The Role of Metal Foams for Sustainability and Energy Transition
Abstract
1. Introduction
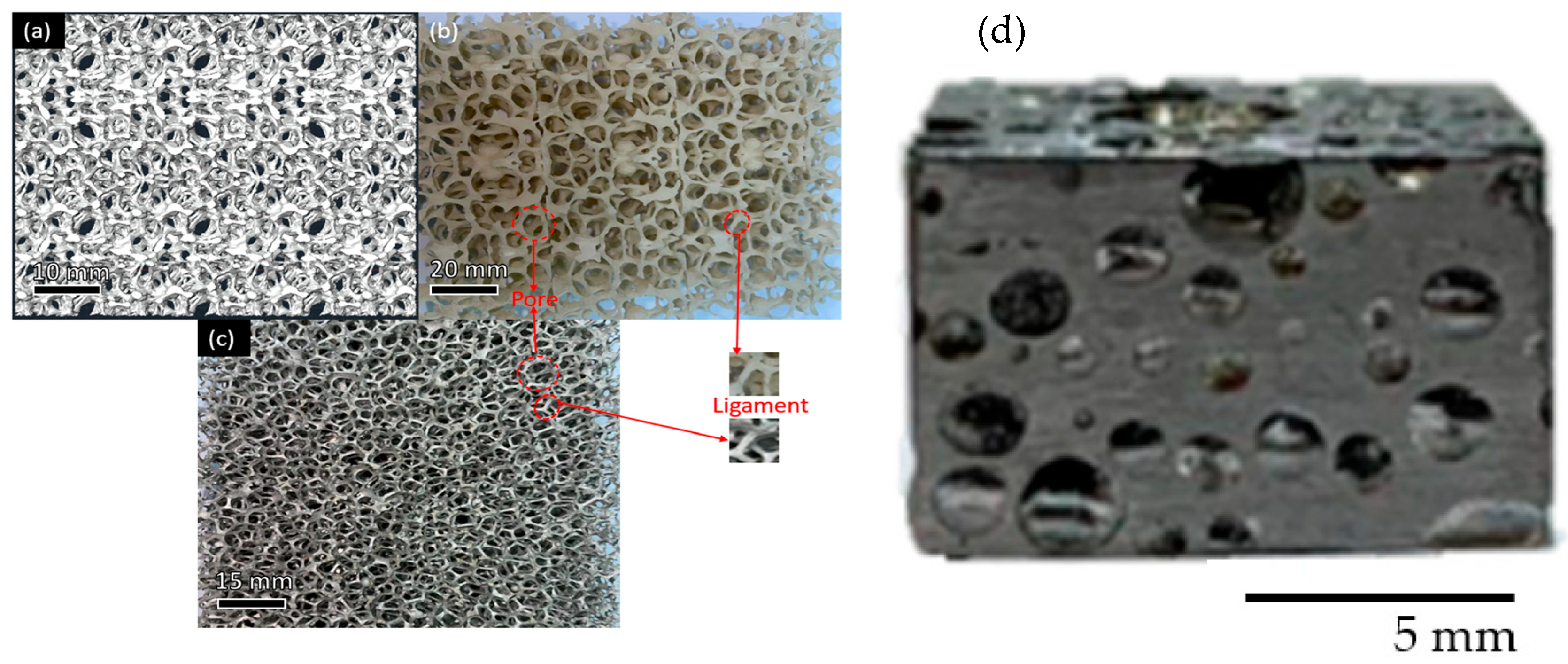
2. Structures and Fabrication of Metal Foams
2.1. Gas Injection Foaming
2.2. Powder Metallurgy Foaming
2.3. Cast Foaming Process
3. Electrocatalysis and Energy Conversion
4. Energy Storage Systems
5. Thermal Management and Heat Transfer
6. Hydrogen Technologies
6.1. Electrode Materials
6.2. Liquid Hydrogen Fuel Tanks
6.3. Catalyst Support
6.4. Fuel Cell Technology
7. Lightweight and Multifunctional Structural Materials
8. Conclusions and Future Outlook
- Future research must focus on developing standardized protocols for manufacturing high-quality and repeatable metal foams;
- Further investigations could conduct comprehensive life-cycle assessments to provide a more accurate understanding of the environmental impacts involved in metal foam production and their full life cycle;
- Future studies must explore the feasibility and impact of using more sustainable raw materials, including waste, in the production of metal foams;
- Further research could be conducted on the eco-design of metal foams, considering factors such as recyclability, durability, and repairability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge Solid State Science Series; Cambridge University Press: Cambridge, UK, 1997; ISBN 978-0-521-49911-8. [Google Scholar]
- Evans, A. Multifunctionality of Cellular Metal Systems. Prog. Mater. Sci. 1998, 43, 171–221. [Google Scholar] [CrossRef]
- Costanza, G.; Solaiyappan, D.; Tata, M.E. Properties, Applications and Recent Developments of Cellular Solid Materials: A Review. Materials 2023, 16, 7076. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, Characterisation and Application of Cellular Metals and Metal Foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Dineshkumar, J.; Jesudas, T.; Elayaraja, R. Characteristics, Applications and Processing of Aluminium Foams—A Review. Mater. Today Proc. 2021, 42, 1773–1776. [Google Scholar] [CrossRef]
- Patel, N.; Mittal, G.; Agrawal, M.; Pradhan, A.K. Aluminum Foam Production, Properties, and Applications: A Review. Int. J. Met. 2024, 18, 2181–2198. [Google Scholar] [CrossRef]
- Jiang, B.; He, C.; Zhao, N.; Nash, P.; Shi, C.; Wang, Z. Ultralight Metal Foams. Sci. Rep. 2015, 5, 13825. [Google Scholar] [CrossRef]
- Yang, X.; Huang, X.; Qiu, X.; Guo, Q.; Zhang, X. Supramolecular Metallic Foams with Ultrahigh Specific Strength and Sustainable Recyclability. Nat. Commun. 2024, 15, 4553. [Google Scholar] [CrossRef] [PubMed]
- Zewdie, F.; Bhatnagar, N. A Composite Metal Foam with Higher Specific Stiffness and Energy Absorption Capacity Developed from Aluminum Alloy (LM24) as a Matrix and Cermet Hollow Spheres of Diameters Ranging from 3 to 6 Mm as Pore-Creating Agents. Int. J. Met. Mater. 2025, 1, 2–20. [Google Scholar] [CrossRef]
- Costanza, G.; Giudice, F.; Sili, A.; Tata, M.E. Correlation Modeling between Morphology and Compression Behavior of Closed-Cell Al Foams Based on X-Ray Computed Tomography Observations. Metals 2021, 11, 1370. [Google Scholar] [CrossRef]
