolic extract of whole plant of Teramnus labialis (Linn.) in rat fed with high fat diet. Int. J. PharmTech Res. 2012, 4, 1233–1237. [Google Scholar]
- Acosta Fernández, Y.; González Morales, A.; Fernandes, P.; Mazorra Calero, C.; Fontes Marrero, D. Quality of Teramnus labialis (L.f.) Spreng seeds harvest in Ciego de Avila, Cuba. Univ. Cienc. 2022, 11, 118–132. [Google Scholar]
- Acosta Fernández, Y.; Fontes Marrero, D.; Martínez Melo, J.; Mazorra Calero, C. Perspectivas de Teramnus labialis (L.f.) Spreng para el desarrollo de sistemas agrícolas en Cuba. Rev. Prod. Anim. 2021, 33, 71–82. [Google Scholar]
- González, Y.; Mendoza, F. Momento de cosecha de las semillas de Teramnus labialis cv. Semilla Clara. Pastos Forrajes 1995, 18, 239–244. [Google Scholar]
- Morris, J.B.; Wang, M.L. Updated review of potential medicinal genetic resources in the USDA, ARS, PGRCU industrial and legume crop germplasm collections. Ind. Crops Prod. 2018, 123, 470–479. [Google Scholar] [CrossRef]
- Acosta, Y.; Hernández, L.; Mazorra, C.; Quintana, N.; Zevallos, B.; Lorenzo, J.C.; Martínez-Montero, M.E.; Fontes, D. Seed cryostorage enhances subsequent plant productivity in the forage species Teramnus labialis (L.f.) Spreng. CryoLetters 2019, 40, 36–44. [Google Scholar]
- Acosta, Y.; Pérez, L.; Escalante, D.; Nápoles, L.; Concepción, O.; Pérez, A.; Pérez, L.S.; Martínez-Montero, M.E.; Fontes, D.; Lorenzo, J.C. Dormancy breaking in Teramnus labialis (L.f.) Spreng seeds through liquid nitrogen exposure is based on the modification of the hilar region, cuticle, and macrosclereid. Acta Physiol. Plant. 2020, 42, 144. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. The great diversity in kinds of seed dormancy: A revision of the Nikolaeva-Baskin classification system for primary seed dormancy. Seed Sci. Res. 2021, 31, 249–277. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Kimura, E.; Islam, M.A. Seed scarification methods and their use in forage legumes. Res. J. Seed Sci. 2012, 5, 38–50. [Google Scholar] [CrossRef]
- Schnadelbach, A.; Veiga-Barbosa, L.; Ruiz, C.; Pita, J.M.; Pérez-García, F. Dormancy breaking and germination of Adenocarpus desertorum, Astragalus gines-lopezii and Hippocrepis grosii (Fabaceae) seeds, three threatened endemic Spanish species. Seed Sci. Technol. 2016, 44, 1–14. [Google Scholar] [CrossRef]
- Chaves Neto, J.R.; Rodrigues, F.S.; Luft, L.; Confortin, T.C.; Todero, I.; dos Santos, M.S.N.; Zabot, G.L.; Mazutti, M.A.; Tres, M.V. Performance of distinct strategies on overcoming dormancy in Senna obtusifolia seeds. Rev. Eng. Agric.-REVENG 2022, 30, 383–391. [Google Scholar] [CrossRef]
- Carruggio, F.; Onofri, A.; Impelluso, C.; Giusso del Galdo, G.; Scopece, G.; Cristaudo, A. Seed dormancy breaking and germination in Bituminaria basaltica and B. bituminosa (Fabaceae). Plants 2020, 9, 1110. [Google Scholar] [CrossRef]
- Muñoz, B.C.; Sánchez, J.; Montejo, L.A.; González, Y.; Reino, J. Germinative evaluation of 20 legume accessions stored under unfavorable conditions. Pastos Forrajes 2009, 32, 263–276. [Google Scholar]
