The Influence of Blue and Red Light on Seed Development and Dormancy in Nicotiana tabacum L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth and Cultivation of Tobacco Plants
2.2. Membranes Isolation and Solubilization
2.3. Denaturing Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4. Absorption Spectroscopy
2.5. Photosynthetic Activity
2.6. Induction of Etiolation
2.7. Germination Tests
2.8. Spectral Analysis of Light
2.9. Data Analysis
3. Results
3.1. In the Dark, Ovaries but Not Sepals Undergo Etiolation
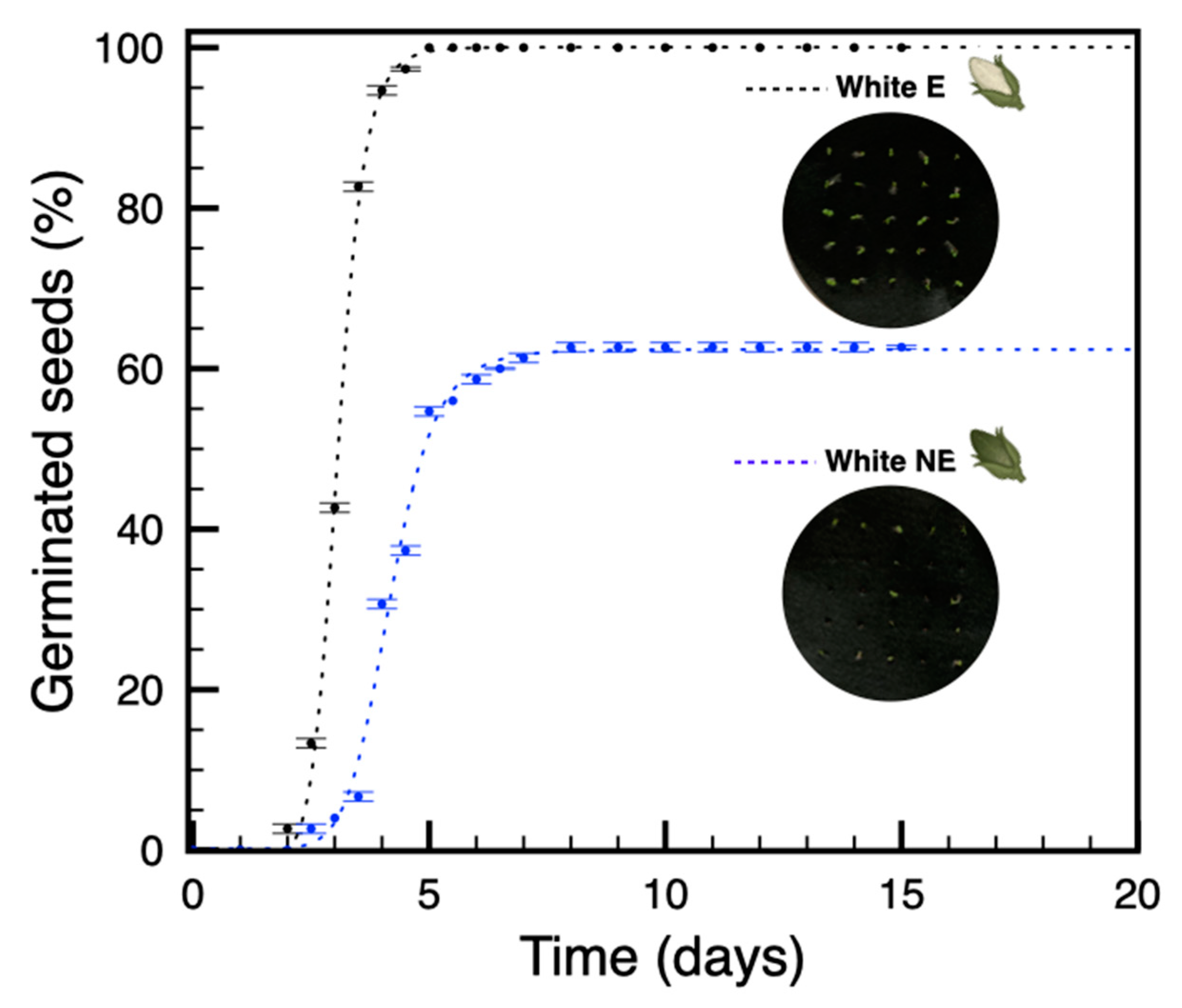
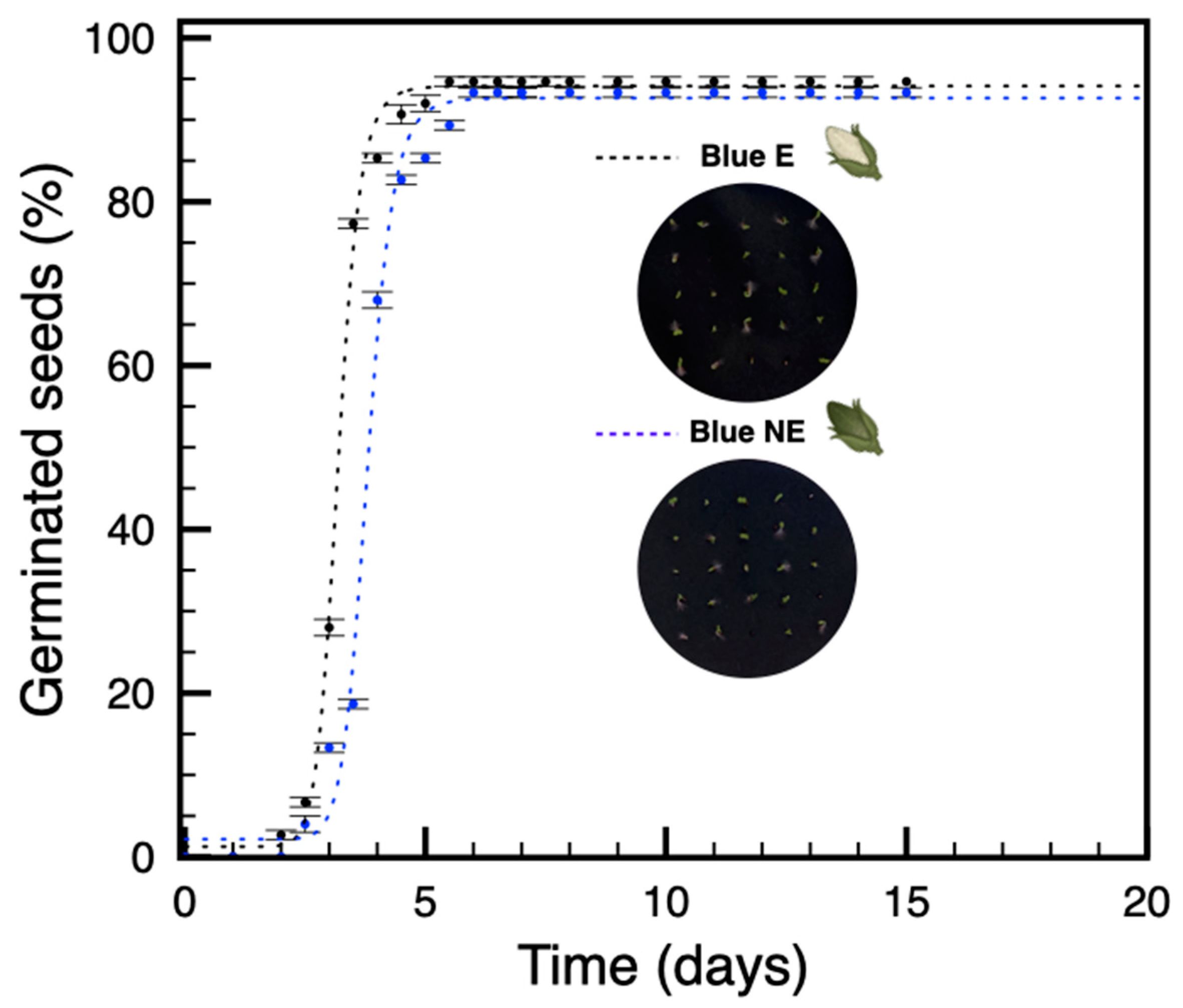
3.2. Seeds from Plants Grown under Blue Light Are Not Dormant
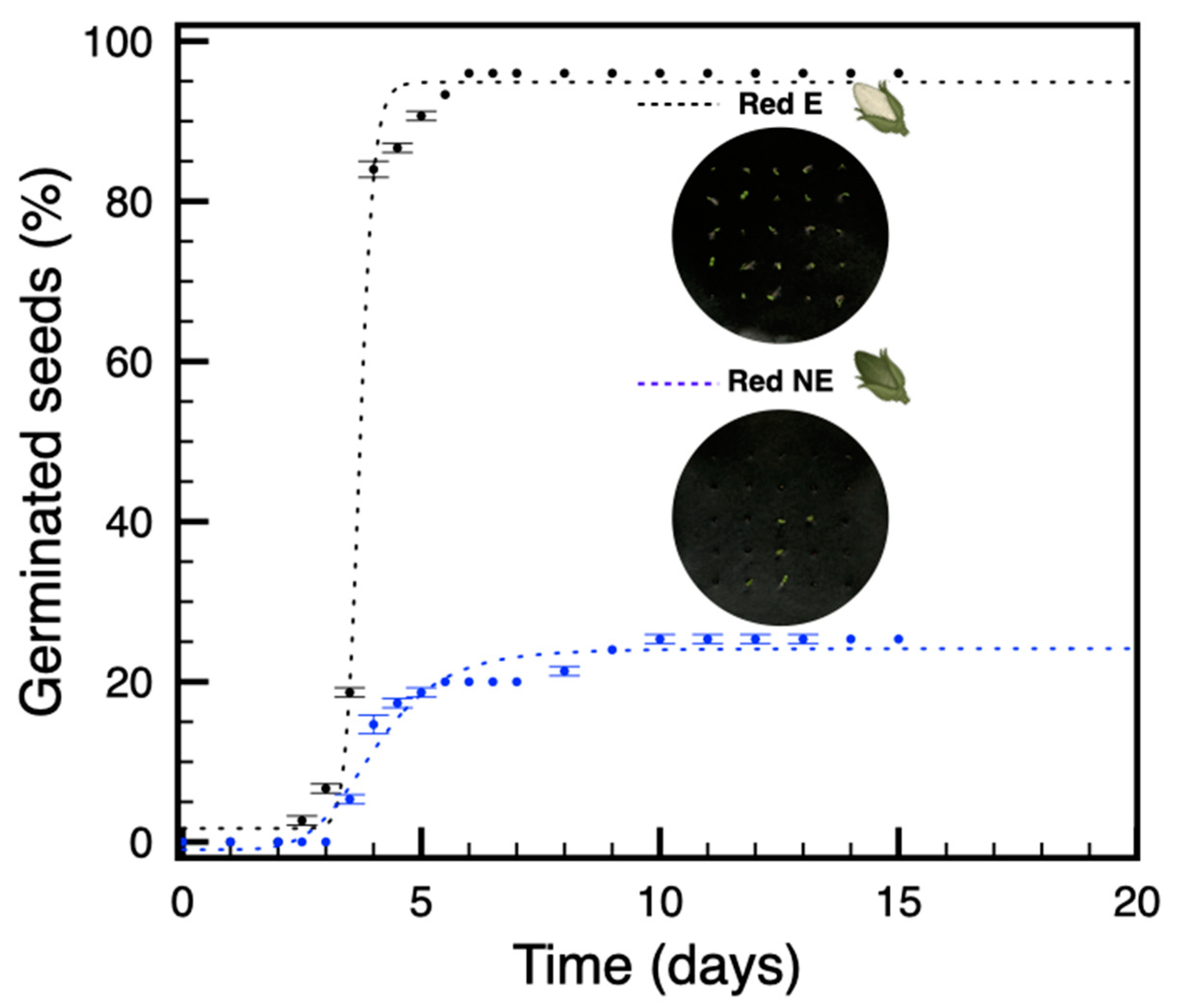
3.3. In Plants Grown under Red Light, Only Seeds from Etiolated Capsules Are Not Dormant
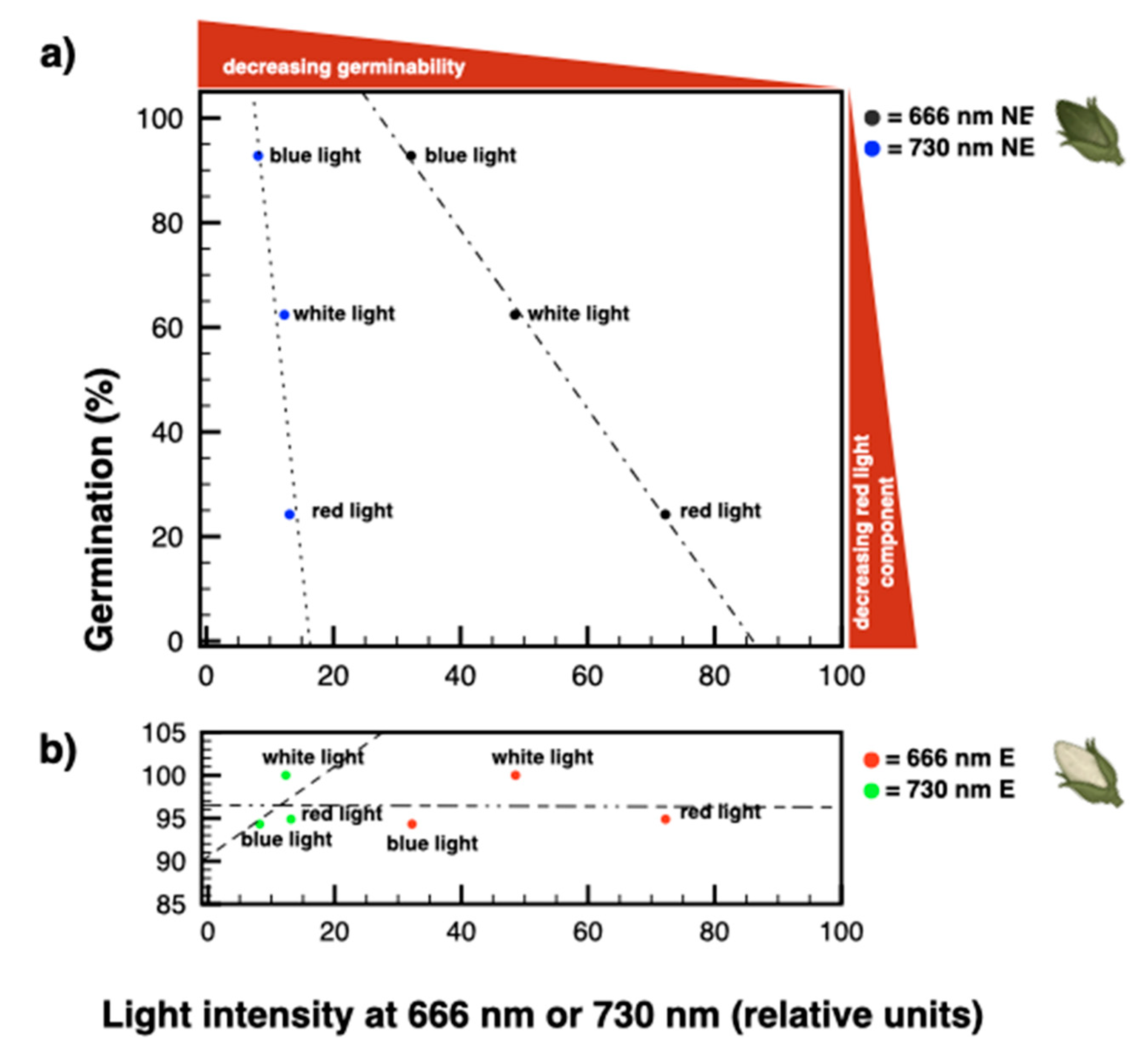
3.4. The Amount and Quality of Red Light Induce a Proportional Increase of Dormancy in Seeds from Non-Etiolated Capsules
3.5. Plant Growth under Different Lights Has Comparable Photosynthetic Rates
4. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Möglich, A.; Yang, X.; Ayers, R.A.; Moffat, K. Structure and Function of Plant Photoreceptors. Annu. Rev. Plant Biol. 2010, 61, 21–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petroutsos, D.; Tokutsu, R.; Maruyama, S.; Flori, S.; Greiner, A.; Magneschi, L.; Cusant, L.; Kottke, T.; Mittag, M.; Hegemann, P.; et al. A blue-light photoreceptor mediates the feedback regulation of photosynthesis. Nature 2016, 537, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-Regulated Plant Growth and Development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [PubMed] [Green Version]