- Albertelli, P.; Esposito, S.; Mussi, V.; Goletti, M.; Monno, M. Effect of Metal Foam on Vibration Damping and Its Modelling. Int. J. Adv. Manuf. Technol. 2021, 117, 2349–2358. [Google Scholar] [CrossRef]
- Arjunan, A.; Baroutaji, A.; Praveen, A.S.; Olabi, A.G.; Wang, C.J. Acoustic Performance of Metallic Foams. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-803581-8. [Google Scholar]
- Thulasikanth, V.; Raghupathy, P. A study on sound-absorption ability of closed-cell aluminium foams. Mater. Technol. 2023, 57, 423–431. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, R. Prediction of Directional Effective Thermal Conductivity of Anisotropic Open-Cell Metal Foam. Int. Commun. Heat Mass Transf. 2025, 166, 109136. [Google Scholar] [CrossRef]
- Costanza, G.; Tata, M.E. Metal Foams: Recent Experimental Results and Further Developments. Metall. Ital. 2011, 103, 3–7. [Google Scholar]
- Karuppasamy, R.; Barik, D. Production Methods of Aluminium Foam: A Brief Review. Mater. Today Proc. 2021, 37, 1584–1587. [Google Scholar] [CrossRef]
- Thulasikanth, V.; Padmanabhan, R. Fabrication of Sustainable Closed-Cell Aluminium Foams Using Recycled Fly Ash and Eggshell Powder. Mater. Today Commun. 2023, 37, 107302. [Google Scholar] [CrossRef]
- Rodinger, T.; Ćorić, D.; Alar, Ž. The Influence of Foaming Agents on Aluminium Foam Cell Morphology. Metals 2023, 13, 1146. [Google Scholar] [CrossRef]
- Tripathi, O.; Singh, D.P.; Dwivedi, V.K.; Agarwal, M. A Focused Review on Aluminum Metallic Foam: Processing, Properties, and Applications. Mater. Today Proc. 2021, 47, 6622–6627. [Google Scholar] [CrossRef]
- Islam, M.A.; Kader, M.A.; Escobedo, J.P. Dynamic Perforation Behavior of Closed-Cell Aluminium Foams and Foam Core Sandwich Panels with Various Shaped Projectile Tips. Mech. Res. Commun. 2025, 148, 104472. [Google Scholar] [CrossRef]
- Behymer, N.; Morsi, K. Review: Closed-Cell Metallic Foams Produced via Powder Metallurgy. Metals 2023, 13, 959. [Google Scholar] [CrossRef]
- Jozić, S.; Lela, B.; Krolo, J.; Jakovljević, S. Production of Open-Cell Metal Foams by Recycling of Aluminum Alloy Chips. Materials 2023, 16, 3930. [Google Scholar] [CrossRef] [PubMed]
- Costanza, G.; Dodbiba, G.; Tata, M.E. Optimization of the Process Parameters for the Manufacturing of Open-Cells Iron Foams with High Energy Absorption. Procedia Struct. Integr. 2016, 2, 2277–2282. [Google Scholar] [CrossRef][Green Version]
- Hassan, A.; Alnaser, I.A. A Review of Different Manufacturing Methods of Metallic Foams. ACS Omega 2024, 9, 6280–6295. [Google Scholar] [CrossRef] [PubMed]
- Costanza, G.; Gusmano, G.; Montanari, R.; Tata, M.E. Manufacturing Routes and Application of Metals Foams. Metall. Ital. 2003, 95, 31–35. [Google Scholar]
- Kulshreshtha, A.; Dhakad, S. Preparation of Metal Foam by Different Methods: A Review. Mater. Today Proc. 2020, 26, 1784–1790. [Google Scholar] [CrossRef]