- Mazorra-Calero, C.A.; Fontes-Marrero, D.; Donis-García, L.H.; Martínez-Melo, J.; Acosta-Fernández, Y.; Espinosa-Alemán, I.; Lavinge, C.; Fernandes, P.; González-Morales, A. Technological and socioeconomic diagnosis of the establishment of Psidium guajava L. and Teramnus labialis in Ciego de Ávila, Cuba. Pastos Forrajes 2016, 39, 259–264. [Google Scholar]
- González, Y.; Mendoza, F. Comportamiento de la germinación de Teramnus labialis Cv. Semilla Clara. II. Tratamientos antes de almacenar. Pastos Forrajes 1991, 14, 227–234. [Google Scholar]
- Fontes, D.; Mazorra, C.; Pulido, L.; Cubillas, N.; Hernández, N.; Lazo, M.; Rodríguez, L.; Rodríguez, W. Teramnus labialis: Leguminosa promisoria para la producción diversificada en fincas citrícolas. Zootec. Trop. 2008, 26, 351–354. [Google Scholar]
- Acosta, Y.; Pérez, L.; Linares, C.; Hernández, L.; Escalante, D.; Pérez, A.; Zevallos, B.; Yabor, L.; Martínez-Montero, M.E.; Cejas, I.; et al. Effects of Teramnus labialis (L.f.) Spreng seed cryopreservation on subsequent seed and seedling growth and biochemistry. Acta Physiol. Plant. 2020, 42, 7. [Google Scholar] [CrossRef]
- Acosta, Y.; Fontes, D.; Martínez-Montero, M.E. Liquid Nitrogen as promotor of seeds germination and seedling growth in tropical legumes. INGE CUC 2021, 17, 2. [Google Scholar]
- Acosta, Y.; Pérez, L.; Escalante, D.; Mazorra-Calero, C.; Martínez-Melo, J.; Martínez-Montero, M.E.; Fortes, D.; Hajari, E.; Lorenzo, J.C.; Fontes, D. Exposure of Teramnus Labialis (L.f.) Spreng seeds to Liquid Nitrogen does not affect nutritional status of field grown adult plants. CryoLetters 2021, 42, 106–110. [Google Scholar] [PubMed]
- Kholina, A.B.; Voronkova, N.M. Seed cryopreservation of some medicinal legumes. J. Bot. 2012, 2012, 186891. [Google Scholar] [CrossRef]
- Xia, L.; Wei, Y.; Hubiao, Y.; Xinyong, L.; Hengfu, H.; Rongshu, D.; Zhiyong, W.; Yiming, L. Comparison of different seed scarification methods for breaking dormancy of seeds of Vigna marina and Canavalia rosea. J. Trop. Biol. 2022, 13, 281–286. [Google Scholar] [CrossRef]
- Paula, J.C.B.D.; Guariz, H.R.; Ribeiro Júnior, W.A.; Shimizu, G.D.; Faria, R.T.D.; Oliveira, H.C.D. Cryopreservation of seeds of the Brazilian native species Aroeira-do-sertão (Astronium urundeuva M. Allemão Engl.). Rev. Caatinga 2022, 35, 915–924. [Google Scholar] [CrossRef]
- Mira, S.; Schnadelbach, A.; Correa, E.C.; Pérez-García, F.; González-Benito, M.E. Variability of physical dormancy in relation to seed mechanical properties of three legume species. Seed Sci. Technol. 2017, 45, 540–556. [Google Scholar] [CrossRef]
- Farida, A.; Rabeha, C.; Aissa, A. Effect of different scarification techniques on the germination of some endemic Medicago species in Algeria. PONTE Int. J. Sci. Res. 2020, 76, 62–74. [Google Scholar] [CrossRef]
- González-Benito, M.E.; Salinas, P.; Amigo, P. Effect of seed moisture content and cooling rate in liquid nitrogen on legume seed germination and seedling vigour. Seed Sci. Technol. 2003, 31, 423–434. [Google Scholar] [CrossRef]