- Collu, G.; Farci, D.; Esposito, F.; Pintus, F.; Kirkpatrick, J.; Piano, D. New insights into the operative network of FaEO, an enone oxidoreductase from Fragaria x ananassa Duch. Plant Mol. Biol. 2017, 94, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ljung, K.; Breton, G.; Schmitz, R.J.; Pruneda-Paz, J.; Cowing-Zitron, C.; Cole, B.J.; Ivans, L.J.; Pedmale, U.V.; Jung, H.-S.; et al. Linking photoreceptor excitation to changes in plant architecture. Genes Dev. 2012, 26, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Paik, I.; Huq, E. Plant photoreceptors: Multi-functional sensory proteins and their signaling networks. Semin. Cell Dev. Biol. 2019, 92, 114–121. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Piano, D.; Cocco, E.; Guadalupi, G.; Kalaji, H.M.; Kirkpatrick, J.; Farci, D. Characterization under quasi-native conditions of the capsanthin/capsorubin synthase from Capsicum annuum L. Plant Physiol. Biochem. 2019, 143, 165–175. [Google Scholar] [CrossRef]
- Contreras, S.; Bennett, M.A.; Metzger, J.D.; Tay, D. Maternal Light Environment During Seed Development Affects Lettuce Seed Weight, Germinability, and Storability. HortScience 2008, 43, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.-W.; Liu, S.; Lin, R. The role of light in regulating seed dormancy and germination. J. Integr. Plant Biol. 2020, 62, 1310–1326. [Google Scholar] [CrossRef]
