Modeling and Analysis of Corrosion of Aluminium Alloy 6060 Using Electrochemical Impedance Spectroscopy (EIS)
Abstract
1. Introduction
2. Materials and Methods
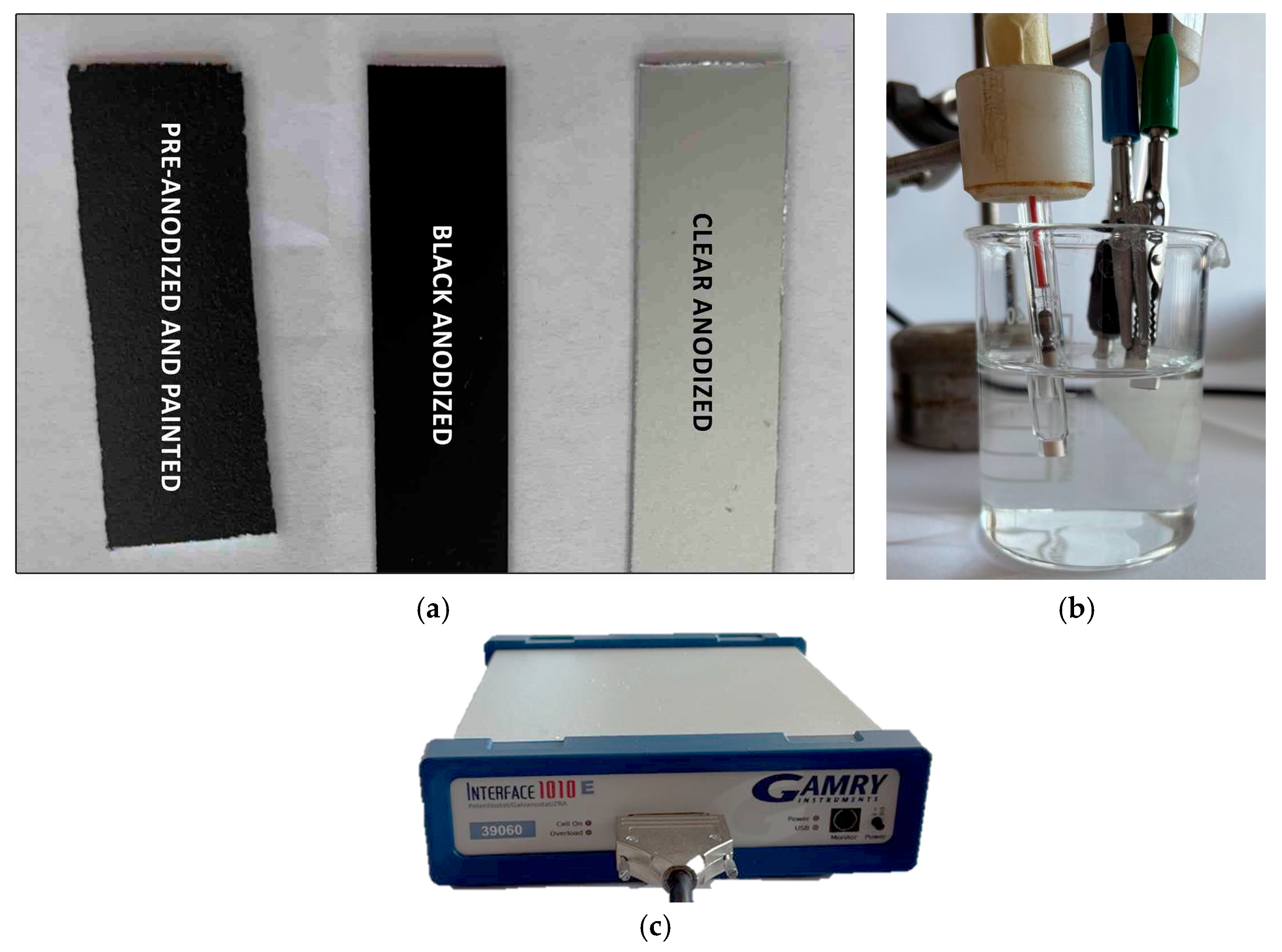
2.1. Materials and Coating Production
2.2. Experimental Procedure
3. Results and Discussion
3.1. Surface Morphology

3.2. Electrochemical Characterization
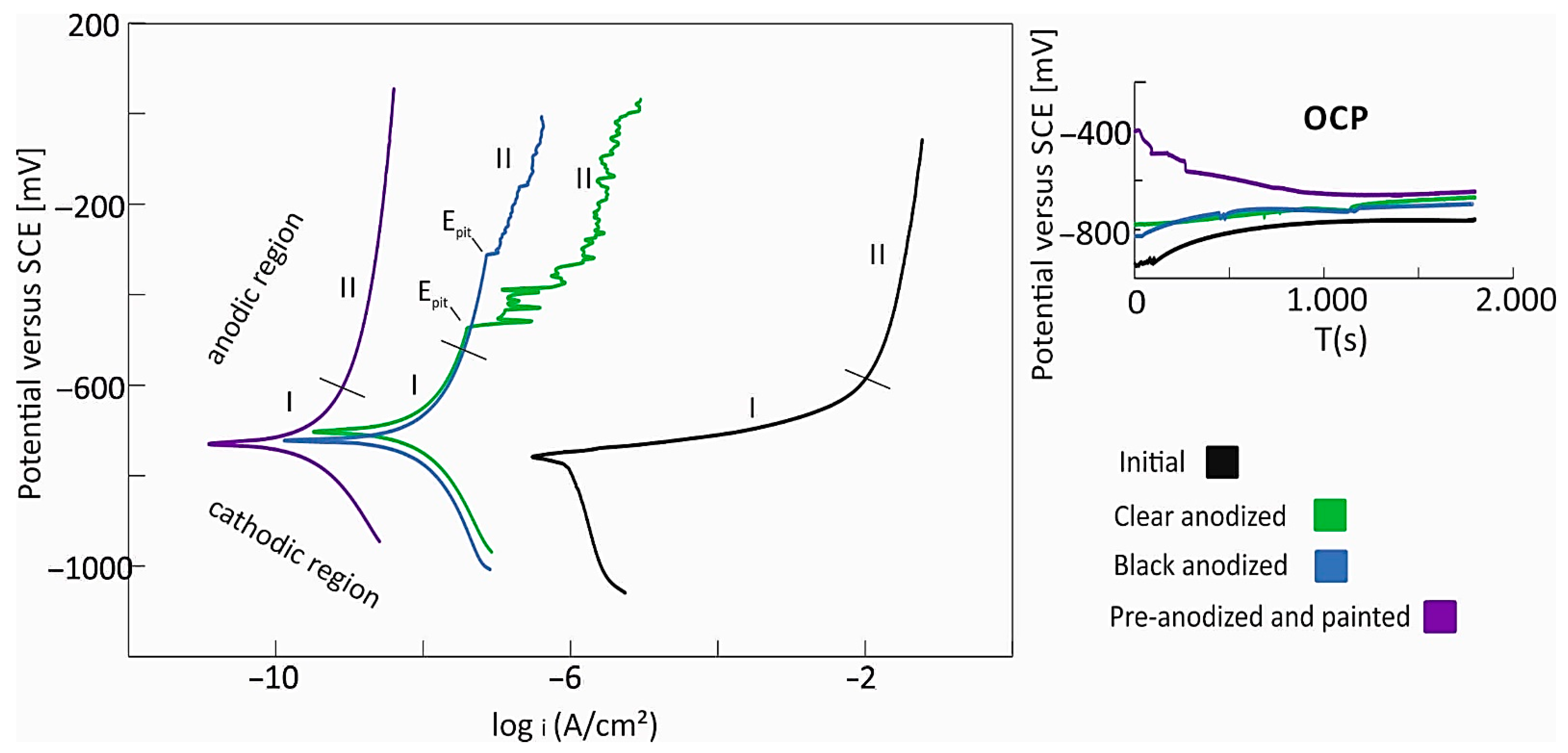
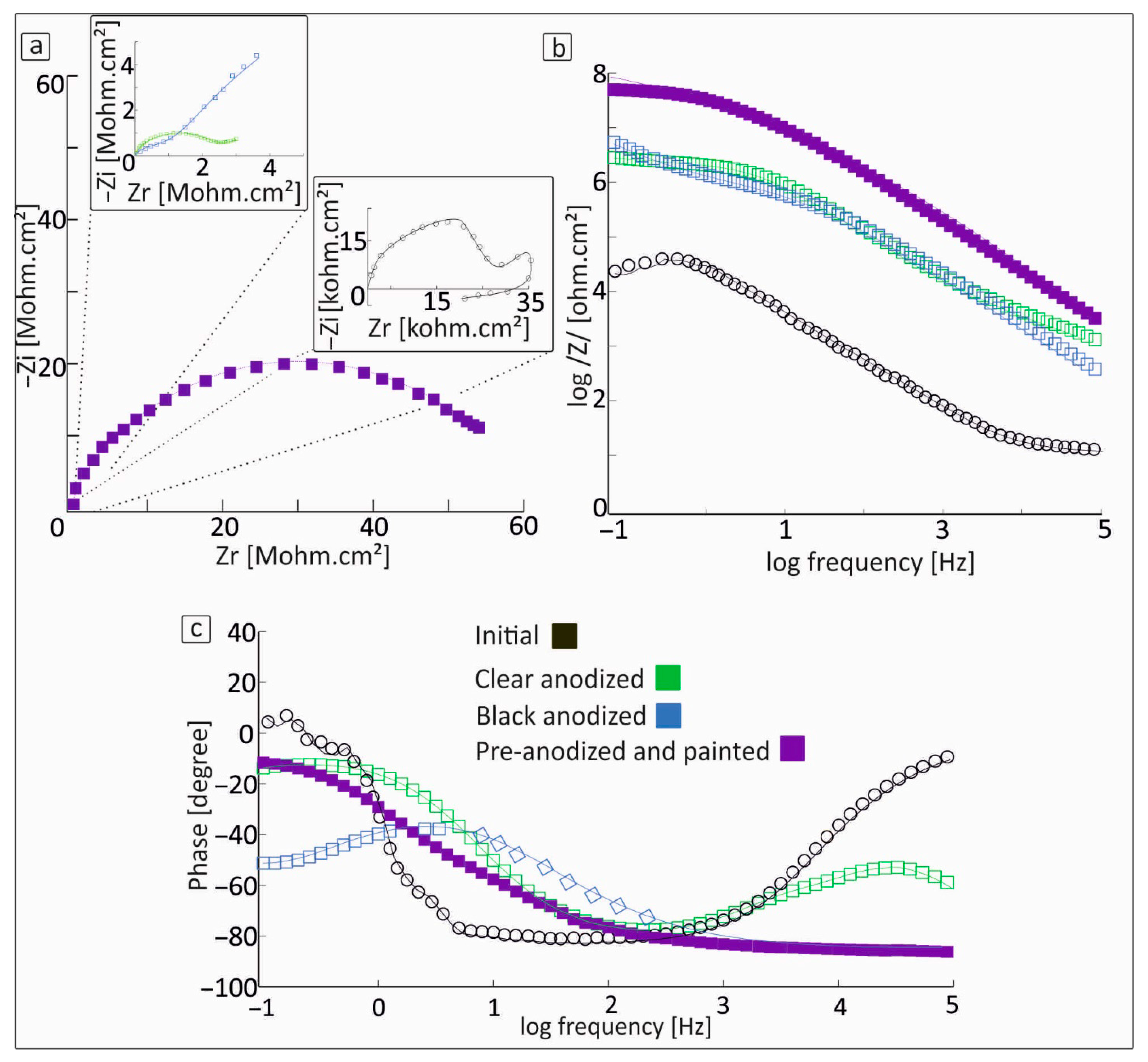
3.3. Proposed Equivalent Electrical Circuit Models

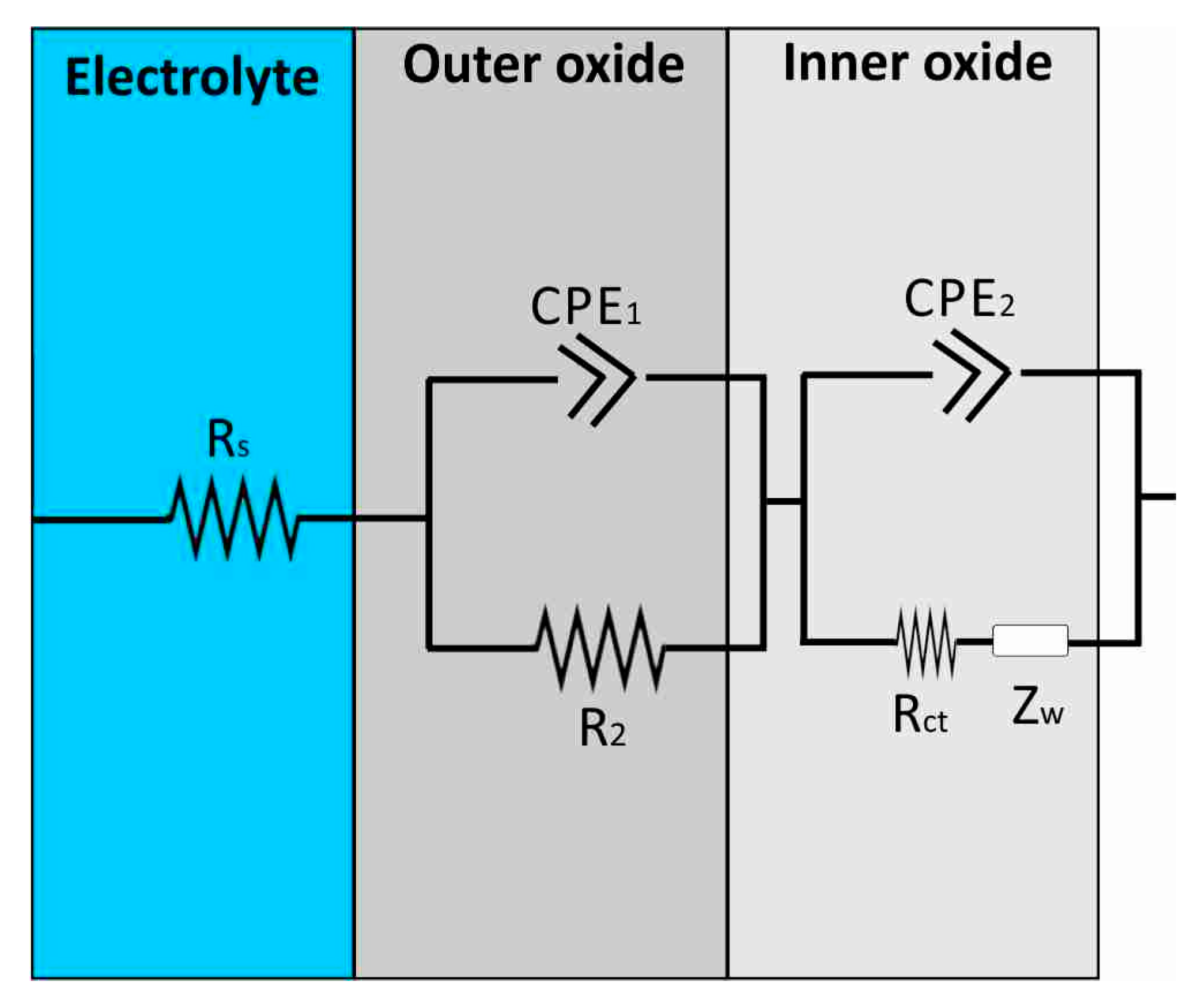
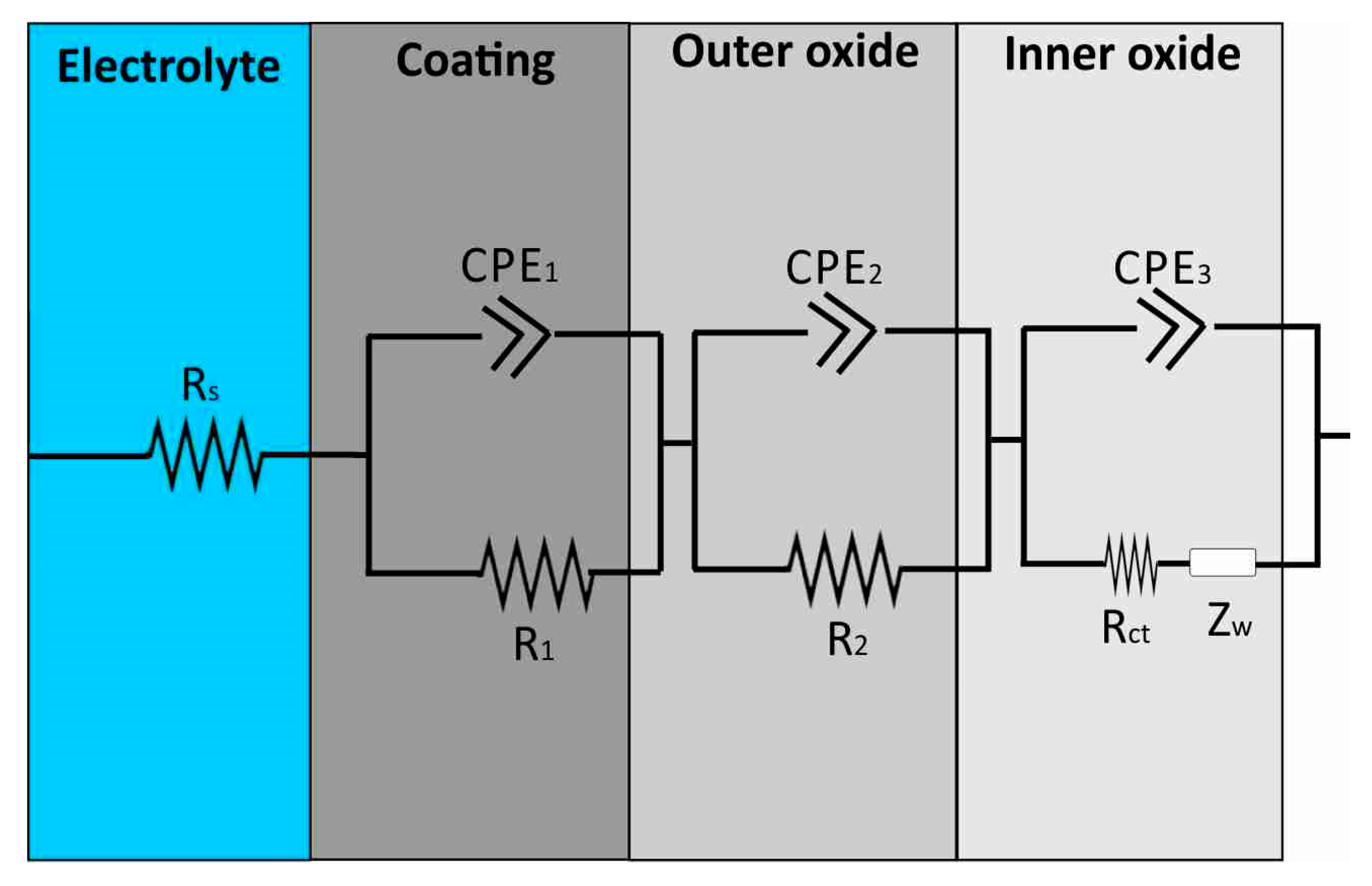
| Al Samples | Rs (Ω) | CPE1 (S × sa) | α1 | CPE2 (S × sa) | α2 | CPE3 (S × sa) | α3 | R1 (MΩ) | R2 | Rct | W (S × s1/2) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | 6.63 | 9.95 × 10−6 | 0.87 | - | - | - | - | - | - | 13 kΩ | - |
| Clear anodized | 26.3 | 6 × 10−10 | 1 | 2 × 10−9 | 0.95 | - | - | - | 0.7 ΜΩ | 1.48 ΜΩ | 1.2 × 10−6 |
| Black Anodized | 1 | 4 × 10−10 | 0.92 | 2.9 × 10−9 | 0.9 | - | - | - | 4.21 MΩ | 0.74 ΜΩ | 68 × 10−5 |
| Pre-anodized and painted | 0.1 | 1.74 × 10−9 | 0.95 | 1.8 × 10−9 | 0.93 | 1.4 × 10−9 | 0.9 | 7.5 | 2 kΩ | 0.47 MΩ | 1.3 × 10−5 |
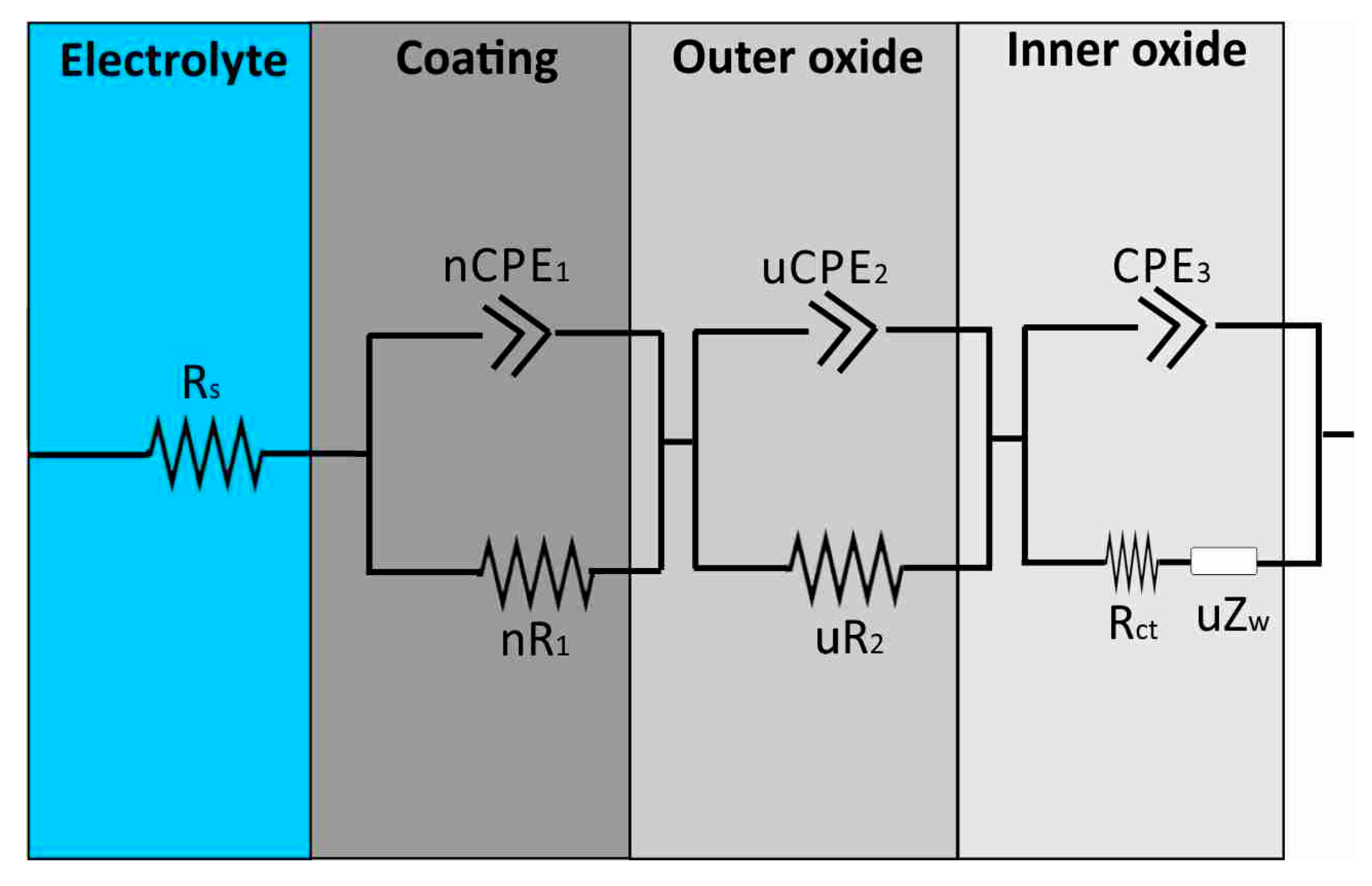
4. Conclusions