- Walters, C.; Pence, V.C. The unique role of seed banking and cryobiotechnologies in plant conservation. Plants People Planet 2021, 3, 83–91. [Google Scholar] [CrossRef]
- Acharya, S.N.; Kokko, E.G.; Fraser, J. Storage duration and freeze-thaw effects on germination and emergence of cicer milkvetch (Astragalus cicer) seeds. J. Seed Technol. 1993, 17, 9–21. [Google Scholar]
- Acharya, S.N.; Stout, D.G.; Brooke, B.; Thompson, D. Cultivar and storage effects on germination and hard seed content of alfalfa. Can. J. Plant Sci. 1999, 79, 201–208. [Google Scholar] [CrossRef]
- Kameswara, N.; Hanson, J.; Dulloo, M.E.; Ghosh, K.; Novell, D.; Larinde, M. Manual Para el Manejo de Semillas en Bancos de Germoplasma, 8th ed.; Bioversity International: Rome, Italy, 2007. [Google Scholar]
- Altare, M.; Trione, S.; Guevara, J.C.; Cony, M. Stimulation and promotion of germination in Opuntia ficus-indica seeds. J. Prof. Assoc. Cactus Dev. 2006, 8, 10. [Google Scholar]
- Silva, L.J.d.; Medeiros, A.D.d.; Oliveira, A.M.S. SeedCalc, a new automated R software tool for germination and seedling length data processing. J. Seed Sci. 2019, 41, 250–257. [Google Scholar] [CrossRef]
- ISTA (Ed.) International Rules for Seed Testing; ISTA: Bassersdorf, Switzerland, 2016; p. 192. [Google Scholar]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill: London, UK; New York, NY, USA, 1940. [Google Scholar]
- Smolikova, G.; Leonova, T.; Vashurina, N.; Frolov, A.; Medvedev, S. Desiccation Tolerance as the Basis of Long-Term Seed Viability. Int. J. Mol. Sci. 2021, 22, 101. [Google Scholar] [CrossRef]
- Ballesteros, D.; Pritchard, H.W.; Walters, C. Dry architecture: Towards the understanding of the variation of longevity in desiccation-tolerant germplasm. Seed Sci. Res. 2020, 30, 142–155. [Google Scholar] [CrossRef]
- Walters, C. Orthodoxy, recalcitrance and in-between: Describing variation in seed storage characteristics using threshold responses to water loss. Planta 2015, 242, 397–406. [Google Scholar] [CrossRef]
- Walters, C.; Fleming, M.B.; Hill, L.M.; Dorr, E.J.; Richards, C.M. Stress-response relationships related to ageing and death of orthodox seeds: A study comparing viability and RNA integrity in soya bean (Glycine max) cv. Williams 82. Seed Sci. Res. 2020, 30, 161–172. [Google Scholar] [CrossRef]
- Freire, J.; Rouws, J.; Breier, T.; Ataíde, G. Drying and storage of Melanoxylon brauna Schott. Seeds. Braz. J. Biol. 2021, 81, 464–473. [Google Scholar] [CrossRef]
- Gama-Arachchige, N.S.; Baskin, J.M.; Geneve, R.L.; Baskin, C.C. Identification and characterization of the water gap in physically dormant seeds of Geraniaceae, with special reference to Geranium carolinianum. Ann. Bot. 2010, 105, 977–990. [Google Scholar] [CrossRef]
- Jaganathan, G.K.; Song, D.; Liu, W.; Han, Y.; Liu, B. Relationship between seed moisture content and acquisition of impermeability in Nelumbo nucifera (Nelumbonaceae). Acta Bot. Bras. 2017, 31, 639–644. [Google Scholar] [CrossRef]
- Jaganathan, G.K.; Han, Y.; Song, D.; Selvam, P.; Liu, B. Maternal and burial environment determine the physical dormancy release in tropical Senna auriculata (Fabaceae) seeds. J. For. Res. 2019, 30, 1343–1351. [Google Scholar] [CrossRef]