- Galvão, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roso, R.; Nunes, U.R.; Müller, C.A.; Paranhos, J.T.; Lopes, S.J.; Dornelles, S.H.B.; Bertagnolli, C.M.; Huth, C.; Forte, C.T.; Menegaes, J.F. Light quality and dormancy overcoming in seed germination of Echium plantagineum L. (Boraginaceae). Braz. J. Biol. 2021, 81, 650–656. [Google Scholar] [CrossRef]
- Hernández-Adasme, C.; Silva, H.; Escalona, V. Germination of green and red lettuce cultivars is influenced by LED light spectrum. Acta Hortic. 2022, 1337, 361–366. [Google Scholar] [CrossRef]
- Nemahunguni, N.K.; Gupta, S.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. The effect of biostimulants and light wavelengths on the physiology of Cleome gynandra seeds. Plant Growth Regul. 2019, 90, 467–474. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, Z.; Wei, Z.; Hu, X. Effect of maternal environment on seed germination and seed yield components of Thlaspi arvense. Ind. Crop. Prod. 2022, 181, 114790. [Google Scholar] [CrossRef]
- Ptushenko, O.S.; Ptushenko, V.V.; Solovchenko, A.E. Spectrum of Light as a Determinant of Plant Functioning: A Historical Perspective. Life 2020, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Zhong, M.; Zeng, B.; Tang, D.; Yang, J.; Qu, L.; Yan, J.; Wang, X.; Li, X.; Liu, X.; Zhao, X. The blue light receptor CRY1 interacts with GID1 and DELLA proteins to repress GA signaling during photomorphogenesis in Arabidopsis. Mol. Plant 2021, 14, 1328–1342. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2021, 41, 742–780. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta 2019, 1861, 148131. [Google Scholar] [CrossRef]
- Hayes, R.G.; Klein, W.H. Spectral quality influence of light during development of Arabidopsis thaliana plants in regulating seed germination. Plant Cell Physiol. 1974, 15, 643–653. [Google Scholar] [CrossRef]