- Surface treatments significantly improve corrosion resistance. Anodized samples demonstrate an approximately 30 times lower corrosion rate, while the pre-anodized and painted samples offer the highest protection, with a corrosion rate nearly 1000 times lower than that of the untreated aluminum.
- The clear anodized sample showed an almost ideal dielectric behavior (α~1) and a charge transfer resistance of 1.48 MΩ, indicating a uniform oxide. The clear anodized sample had higher anodic resistance but lower Rct (0.74 MΩ), suggesting reduced oxide quality, likely due to porosity or dye effects.
- The pre-anodized and painted samples demonstrate a complex multilayer electrochemical behavior that explains its superior protective performance. The outer layer exhibits very high resistance (7.5 MΩ) and excellent dielectric properties (α = 0.95), indicating an effective barrier against corrosion.
- The high values of resistance for the anodized samples indicate that the sealing process was highly effective.
- The thickness of the anodized layers correlates well with the SEM results.
Author Contributions
Funding
Conflicts of Interest
References
- Yashpal; Jawalkar, C.S.; Kant, S. A review on use of aluminium alloys in aircraft components. i-Manag. J. Mater. Sci. 2015, 3, 33–38. [Google Scholar] [CrossRef]
- Egorkin, V.S.; Vyaliy, I.E.; Gnedenkov, A.S.; Kharchenko, U.V.; Sinebryukhov, S.L.; Gnedenkov, S.V. Corrosion properties of the composite coatings formed on PEO pretreated AlMg3 aluminum alloy by dip-coating in polyvinylidenefluoride-polytetrafluoroethylene suspension. Polymers 2024, 16, 2945. [Google Scholar] [CrossRef] [PubMed]
- Peltier, F.; Thierry, D. Development of a reliable accelerated corrosion test for painted aluminum alloys used in the aerospace industry. Corros. Mater. Degrad. 2024, 5, 427–438. [Google Scholar] [CrossRef]
- Gyarmati, G.; Erdélyi, J. Intermetallic phase control in cast aluminum alloys by utilizing heterogeneous nucleation on oxides. Metals 2025, 15, 404. [Google Scholar] [CrossRef]
- Akhyar, H.; Farhan, A. Cooling rate, hardness and microstructure of aluminum cast alloys. Mater. Sci. Mater. Rev. 2018, 2, 1. [Google Scholar] [CrossRef]
- Shah, S.; Gopal, A.; Thronsen, E.; Hatzoglou, C.; Holmedal, B. Precipitation, mechanical properties and early slant ductile fracture in cyclic and naturally aged Al-Zn-Mg(-Cu) alloys. Mater. Des. 2022, 222, 111026. [Google Scholar] [CrossRef]
- Nasim, W.; Mungole, T.; Efe, M.; Rohatgi, A. Simultaneous improvement in strength and ductility in 7xxx Al through a combination of natural aging and deformation. Mater. Sci. Eng. A 2023, 884, 145566. [Google Scholar] [CrossRef]