- Costanza, G.; Tata, M.E.; Trillicoso, G. Al Foams Manufactured by PLA Replication and Sacrifice. Int. J. Lightweight Mater. Manuf. 2021, 4, 62–66. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, M.; Yang, R.; Shang, S.; Zhang, P.; Chen, N.; Tang, L.; Chen, X. Cell Size Controlling of Closed-Cell Aluminum Foams. J. Mater. Res. Technol. 2025, 36, 1294–1313. [Google Scholar] [CrossRef]
- Verran, G.O.; Kurzawa, U. An Experimental Study of Aluminum Can Recycling Using Fusion in Induction Furnace. Resour. Conserv. Recycl. 2008, 52, 731–736. [Google Scholar] [CrossRef]
- Mustapha, K.A.; Shikh Anuar, F.; Mohd Saat, F.A.-Z. Prediction of Slip Velocity at the Interface of Open-Cell Metal Foam Using 3D Printed Foams. Colloids Interfaces 2022, 6, 80. [Google Scholar] [CrossRef]
- Mahadev; Sreenivasa, C.G.; Shivakumar, K.M. A Review on Prodution of Aluminium Metal Foams. IOP Conf. Ser. Mater. Sci. Eng. 2018, 376, 012081. [Google Scholar] [CrossRef]
- Poot Manzanilla, A.J.; Cruz Ramírez, A.; Colin García, E.; Romero Serrano, J.A.; Sánchez Alvarado, R.G.; Suárez Rosales, M.Á. Production of Refined and Modified Closed-Cell Aluminum Foams by Melt-Foaming Method. Metals 2023, 13, 622. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Chen, X.; Shi, T.; Liu, Z.; Wang, N. Fabrication of Aluminum Foams with Small Pore Size by Melt Foaming Method. Metall. Mater. Trans. B 2017, 48, 754–762. [Google Scholar] [CrossRef]
- Mudge, A.; Morsi, K. Fabrication of Uniform and Rounded Closed-Cell Aluminum Foams Using Novel Foamable Precursor Particles (FPPs). Metals 2024, 14, 120. [Google Scholar] [CrossRef]
- Wang, N.; Maire, E.; Chen, X.; Adrien, J.; Li, Y.; Amani, Y.; Hu, L.; Cheng, Y. Compressive Performance and Deformation Mechanism of the Dynamic Gas Injection Aluminum Foams. Mater. Charact. 2019, 147, 11–20. [Google Scholar] [CrossRef]
- Babcsan, N.; Beke, S.; Makk, P.; Soki, P.; Számel, G.; Degischer, H.P.; Mokso, R. ALUHAB—The Superior Aluminium Foam. In ICAA13 Pittsburgh; Springer: Cham, Switzerland, 2012; pp. 1005–1010. ISBN 978-3-319-48761-8. [Google Scholar]
- Wang, N.; Chen, X.; Li, Y.; Liu, Z.; Zhao, Z.; Cheng, Y.; Liu, Y.; Zhang, H. The Cell Size Reduction of Aluminum Foam with Dynamic Gas Injection Based on the Improved Foamable Melt. Colloids Surf. A Physicochem. Eng. Asp. 2017, 527, 123–131. [Google Scholar] [CrossRef]
- Noack, M.A.; Bülk, F.; Wang, N.; Banhart, J.; García-Moreno, F. Aluminium Foam with Sub-Mm Sized Cells Produced Using a Rotating Gas Injector. Mater. Sci. Eng. B 2021, 273, 115427. [Google Scholar] [CrossRef]
- Baumgärtner, F.; Duarte, I.; Banhart, J. Industrialization of Powder Compact Toaming Process. Adv. Eng. Mater. 2000, 2, 168–174. [Google Scholar] [CrossRef]
- Banhart, J.; Seeliger, H. Recent Trends in Aluminum Foam Sandwich Technology. Adv. Eng. Mater. 2012, 14, 1082–1087. [Google Scholar] [CrossRef]
- Banhart, J.; Seeliger, H.-W. Aluminium Foam Sandwich Panels: Manufacture, Metallurgy and Applications. Adv. Eng. Mater. 2008, 10, 793–802. [Google Scholar] [CrossRef]
- Yuan, G.; Li, Y.; Hu, L.; Fu, W. Preparation of Shaped Aluminum Foam Parts by Investment Casting. J. Mater. Process. Technol. 2023, 314, 117897. [Google Scholar] [CrossRef]