- Cammarisano, L.; Donnison, I.S.; Robson, P.R.H. The Effect of Red & Blue Rich LEDs vs Fluorescent Light on Lollo Rosso Lettuce Morphology and Physiology. Front. Plant Sci. 2021, 12, 603411. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Viršilė, A.; Miliauskienė, J.; Haimi, P.J.; Laužikė, K.; Brazaitytė, A.; Duchovskis, P. The Physiological Response of Lettuce to Red and Blue Light Dynamics Over Different Photoperiods. Front. Plant Sci. 2021, 11, 610174. [Google Scholar] [CrossRef] [PubMed]
- Milberg, P.; Andersson, L.; Thompson, K. Large-seeded spices are less dependent on light for germination than small-seeded ones. Seed Sci. Res. 2000, 10, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Pinna, M.S.; Mattana, E.; Cañadas, E.M.; Bacchetta, G. Effects of pre-treatments and temperature on seed viability and germination of Juniperus macrocarpa Sm. Comptes Rendus. Biol. 2014, 337, 338–344. [Google Scholar] [CrossRef]
- Cuena-Lombraña, A.; Porceddu, M.; Dettori, C.A.; Bacchetta, G. Predicting the consequences of global warming on Gentiana lutea germination at the edge of its distributional and ecological range. PeerJ 2020, 8, e8894. [Google Scholar] [CrossRef]
- Handa, A.K.; Tiznado-Hernández, M.E.; Mattoo, A.K. Fruit development and ripening: A molecular perspective. In Plant Biotechnology and Agriculture; Academic Press: Cambridge, MA, USA, 2012; pp. 405–424. [Google Scholar]
- El-Keblawy, A.; Soliman, S.; Al-Khoury, R.; Ghauri, A.; Al Rammah, H.; Hussain, S.E.; Rashid, S.; Manzoor, Z. Effect of maturation conditions on light and temperature requirements during seed germination of Citrullus colocynthis from the Arabian Desert. Plant Biol. 2018, 21, 292–299. [Google Scholar] [CrossRef]