- Vidak, M.; Lazarević, B.; Javornik, T.; Šatović, Z.; Carović-Stanko, K. Seed Water Absorption, Germination, Emergence and Seedling Phenotypic Characterization of the Common Bean Landraces Differing in Seed Size and Color. Seeds 2022, 1, 324–339. [Google Scholar] [CrossRef]
- Mai-Hong, T.; Hong, T.D.; Hien, N.T.; Ellis, R.H. Onset of germinability, desiccation tolerance and hardseededness in developing seeds of Peltophorum pterocarpum (DC) K. Heyne (Caesalpinioideae). Seed Sci. Res. 2003, 13, 323–327. [Google Scholar] [CrossRef]
- Bolingue, W.; Ly, V.B.; Leprince, O.; Buitink, J. Characterization of dormancy behaviour in seeds of the model legume Medicago truncatula. Seed Sci. Res. 2010, 20, 97–107. [Google Scholar] [CrossRef]
- Qu, X.; Baskin, J.M.; Baskin, C.C. Whole-seed development in Sicyos angulatus (Cucurbitaceae, Sicyeae) and a comparison with the development of water-impermeable seeds in five other families. Plant Species Biol. 2010, 25, 185–192. [Google Scholar] [CrossRef]
- Jayasuriya, K.M.G.G.; Wijetunga, A.S.T.B.; Baskin, J.M.; Baskin, C.C. Seed dormancy and storage behaviour in tropical Fabaceae: A study of 100 species from Sri Lanka. Seed Sci. Res. 2013, 23, 257–269. [Google Scholar] [CrossRef]
- Plitta-Michalak, B.P.; Naskręt-Barciszewska, M.Z.; Barciszewski, J.; Chmielarz, P.; Michalak, M. Epigenetic Integrity of Orthodox Seeds Stored under Conventional and Cryogenic Conditions. Forests 2021, 12, 288. [Google Scholar] [CrossRef]
- Ballesteros, D.; Pence, V.C. Survival and death of seeds during liquid nitrogen storage: A case study on seeds with short lifespans. CryoLetters 2017, 38, 278–289. [Google Scholar]
- Srivastava, A.K.; Suresh Kumar, J.; Suprasanna, P. Seed ‘primeomics’: Plants memorize their germination under stress. Biol. Rev. 2021, 96, 1723–1743. [Google Scholar] [CrossRef]
- Jaganathan, G.K. Do Fabaceae species with physical dormancy occur mostly in the temperate ecosystems? A rebuttal to using global biodiversity information facility (GBIF) analysis. Plant Sci. Today 2020, 7, 109–111. [Google Scholar] [CrossRef]
- van der Walt, K.; Burritt, D.J.; Nadarajan, J. Impacts of Rapid Desiccation on Oxidative Status, Ultrastructure and Physiological Functions of Syzygium maire (Myrtaceae) Zygotic Embryos in Preparation for Cryopreservation. Plants 2022, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Cejas, I.; Rumlow, A.; Turcios, A.; Engelmann, F.; Martínez, M.E.; Yabor, L.; Papenbrock, J.; Lorenzo, J.C. Exposure of common bean seeds to liquid nitrogen modifies mineral composition of young plantlet leaves. Am. J. Plant Sci. 2016, 7, 1612–1617. [Google Scholar] [CrossRef]
- Naderi, M.A.; Jebelli, M.; Jafari, A.A. Cryopreservation of Smirnovia iranica (Sabeti) seeds and evaluation of cryopreserved seeds under laboratory, greenhouse and natural habitat conditions. J. Rangel. Sci. 2017, 7, 122–137. [Google Scholar]
- Villalobos, A.; Campbell, R.; Díaz, R.; Martínez, J.; Escalante, D.; Martinez-Montero, M.E.; Quintana, N.; Yabor, L.; Hoefer, M.; Sershen; et al. Chickpea seed cryostorage alters germinant but not adult plant growth. Biologia 2020, 76, 55–61. [Google Scholar] [CrossRef]