- Dai, Y.; Yan, L.; Hao, J. Review on Micro-Alloying and Preparation Method of 7xxx Series Aluminum Alloys: Progresses and Prospects. Materials 2022, 15, 1216. [Google Scholar] [CrossRef]
- Minjung, K.; Cheolhee, K. A Review of Joining Processes for High Strength 7xxx Series Aluminum Alloys. J. Weld. Join. 2017, 35, 79–88. [Google Scholar] [CrossRef]
- Choi, Y.; Moon, C.; Lee, M.-G. Experimental study on the mechanical properties of 7xxx aluminium alloy sheet under different heat treatment conditions. IOP Conf. Ser. Mater. Sci. Eng. 2019, 651, 012080. [Google Scholar] [CrossRef]
- Guo, F.; Cao, Y.; Wang, K.; Zhang, P.; Cui, Y.; Hu, Z.; Xie, Z. Effect of the Anodizing Temperature on Microstructure and Tribological Properties of 6061 Aluminum Alloy Anodic Oxide Films. Coatings 2022, 12, 31. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.; Cabral-Miramontes, N.; Nieves-Mendoza, D.; Lara-Banda, M.; Maldonado-Bandala, E.; Olguín-Coca, J.; Lopez-Leon, L.D.; Estupiñan-Lopez, F.; Calderon, F.A.; Gaona Tiburcio, C. Anodizing of AA2024 Aluminum–Copper Alloy in Citric-Sulfuric Acid Solution: Effect of Current Density on Corrosion Resistance. Coatings 2024, 14, 816. [Google Scholar] [CrossRef]