- Farci, D.; Haniewicz, P.; Cocco, E.; De Agostini, A.; Cortis, P.; Kusaka, M.; Loi, M.C.; Piano, D. The Impact of Fruit Etiolation on Quality of Seeds in Tobacco. Front. Plant Sci. 2020, 11, 563971. [Google Scholar] [CrossRef]
- Haniewicz, P.; Floris, D.; Farci, D.; Kirkpatrick, J.; Loi, M.C.; Büchel, C.; Bochtler, M.; Piano, D. Isolation of Plant Photosystem II Complexes by Fractional Solubilization. Front. Plant Sci. 2015, 6, 1100. [Google Scholar] [CrossRef] [Green Version]
- Farci, D.; Collu, G.; Kirkpatrick, J.; Esposito, F.; Piano, D. RhVI1 is a membrane-anchored vacuolar invertase highly expressed in Rosa hybrida L. petals. J. Exp. Bot. 2016, 67, 3303–3312. [Google Scholar] [CrossRef] [Green Version]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta BBA Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- De Agostini, A.; Caltagirone, C.; Caredda, A.; Cicatelli, A.; Cogoni, A.; Farci, D.; Guarino, F.; Garau, A.; Labra, M.; Lussu, M.; et al. Heavy metal tolerance of orchid populations growing on abandoned mine tailings: A case study in Sardinia Island (Italy). Ecotoxicol. Environ. Saf. 2019, 189, 110018. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Yamaki, T. Interaction of gibberellin a3, and inorganic phosphate in tobacco seed germination. Plant Cell Physiol. 1962, 3, 175–187. [Google Scholar] [CrossRef]