- Zevallos, B.; Cejas, I.; Engelmann, F.; Carputo, D.; Scarano, M.T.; Yanes, E.; Martínez-Montero, M.E.; Lorenzo, J.C. Phenotypic and molecular characterization of plants regenerated from non-cryopreserved and cryopreserved wild Solanum lycopersicum Mill. seeds. CryoLetters 2014, 35, 216–225. [Google Scholar]
- Stegani, V.; Alves, G.A.C.; Bertoncelli, D.J.; de Faria, R.T.V. Criopreservação de sementes de rainha do abismo (Sinningia leucotricha). Rev. Bras. Hortic. Ornam. 2017, 23, 15–21. [Google Scholar] [CrossRef]
- Vettorazzi, R.G.; Carvalho, V.S.; Teixeira, M.C.; Campostrini, E.; Da Cunha, M.; de Matos, E.M.; Viccini, L.F. Cryopreservation of immature and mature seeds of Brazilian orchids of the genus Cattleya. Sci. Hortic. 2019, 256, 108603. [Google Scholar] [CrossRef]
- Villalobos, A.; Arguedas, M.; Escalante, D.; Martínez, J.; Zevallos, B.E.; Cejas, I.; Yabor, L.; Martínez-Montero, M.E.; Sershen; Lorenzo, J.C. Cryopreservation of Sorghum seeds modifies germination and seedling growth but not field performance of adult plants. J. Appl. Bot. Food Qual. 2019, 92, 94–99. [Google Scholar] [CrossRef]
- Vendrame, W.; Takane, R.; Cardoso, L.; Alvarez, L.; Tadeu, R. Cryopreservation of seeds of Melocactus zehntneri Braun ex Ritter f. and Cereus gounellei Luetzelb ex Schum k. by the vitrification method. Agron. Sci. Biotechnol. 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Jastrzębowski, S.; Ukalska, J.; Kantorowicz, W.; Klisz, M.; Wojda, T.; Sułkowska, M. Effects of thermal-time artificial scarification on the germination dynamics of black locust (Robinia pseudoacacia L.) seeds. Eur. J. For. Res. 2017, 136, 471–479. [Google Scholar] [CrossRef]
- Wu, G.; Jaganathan, G.K.; Song, D.; Liu, B. Cryopreservation of selected physical dormant species with special focus on dormancy breaking time. Res. J. Seed Sci. 2017, 10, 38–42. [Google Scholar]
- Pullman, G.S.; Bucalo, K.; Determann, R.O.; Cruse-Sanders, J.M. Seed Cryopreservation and Germination of Rhus glabra and the Critically Endangered Species Rhus michauxii. Plants 2021, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; De la Cuadra, C. Evaluation of different scarification methods to remove hardseededness in Trifolium subterraneum and Medicago polymorpha accessions of the Spanish base genebank. Seed Sci. Technol. 2004, 32, 671–681. [Google Scholar] [CrossRef]
- Issac, M.; Kuriakose, P.; Leung, S.; Costa, A.B.; Johnson, S.; Bucalo, K.; Stober, J.M.; Determann, R.O.; Rogers, W.L.; Cruse-Sanders, J.M.; et al. Seed Cryopreservation, Germination, and Micropropagation of Eastern Turkeybeard, Xerophyllum asphodeloides (L.) Nutt.: A Threatened Species from the Southeastern United States. Plants 2021, 10, 1462. [Google Scholar] [CrossRef]
- Pritchard, H.W.; Manger, K.R.; Prendergast, F.G. Changes in Trifolium arvense seed quality following alternating temperature treatment using liquid nitrogen. Ann. Bot. 1988, 62, 1–11. [Google Scholar] [CrossRef]