- Bhat, K.U.; Panemangalore, D.B.; Kuruveri, S.B.; Merbin, J.; Menezes, P.L. Surface Modification of 6xxx Series Aluminum Alloys. Coatings 2022, 12, 180. [Google Scholar] [CrossRef]
- Rahman, N.A.; Jol, C.J.; Linus, A.A.; Jan, B.H.M.; Parabi, A.; Ming, C.K.; Parabi, A.S.L.; James, A.; Shamsol, N.S.; John, S.B.; et al. Corrosion resistance and electrochemical adaptation of aluminium in brackish peat water sources under seawater intrusion in the rural tropical peatlands of Borneo. Sustain. Chem. Clim. Action 2025, 6, 100074. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Chen, W.; Hong, H.; Zhang, T. Long-Term Corrosion Behavior of 434 Stainless Steel Coatings on T6061 Aluminum Alloy in Chloride Environments. Coatings 2025, 15, 144. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Kou, L.; Li, X.; Qiao, X.; Xu, G. Multiscale simulation of pitting and intergranular corrosion in aluminium alloys based on microstructural characteristics. Corros. Sci. 2025, 255, 113093. [Google Scholar] [CrossRef]
- Zhou, Z.; Ge, X.; Fan, W.; Shan, B.; Yang, J.; Zhao, X. Corrosion behavior of 7B04 aluminum alloy under the synergistic effect of Lacticaseibacillus paracasei and Acinetobacter lwoffi. Arab. J. Chem. 2023, 16, 105308. [Google Scholar] [CrossRef]