- Ballaré, C.L. Phytochrome Responses: Think Globally, Act Locally. Trends Plant Sci. 2017, 22, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Brazel, A.J.; Ó’Maoiléidigh, D.S. Photosynthetic activity of reproductive organs. J. Exp. Bot. 2019, 70, 1737–1754. [Google Scholar] [CrossRef]
- McCullough, J.M.W.; Shropshire, W., Jr. Physiological predetermination of germination responses in Arabidopsis thaliana (1.) HEYNH. Plant Cell Physiol. 1970, 11, 139–148. [Google Scholar] [CrossRef]
- Donohue, K.; Heschel, M.S.; Butler, C.M.; Barua, D.; Sharrock, R.A.; Whitelam, G.C.; Chiang, G.C.K. Diversification of phytochrome contributions to germination as a function of seed-maturation environment. New Phytol. 2007, 177, 367–379. [Google Scholar] [CrossRef]
- Casal, J.J.; Sánchez, R.A. Phytochromes and seed germination. Seed Sci. Res. 1998, 8, 317–329. [Google Scholar] [CrossRef]
- Koornneef, M.; Bentsink, L.; Hilhorst, H. Seed dormancy and germination. Curr. Opin. Plant Biol. 2002, 5, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Toyomasu, T.; Kawaide, H.; Mitsuhashi, W.; Inoue, Y.; Kamiya, Y. Phytochrome Regulates Gibberellin Biosynthesis during Germination of Photoblastic Lettuce Seeds. Plant Physiol. 1998, 118, 1517–1523. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, M.; Hanada, A.; Yamauchi, Y.; Kuwahara, A.; Kamiya, Y.; Yamaguchi, S. Gibberellin Biosynthesis and Response during Arabidopsis Seed Germination. Plant Cell 2003, 15, 1591–1604, Erratum in Plant Cell 2004, 16, 783. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.; Hanada, A.; Kuwahara, A.; Endo, A.; Okamoto, M.; Yamauchi, Y.; North, H.; Marion-Poll, A.; Sun, T.-P.; Koshiba, T.; et al. Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 2006, 48, 354–366. [Google Scholar] [CrossRef] [PubMed]