- Lin, H.-S.; Lin, L.-Y. Improving Visible-Light Responses and Electric Conductivities by Incorporating Sb2S3 and Reduced Graphene Oxide in a WO3 Nanoplate Array for Photoelectrochemical Water Oxidation. Electrochim. Acta 2017, 252, 235–244. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Lin, L.-Y. Synthesis of Monoclinic BiVO4 Nanorod Array for Photoelectrochemical Water Oxidation: Seed Layer Effects on Growth of BiVO4 Nanorod Array. Electrochim. Acta 2018, 285, 164–171. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Lin, L.-Y.; Li, X. Efficient Battery Supercapacitor Hybrid Devices with Quaternary Metal Oxide Electrodes Based on Nickel and Cobalt. J. Energy Storage 2019, 25, 100826. [Google Scholar] [CrossRef]
- Hong, W.-L.; Lin, L.-Y. Design of Nickel Cobalt Oxide and Nickel Cobalt Oxide@nickel Molybdenum Oxide Battery-Type Materials for Flexible Solid-State Battery Supercapacitor Hybrids. J. Power Sources 2019, 435, 226797. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Lin, L.-Y. Morphology Variation for the Nickel Cobalt Molybdenum Copper Oxide with Different Metal Ratios and Their Application on Energy Storage. Electrochim. Acta 2019, 298, 745–755. [Google Scholar] [CrossRef]
- Yan, X.; Xia, M.; Liu, H.; Zhang, B.; Chang, C.; Wang, L.; Yang, G. An Electron-Hole Rich Dual-Site Nickel Catalyst for Efficient Photocatalytic Overall Water Splitting. Nat. Commun. 2023, 14, 1741. [Google Scholar] [CrossRef]
- Lee, P.-Y.; Lin, L.-Y.; Yougbaré, S. Sulfurization of Nickel–Cobalt Fluoride Decorating Ammonia Ions as Efficient Active Material of Supercapacitor. J. Solid State Chem. 2022, 313, 123345. [Google Scholar] [CrossRef]
- Ye, Z.-H.; Lin, L.-Y.; Yu, C.-F. Nickel Precursor-Free Synthesis of Nickel Cobalt Sulfide on Ni Foam: Effects of the pH Value on the Morphology and the Energy-Storage Ability. J. Energy Storage 2016, 8, 60–68. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Lin, L.-Y.; Huang, Y.-Y.; Hong, W.-L. Nickel Precursor-Free Synthesis of Nickel Cobalt-Based Ternary Metal Oxides for Asymmetric Supercapacitors. Electrochim. Acta 2018, 281, 692–699. [Google Scholar] [CrossRef]
- Xiao, C.-Y.; Chen, T.-Y.; Chung, R.-J.; Yougbaré, S.; Lin, L.-Y.; Wu, Y.-F. Binder-Free Synthesis of Metal Organic Framework Derived Cobalt Sulfide on Carbon Cloth for Efficient Energy Storage Devices. J. Energy Storage 2022, 55, 105622. [Google Scholar] [CrossRef]
- Cheng, T.-M.; Huang, H.-W.; Chen, T.-Y.; Yougbaré, S.; Lin, L.-Y.; Wu, Y.-F. Facile Synthesis of Metal Organic Framework-Derived Cobalt Sulfide on Ni Foam as Binder-Free Electrode of Battery Supercapacitor Hybrid. J. Energy Storage 2022, 56, 106110. [Google Scholar] [CrossRef]
- Pierożyński, B.; Kuczyński, M.; Mikołajczyk, T. Simple Nickel Foam Modification Procedures for Enhanced Ni Foam Supercapacitor Applications. Crystals 2024, 14, 777. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Sambasivam, S.; Raghavendra, K.V.G.; Vinodh, R.; Obaidat, I.M.; Kim, H.-J. Facile Synthesis of Hierarchical Flower-like NiMoO4-CoMoO4 Nanosheet Arrays on Nickel Foam as an Efficient Electrode for High Rate Hybrid Supercapacitors. J. Energy Storage 2020, 30, 101550. [Google Scholar] [CrossRef]