- Cui, T.; Wu, J.; Song, J.; Meng, D.; Jin, X.; Tian, H.; Cui, Z. Atmospheric Corrosion Behavior of Typical Aluminum Alloys in Low-Temperature Environment. Metals 2025, 15, 277. [Google Scholar] [CrossRef]
- Malaret, F. Exact calculation of corrosion rates by the weight-loss method. Exp. Results 2022, 3, e13. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Burattini, M.; Miglio, R. Study of the Corrosion Resistance of Austenitic Stainless Steels during Conversion of Waste to Biofuel. Materials 2017, 10, 325. [Google Scholar] [CrossRef]
- Laurent, C.; Scenini, F.; Monetta, T.; Bellucci, F.; Curioni, M. The contribution of hydrogen evolution processes during corrosion of aluminium and aluminium alloys investigated by potentiodynamic polarisation coupled with real-time hydrogen measurement. npj Mater. Degrad. 2017, 1, 6. [Google Scholar] [CrossRef]
- ISO 9227:2022/Amd 1:2024; Corrosion Tests in Artificial Atmospheres-Salt Spray Tests. International Organization for Standardization: Geneva, Switzerland, 2024.
- ISO 4623-2:2016; Paints and varnishes — Determination of resistance to filiform corrosion — Part 2: Aluminium substrates. International Organization for Standardization: Geneva, Switzerland, 2016.