| Capsule Type | Capsule Part | F0/Fm | Y (%) * | Chl a ** (Relative) | Chl b ** (Relative) | Chl a/b (Ratio) |
|---|---|---|---|---|---|---|
 |  | 0.35 ± 0.01 | 65.3 ± 0.33 | 1 | 1 | 1.82 |
 | n.a. *** | n.a. *** | 0.96 | 0.94 | 1.87 | |
 |  | 0.57 ± 0.06 | 43.3 ± 0.21 | 0.013 | 0.019 | 2.20 |
 | n.a. *** | n.a. *** | 0.72 | 0.78 | 1.99 |
| White Light (Control) | Blue Light | Red Light | |
|---|---|---|---|
| F0/Fm | 0.286 ± 0.004 | 0.290 ± 0.003 | 0.271 ± 0.002 |
| Y (%) * | 71.367 ± 0.416 | 70.933 ± 0.321 | 72.767 ± 0.231 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocco, E.; Farci, D.; Haniewicz, P.; Schröder, W.P.; Maxia, A.; Piano, D. The Influence of Blue and Red Light on Seed Development and Dormancy in Nicotiana tabacum L. Seeds 2022, 1, 152-163. https://doi.org/10.3390/seeds1030014
Cocco E, Farci D, Haniewicz P, Schröder WP, Maxia A, Piano D. The Influence of Blue and Red Light on Seed Development and Dormancy in Nicotiana tabacum L. Seeds. 2022; 1(3):152-163. https://doi.org/10.3390/seeds1030014
Chicago/Turabian StyleCocco, Emma, Domenica Farci, Patrycja Haniewicz, Wolfgang P. Schröder, Andrea Maxia, and Dario Piano. 2022. "The Influence of Blue and Red Light on Seed Development and Dormancy in Nicotiana tabacum L." Seeds 1, no. 3: 152-163. https://doi.org/10.3390/seeds1030014
APA StyleCocco, E., Farci, D., Haniewicz, P., Schröder, W. P., Maxia, A., & Piano, D. (2022). The Influence of Blue and Red Light on Seed Development and Dormancy in Nicotiana tabacum L. Seeds, 1(3), 152-163. https://doi.org/10.3390/seeds1030014