- Wang, Z.; Li, P.; Pu, L.; Hong, P.; Ran, Y.; Qian, W.; Wang, Y. Multi-Sized Nanosheets Cobalt-Iron Layered Double Hydroxide Grown on Nickel Foam as High Performance Supercapacitor Electrode Material. J. Energy Storage 2021, 33, 102088. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Huang, W. Porous Graphene Oxide Prepared on Nickel Foam by Electrophoretic Deposition and Thermal Reduction as High-Performance Supercapacitor Electrodes. Materials 2017, 10, 936. [Google Scholar] [CrossRef]
- Hamidi, E.; Ganesan, P.B.; Sharma, R.K.; Yong, K.W. Computational Study of Heat Transfer Enhancement Using Porous Foams with Phase Change Materials: A Comparative Review. Renew. Sustain. Energy Rev. 2023, 176, 113196. [Google Scholar] [CrossRef]
- Variji, N.; Siavashi, M.; Tahmasbi, M.; Bidabadi, M. Analysis of the Effects of Porous Media Parameters and Inclination Angle on the Thermal Storage and Efficiency Improvement of a Photovoltaic-Phase Change Material System. J. Energy Storage 2022, 50, 104690. [Google Scholar] [CrossRef]
- Ali, H.M. Heat Transfer Augmentation of Porous Media (Metallic Foam) and Phase Change Material Based Heat Sink with Variable Heat Generations: An Experimental Evaluation. Sustain. Energy Technol. Assess. 2022, 52, 102218. [Google Scholar] [CrossRef]
- Huo, Y.; Yin, M.; Rao, Z. Heat Transfer Enhanced by Angle-Optimized Fan-Shaped Porous Medium in Phase Change Thermal Energy Storage System at Pore Scale. Int. J. Therm. Sci. 2022, 172, 107363. [Google Scholar] [CrossRef]
- Asefi, G.; Ma, T.; Wang, R. Parametric Investigation of Photovoltaic-Thermal Systems Integrated with Porous Phase Change Material. Appl. Therm. Eng. 2022, 201, 117727. [Google Scholar] [CrossRef]
- NematpourKeshteli, A.; Iasiello, M.; Langella, G.; Bianco, N. Increasing Melting and Solidification Performances of a Phase Change Material-Based Flat Plate Solar Collector Equipped with Metal Foams, Nanoparticles, and Wavy Wall-Y-Shaped Surface. Energy Convers. Manag. 2023, 291, 117268. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Chen, Z.; Gan, G.; Su, Y. Impact of Porous Host Materials on the Compromise of Thermochemical Energy Storage Performance. Renew. Energy 2025, 245, 122784. [Google Scholar] [CrossRef]
- Fiedler, T.; Movahedi, N. Compact Aluminium Foam Heat Exchangers. Metals 2023, 13, 1440. [Google Scholar] [CrossRef]
- Pulvirenti, B.; Celli, M.; Barletta, A. Flow and Convection in Metal Foams: A Survey and New CFD Results. Fluids 2020, 5, 155. [Google Scholar] [CrossRef]
- Weng, S.; An, Q.; Xu, Y.; Jiao, Y.; Chen, J. In-Situ Formation of NiFe-MOF on Nickel Foam as a Self-Supporting Electrode for Flexible Electrochemical Sensing and Energy Conversion. Chemosensors 2023, 11, 242. [Google Scholar] [CrossRef]
- Cha, S.-J.; Tak, H.-J.; Hwang, B.-K.; Lee, J.-P.; Kim, J.-H.; Lee, J.-M. Structural Assessment of Independent Type-C Liquid Hydrogen Fuel Tank. J. Mar. Sci. Eng. 2025, 13, 730. [Google Scholar] [CrossRef]