- Chen, J.; Liu, J.; Wang, H.; Li, B.; Hu, Q.; Shao, T.; Yang, R.; Wang, B.; Wan, Q.; Li, Z.; et al. Experimental Study on Neutral Salt Spray Accelerated Corrosion of Metal Protective Coatings for Power-Transmission and Transformation Equipment. Coatings 2023, 13, 480. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J. Salt spray corrosion and electrochemical corrosion properties of anodic oxide film on 7475 aluminum alloy. J. Alloys Compd. 2015, 632, 286–290. [Google Scholar] [CrossRef]
- Usman, B.J.; Scenini, F.; Curioni, M. Corrosion testing of anodized aerospace alloys: Comparison between immersion and salt spray testing using electrochemical impedance spectroscopy. J. Electrochem. Soc. 2020, 167, 041505. [Google Scholar] [CrossRef]
- Doğru Mert, B. Corrosion protection of aluminum by electrochemically synthesized composite organic coating. Corros. Sci. 2015, 103, 88–94. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2014, 12, 1–35. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Méndez-Ramírez, C.T.; Martínez-Ramos, C.; Baltazar-Zamora, M.A.; Santiago-Hurtado, G.; Estupinan-Lopez, F.; Landa-Ruiz, L.; Nieves-Mendoza, D.; Almeraya-Calderon, F. Electrochemical Noise Analysis: An Approach to the Effectivity of Each Method in Different Materials. Materials 2024, 17, 4013. [Google Scholar] [CrossRef]
- Cottis, R.A. Electrochemical noise for corrosion monitoring. In Techniques for Corrosion Monitoring; Woodhead Publishing Series in Metals and Surface Engineering; Elsevier: Amsterdam, The Netherlands, 2008; pp. 86–110. [Google Scholar] [CrossRef]
- Wang, C.; Wu, L.; Xue, F.; Ma, R.; Etim, I.-I.N.; Hao, X.; Dong, J.; Ke, W. Electrochemical noise analysis on the pit corrosion susceptibility of biodegradable AZ31 magnesium alloy in four types of simulated body solutions. J. Mater. Sci. Technol. 2018, 34, 1876–1884. [Google Scholar] [CrossRef]
- Lv, J.; Yue, Q.-X.; Ding, R.; Wang, X.; Gui, T.-J.; Zhao, X.-D. The application of electrochemical noise for the study of metal corrosion and organic anticorrosion coatings: A review. ChemElectroChem 2020, 8, 337–351. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Wen, Y.; Ikeuba, A.I.; Tan, X.; Sun, T.; Liu, X. Electrochemical noise analysis of the hot corrosion of TP347H stainless steel: Effect of temperature and coal ash thickness. Electrochim. Acta 2025, 525, 146105. [Google Scholar] [CrossRef]
- Wang, D.H. (Ed.) Molecular Sensors for Cardiovascular Homeostasis; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Lasia, A. Impedance spectroscopy applied to the study of electrocatalytic processes. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 241–263. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Zulkifli, M.F.R.; Ghazali, M.S.M.; Al-Amiery, A.A.H.; Ismail, N.; Khairina, S.; Izionworu, V.O.; Suriani, M.J.; Nik, W.W. Application of electrochemical impedance spectroscopy technology to evaluate passivation performance of film forming and adsorption types corrosion inhibitors. Int. J. Corros. Scale Inhib. 2022, 11, 1303–1318. [Google Scholar] [CrossRef]
- Bjørgum, A.; Lapique, F.; Walmsley, J.C.; Redford, K. Anodizing as pre-treatment for structural bonding. Int. J. Adhes. Adhes. 2003, 23, 401–412. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, G.; Zhang, Z.; Wang, Y. Research on corrosion resistance of anodized and sealed 6061 aluminum alloy in 3.5% sodium chloride solution. Int. J. Electrochem. Sci. 2023, 18, 100092. [Google Scholar] [CrossRef]
- Gerengi, H.; Slepski, P.; Özgan, E.; Kurtay, M. Investigation of corrosion behavior of 6060 and 6082 aluminum alloys under simulated acid rain conditions. Mater. Corros. 2013, 66, 233–240. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, H.; Zhou, X.; Li, K.; Liao, Y.; Liang, Z.; Liu, L. Corrosion behavior of anodized Al-Cu-Li alloy: The role of intermetallic particle-introduced film defects. Corros. Sci. 2019, 158, 108110. [Google Scholar] [CrossRef]
- Fontinha, I.R.; Eustáquio, E. Influence of exposure conditions and particulate deposition on anodized aluminum corrosion. Corros. Mater. Degrad. 2022, 3, 770–786. [Google Scholar] [CrossRef]
- Liang, M.; Melchers, R.; Chaves, I. Corrosion and pitting of 6060 series aluminium after 2 years exposure in seawater splash, tidal and immersion zones. Corros. Sci. 2018, 140, 286–296. [Google Scholar] [CrossRef]
- Logori, D.; Pezzato, L.; Settimi, A.G.; Hanoz, D.; Dabalà, M. Microstructural and corrosion properties of burnished 6060 aluminum alloy. Appl. Sci. 2021, 11, 4460. [Google Scholar] [CrossRef]
- Franco, M.; Anoop, S.; Rani, R.U.; Sharma, A.K. Porous layer characterization of anodized and black-anodized aluminium by electrochemical studies. ISRN Corros. 2012, 2012, 323676. [Google Scholar] [CrossRef]
- QUALANOD; Quality Label for Sulphuric Acid-Based Anodizing of Aluminium. QUALANOD Secretariat: Michelau, Germany, 2025.
- DIN 17611:2022-08; Anodized Products of Wrought Aluminium and Wrought Aluminium alloys-Technical Conditions of Delivery. Deutsches Institut für Normung (DIN): Berlin, Germany, 2022.