| Seed Moisture Content (%) | Immersion Time (min) | Germinated Seeds (%) | Hard Seeds (%) | Dead Seeds (%) | MGT (Days) | GI (Seeds Days−1) | T50 (Days) |
|---|---|---|---|---|---|---|---|
| 10 | 15 | 75 ± 1.9 (b) | 11 ± 1.01 (b) | 14 ± 0.98 (a) | 9.12 ± 0.56 (a) | 1.06 ± 0.09 (b) | 7.93 ± 0.48 (a) |
| 30 | 87 ± 1.63 (a) | 0 ± 0.00 (c) | 13 ± 1.02 (a) | 2.81 ± 0.21 (b) | 7.56 ± 0.63 (a) | 1.88 ± 0.09 (b) | |
| 45 | 88 ± 2.51 (a) | 0 ± 0.00 (c) | 12 ± 1.03 (a) | 2.64 ± 0.19 (b) | 6.97 ± 0.61 (a) | 1.93 ± 0.18 (b) | |
| 60 | 85 ± 2.58 (a) | 0 ± 0.00 (c) | 15 ± 1.11 (a) | 2.86 ± 0.25 (b) | 7.15 ± 0.58 (a) | 1.99 ± 0.12 (b) | |
| 8 | 15 | 70 ± 2.46 (b) | 22 ± 1.93 (a) | 8 ± 0.31 (a) | 9.06 ± 0.45 (a) | 1.02 ± 0.08 (b) | 7.85 ± 0.66 (a) |
| 30 | 91 ± 1.91 (a) | 0 ± 0.00 (c) | 9 ± 0.68 (a) | 2.78 ± 0.16 (b) | 7.28 ± 0.45 (a) | 1.86 ± 0.15 (b) | |
| 45 | 90 ± 1.92 (a) | 0 ± 0.00 (c) | 13 ± 1.01 (a) | 2.89 ± 0.22 (b) | 7.76 ± 0.65 (a) | 1.95 ± 0.12 (b) | |
| 60 | 90 ± 1.23 (a) | 0 ± 0.00 (c) | 10 ± 0.58 (a) | 2.68 ± 0.21 (b) | 7.11 ± 0.57 (a) | 1.96 ± 0.11 (b) | |
| 6 | 15 | 74 ± 2.26 (b) | 16 ± 1.35 (ab) | 14 ± 1.12 (a) | 9.05 ± 0.49 (a) | 1.12 ± 0.09 (b) | 7.44 ± 0.61 (a) |
| 30 | 89 ± 2.56 (a) | 0 ± 0.00 (c) | 11 ± 1.01 (a) | 2.61 ± 0.23 (b) | 7.31 ± 0.38 (a) | 1.94 ± 0.15 (b) | |
| 45 | 87 ± 2.58 (a) | 0 ± 0.00 (c) | 13 ± 1.11 (a) | 2.74 ± 0.25 (b) | 7.05 ± 0.45 (a) | 1.98 ± 0.13 (b) | |
| 60 | 89 ± 2.58 (a) | 0 ± 0.00 (c) | 11 ± 0.99 (a) | 2.63 ± 0.21 (b) | 7.28 ± 0.38 (a) | 1.89 ± 0.12 (b) | |
| 4 | 15 | 67 ± 4.12 (b) | 22 ± 1.83 (a) | 11 ± 0.98 (a) | 8.86 ± 0.37 (a) | 0.95 ± 0.09 (b) | 7.45 ± 0.52 (a) |
| 30 | 88 ± 2.13 (a) | 0 ± 0.00 (c) | 12 ± 1.03 (a) | 2.58 ± 0.16 (b) | 6.98 ± 0.56 (a) | 1.89 ± 0.14 (b) | |
| 45 | 89 ± 1.63 (a) | 0 ± 0.00 (c) | 11 ± 0.52 (a) | 2.63 ± 0.23 (b) | 7.01 ± 0.51 (a) | 1.86 ± 0.11 (b) | |
| 60 | 86 ± 1.91 (a) | 0 ± 0.00 (c) | 14 ± 1.38 (a) | 2.55 ± 0.28 (b) | 7.26 ± 0.57 (a) | 1.81 ± 0.15 (b) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta Fernández, Y.; Pérez Gómez, L.; Fontes Marrero, D.; Martinez Montero, M.E. Dormancy Breaking of Teramnus labialis (L.f.) Spreng Seeds Is Affected by the Extent of Liquid Nitrogen Exposure. Seeds 2023, 2, 138-148. https://doi.org/10.3390/seeds2010011
Acosta Fernández Y, Pérez Gómez L, Fontes Marrero D, Martinez Montero ME. Dormancy Breaking of Teramnus labialis (L.f.) Spreng Seeds Is Affected by the Extent of Liquid Nitrogen Exposure. Seeds. 2023; 2(1):138-148. https://doi.org/10.3390/seeds2010011
Chicago/Turabian StyleAcosta Fernández, Yanier, Lianny Pérez Gómez, Dayami Fontes Marrero, and Marcos Edel Martinez Montero. 2023. "Dormancy Breaking of Teramnus labialis (L.f.) Spreng Seeds Is Affected by the Extent of Liquid Nitrogen Exposure" Seeds 2, no. 1: 138-148. https://doi.org/10.3390/seeds2010011
APA StyleAcosta Fernández, Y., Pérez Gómez, L., Fontes Marrero, D., & Martinez Montero, M. E. (2023). Dormancy Breaking of Teramnus labialis (L.f.) Spreng Seeds Is Affected by the Extent of Liquid Nitrogen Exposure. Seeds, 2(1), 138-148. https://doi.org/10.3390/seeds2010011