- Gancarczyk, A.; Sindera, K.; Iwaniszyn, M.; Piątek, M.; Macek, W.; Jodłowski, P.J.; Wroński, S.; Sitarz, M.; Łojewska, J.; Kołodziej, A. Metal Foams as Novel Catalyst Support in Environmental Processes. Catalysts 2019, 9, 587. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, S.; Schäfer, D.; Peters, R.; Kunz, F.; Eichel, R.-A. Numerical Modeling and Simulation of the Solid Oxide Cell Stacks and Metal Interconnect Oxidation with OpenFOAM. Energies 2023, 16, 3827. [Google Scholar] [CrossRef]
- Sahu, S.K.; Sreekanth, P.S.R.; Reddy, S.V.K. A Brief Review on Advanced Sandwich Structures with Customized Design Core and Composite Face Sheet. Polymers 2022, 14, 4267. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Zhong, C.; Zhang, Y.; Hao, S.; Sun, R. Piezoelectric Composite Vibrator with a Bilaminated Structure for Bending Vibration. Appl. Sci. 2019, 9, 4191. [Google Scholar] [CrossRef]
- Meng, H.; Li, G. A Review of Stimuli-Responsive Shape Memory Polymer Composites. Polymer 2013, 54, 2199–2221. [Google Scholar] [CrossRef]
- Lehmhus, D.; Vesenjak, M.; Schampheleire, S.; Fiedler, T. From Stochastic Foam to Designed Structure: Balancing Cost and Performance of Cellular Metals. Materials 2017, 10, 922. [Google Scholar] [CrossRef] [PubMed]
- Kalia, K.; Ameli, A. Additive Manufacturing of Functionally Graded Foams: Material Extrusion Process Design, Part Design, and Mechanical Testing. Addit. Manuf. 2024, 79, 103945. [Google Scholar] [CrossRef]







| Material | Specific Capacitance | Current Density | Capacity Retention | Cycles |
|---|---|---|---|---|
| Ni-Fe LDH on NF | 2078 mF cm−2 | 5 mA cm−2 | 42.6% | 500 |
| Sulfidation of Ni-Fe LDHS | 992 mF cm−2 | 2 mA cm−2 | 64.5% | 2000 |
| Co-Fe LDHs on NF | 3340 mF cm−2 | 1 mA cm−2 | 85.1% | 4000 |
| V-CaCl2 | EP-CaCl2 | Pm-CaCl2 | SG-CaCl2 | V-CaCl2 |
|---|---|---|---|---|
| Discharging reaction rate | Slowest | Slow | Fast | Fastest |
| Temperature decay rate | Fast | Slow | Fast | Slow |
| Reaction bed pressure drop | Low | Low | Medium | Very high |
| Low-temperature charging reaction rate | Fast | Slow | Fastest | Fast |
| High-temperature charging reaction rate | Slow | Medium | Fast | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceci, A.; Costanza, G.; Giudice, F.; Sili, A.; Tata, M.E. The Role of Metal Foams for Sustainability and Energy Transition. Alloys 2025, 4, 16. https://doi.org/10.3390/alloys4030016
Ceci A, Costanza G, Giudice F, Sili A, Tata ME. The Role of Metal Foams for Sustainability and Energy Transition. Alloys. 2025; 4(3):16. https://doi.org/10.3390/alloys4030016
Chicago/Turabian StyleCeci, Alessandra, Girolamo Costanza, Fabio Giudice, Andrea Sili, and Maria Elisa Tata. 2025. "The Role of Metal Foams for Sustainability and Energy Transition" Alloys 4, no. 3: 16. https://doi.org/10.3390/alloys4030016
APA StyleCeci, A., Costanza, G., Giudice, F., Sili, A., & Tata, M. E. (2025). The Role of Metal Foams for Sustainability and Energy Transition. Alloys, 4(3), 16. https://doi.org/10.3390/alloys4030016