- Ofoegbu, S.U.; Fernandes, F.A.O.; Pereira, A.B. The Sealing Step in Aluminum Anodizing: A Focus on Sustainable Strategies for Enhancing Both Energy Efficiency and Corrosion Resistance. Coatings 2020, 10, 226. [Google Scholar] [CrossRef]
- Mrówka, G.; Sieniawski, J. Analysis of Intermetallic Phases in 2024 Aluminium Alloy. SSP 2013, 197, 238–243. [Google Scholar] [CrossRef]
- Shih, H.-H.; Huang, Y.-C. Study on the black electrolytic coloring of anodized aluminum in cupric sulfate. J. Mater. Process. Technol. 2008, 208, 24–28. [Google Scholar] [CrossRef]
- Mrówka, G.; Sieniawski, J.; Wierzbińska, M. Analysis of intermetallic particles in AlSi1MgMn aluminium alloy. J. Achiev. Mater. Manuf. Eng. 2007, 20, 155–158. [Google Scholar]
- Bara, M.; Niedźwiedź, M.; Skoneczny, W. Influence of Anodizing Parameters on Surface Morphology and Surface-Free Energy of Al2O3 Layers Produced on EN AW-5251 Alloy. Materials 2019, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Sukiman, N.L.; Cavanaugh, M.K.; Hinton, B.R.W.; Hutchinson, C.R.; Birbilis, N. Metastable pitting characteristics of aluminium alloys measured using current transients during potentiostatic polarization. Electrochim. Acta 2012, 66, 245–254. [Google Scholar] [CrossRef]
- Allachi, H.; Chaouket, F.; Draoui, K. Protection against corrosion in marine environments of AA6060 aluminium alloy by cerium chlorides. J. Alloys Compd. 2010, 491, 223–229. [Google Scholar] [CrossRef]
- Gamry Instruments. Getting Started with Electrochemical Corrosion Measurement—Calculation of Corrosion Rate from Corrosion Current. Available online: https://www.gamry.com/application-notes/corrosion-coatings/basics-of-electrochemical-corrosion-measurements/ (accessed on 1 June 2025).
- Iqbal, M.A.; Maia, F.; Matykina, E.; Arrabal, R.; Mohedano, M.; Vega, J.M. Active corrosion protection of AA2024T3 by the synergy of flash-PEO/Ce coating and epoxy coating loaded with LDH/eco-friendly gluconate. Prog. Org. Coat. 2025, 206, 109359. [Google Scholar] [CrossRef]
- Rodič, P.; Kapun, B.; Milošev, I. Complementary corrosion protection of cast AlSi7Mg0.3 alloy using Zr-Cr conversion and polyacrylic/siloxane-silica multilayer coatings. npj Mater. Degrad. 2024, 8, 58. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical impedance spectroscopy—A tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Stergioudi, F.; Vogiatzis, C.A.; Gkrekos, K.; Michailidis, N.; Skolianos, S.M. Electrochemical corrosion evaluation of pure, carbon-coated and anodized Al foams. Corros. Sci. 2015, 91, 151–159. [Google Scholar] [CrossRef]
- Huang, Y.; Shih, H.; Huang, H.; Daugherty, J.; Wu, S.; Ramanathan, S.; Chang, C.; Mansfeld, F. Evaluation of the corrosion resistance of anodized aluminum 6061 using electrochemical impedance spectroscopy (EIS). Corros. Sci. 2008, 50, 3569–3575. [Google Scholar] [CrossRef]
- Córdoba-Torres, P. Relationship between constant-phase element (CPE) parameters and physical properties of films with a distributed resistivity. Electrochim. Acta 2017, 225, 592–604. [Google Scholar] [CrossRef]
- Chang, B.-Y. Conversion of a constant phase element to an equivalent capacitor. J. Electrochem. Sci. Technol. 2020, 11, 318–321. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.; Gaona-Tiburcio, C.; Estupinán-López, F.; Lara-Banda, M.; Zambrano-Robledo, P.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Corrosion Resistance of Hard Coat Anodized AA 6061 in Citric–Sulfuric Solutions. Coatings 2020, 10, 601. [Google Scholar] [CrossRef]
| Sample | Ecorr [mV] | Icorr [A/cm2] | βa | βc | Corrosion Rate [mmpy] |
|---|---|---|---|---|---|
| Initial | −0.756 | 9 × 10−7 | 0.022 | −0.52 | 0.03 |
| Clear anodized | −0.702 | 2.72 × 10−8 | 0.78 | −0.52 | 0.0009 |
| Black anodized | −0.722 | 4 × 10−8 | 1.19 | −0.86 | 0.0014 |
| Pre-anodized and painted | −0.729 | 9.72 × 10−10 | 0.91 | −0.46 | 0.00003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baxevani, A.; Lamprou, E.; Mavropoulos, A.; Stergioudi, F.; Michailidis, N.; Tsoulfaidis, I. Modeling and Analysis of Corrosion of Aluminium Alloy 6060 Using Electrochemical Impedance Spectroscopy (EIS). Alloys 2025, 4, 17. https://doi.org/10.3390/alloys4030017
Baxevani A, Lamprou E, Mavropoulos A, Stergioudi F, Michailidis N, Tsoulfaidis I. Modeling and Analysis of Corrosion of Aluminium Alloy 6060 Using Electrochemical Impedance Spectroscopy (EIS). Alloys. 2025; 4(3):17. https://doi.org/10.3390/alloys4030017
Chicago/Turabian StyleBaxevani, Aikaterini, Eleni Lamprou, Azarias Mavropoulos, Fani Stergioudi, Nikolaos Michailidis, and Ioannis Tsoulfaidis. 2025. "Modeling and Analysis of Corrosion of Aluminium Alloy 6060 Using Electrochemical Impedance Spectroscopy (EIS)" Alloys 4, no. 3: 17. https://doi.org/10.3390/alloys4030017
APA StyleBaxevani, A., Lamprou, E., Mavropoulos, A., Stergioudi, F., Michailidis, N., & Tsoulfaidis, I. (2025). Modeling and Analysis of Corrosion of Aluminium Alloy 6060 Using Electrochemical Impedance Spectroscopy (EIS). Alloys, 4(3), 17. https://doi.org/10.3390/alloys4030017







