1. Introduction
Soft magnetic materials and composites (SMC) have numerous applications in modern portable electronic device applications [
1]. The need of miniaturization and the low profile of inductive components for portable devices has led to the development of new power inductive devices. This is in line with the observed trend from one DC–DC converter in the power circuitry to a point-of-load (POL) concept with many smaller converters as a power source for individual ICs or modules. Moreover, the operating voltages of modules in a device might be different, requiring individual and miniaturized DC–DC converters to convert the power provided by the battery into the various circuit blocks. FeSiCr alloys have been proposed as suitable magnetic materials for the fabrication of power multilayer inductors [
2,
3]. As compared to Ni–Cu–Zn ferrites, which are typically used in multilayer inductors, the soft magnetic alloys have benefits of high saturation magnetization and good DC-superposition characteristics and, hence, are able to withstand higher current densities without a significant reduction of the inductance [
2,
3]. One critical issue is the low resistivity of the alloy. Low power losses,
Pv, of the soft magnetic alloy are required for high-frequency applications. Since eddy current losses
Pe are a major contribution to
Pv, the resistivity of the alloy needs to be increased in order to reduce
Pe. Therefore, it is very important to provide sufficient insulation resistance, which is realized by creating a thin insulating oxide layer at the surface of the alloy particles during annealing and multilayer fabrication. It was demonstrated that high compaction pressures are needed to achieve sufficient densification, and that subsequent annealing in the temperature range between 700 °C and 850 °C leads to the formation of an insulating chromia layer with the resistivity increasing with annealing temperature [
4]. Many other studies have focused on increasing the resistivity by coating the alloy particles. Insulating coatings are typically classified as organic, inorganic, and composite coatings. Organic coatings are primarily thermosetting materials, such as epoxy resins, phenolic resins or polyimides. Examples of inorganic coatings include phosphates [
5], silica [
6], MgO [
7], alumina [
8], or ferrites [
9].
Another problem in multilayer chip fabrication arises from the interaction of FeSiCr with the Ag inner electrode material during thermal processing in air. It was observed that Ag reacts with the Cr
2O
3 layer to form semiconducting AgCrO
2 delafossite-type oxide, reducing the insulation resistance and decreasing the inductor performance [
10]. It has been observed that the annealing of FeSiCr-based multilayer inductors in nitrogen allows us to enhance the inductance by suppressing non-magnetic phase formation, but re-annealing in air is required to generate sufficient insulation resistance [
11]. Thus, it seems that the oxygen partial pressure is another variable in addition to the annealing temperature to tailor the electromagnetic properties of FeSiCr alloys and the corresponding inductors. However, the effect of oxygen partial pressure upon annealing on the oxidation layer formation and the corresponding electromagnetic properties has not been systematically studied. Hence, it is desirable to investigate the role of oxygen partial pressure during annealing for the performance optimization of FeSiCr-based power multilayer inductors. In addition, a new concept of combining FeSiCr and Ni–Cu–Zn ferrites with Ag metallization to give a composite multilayer inductor was proposed recently [
12], and annealing protocols include
T and
pO2 as variables as well.
In this study, we have investigated, for the first time, the influence of oxygen partial pressure during annealing at 900 °C of a FeSiCr alloy on the phase formation, insulating Cr oxide layer formation, resistivity, and permeability. We contrast the effect of the annealing temperature in air with that of the oxygen partial pressure (from 0.21 atm down to 10−5 atm) during the annealing of FeSiCr compacts at 900 °C. The results indicate that annealing at 900 °C under lower pO2 leads to decreasing insulation resistance and higher permeability. Annealing at intermediate pO2, on the other hand, provides sufficient insulation resistance and opens a new process window for the firing of multilayer power inductors.
2. Materials and Methods
A commercial FeSiCr powder was used with a composition of 90.3 wt% Fe, 5.4 wt% Si, and 4.3 wt% Cr (Chung Yo Materials Co., Ltd., Kaohsiung, Taiwan). The powder was mixed with a polyvinyl alcohol solution and dried at 95 °C. Pellets and toroids were uniaxially dry pressed with a pressure of 100 MPa. The samples were heated for binder burnout with 1 K/min to 300 °C and annealed for 5 h in air. In the first experimental series, the samples were further annealed with a 5 K/min heating and cooling rate to temperatures between 700 °C and 900 °C with a dwell time of 1 h. In the second series, the samples were annealed at 900 °C with a 1 h dwell time in a gas atmosphere with oxygen partial pressures between 0.21 atm (21%) and 10−5 atm (0.001%) using a computer-controlled gas-mixing unit with mass flow controllers. The pO2 was measured directly at the sample position in the tube furnace with a zirconia-based oxygen sensor system (Zirox GmbH, Greifswald, Germany).
The specific surface area S of the powders was determined from nitrogen adsorption isotherms (BET, Nova 2000, Quantachrome Instruments, Boynton Beach, FL, USA); a mean particle size dBET was estimated using as dBET = 6/ρ·S (with density ρ; assuming spherical particles). The particle size was characterized using laser diffraction (Mastersizer, Malvern Panalytical GmbH, Kassel, Germany). The shrinkage of a cylindrical compact was measured using a Netzsch DIL402 dilatometer (Netzsch-Gerätebau GmbH, Germany) during heating to 1000 °C with a 5 K/min heating rate. Thermal analysis was performed using a TG/DTA 92-16.18 system (Seteram, Caluire, France). The microstructure of the samples was studied on polished samples with a scanning electron microscope (SEM, Ultra 55, Zeiss Microscopy GmbH, Jena, Germany). Elemental analysis was performed using a Bruker EDX system. The phase formation of the materials was evaluated using X-ray diffraction (XRD) with Cu–Kα radiation (15–90°, 3 s, 0.015°/step, Advance D8, Bruker AXS, Karlsruhe, Germany). Rietveld refinements were performed using the software Topas version 6 (Bruker AXS). The resistivity of the samples was measured using a two-point measurement set-up after contacting the samples with silver paste. The permeability of sintered toroids was measured using an Agilent E4991A impedance/material analyzer (Keysight Techn., Santa Rosa, CA, USA) in the frequency range from 1 MHz to 1 GHz.
3. Results
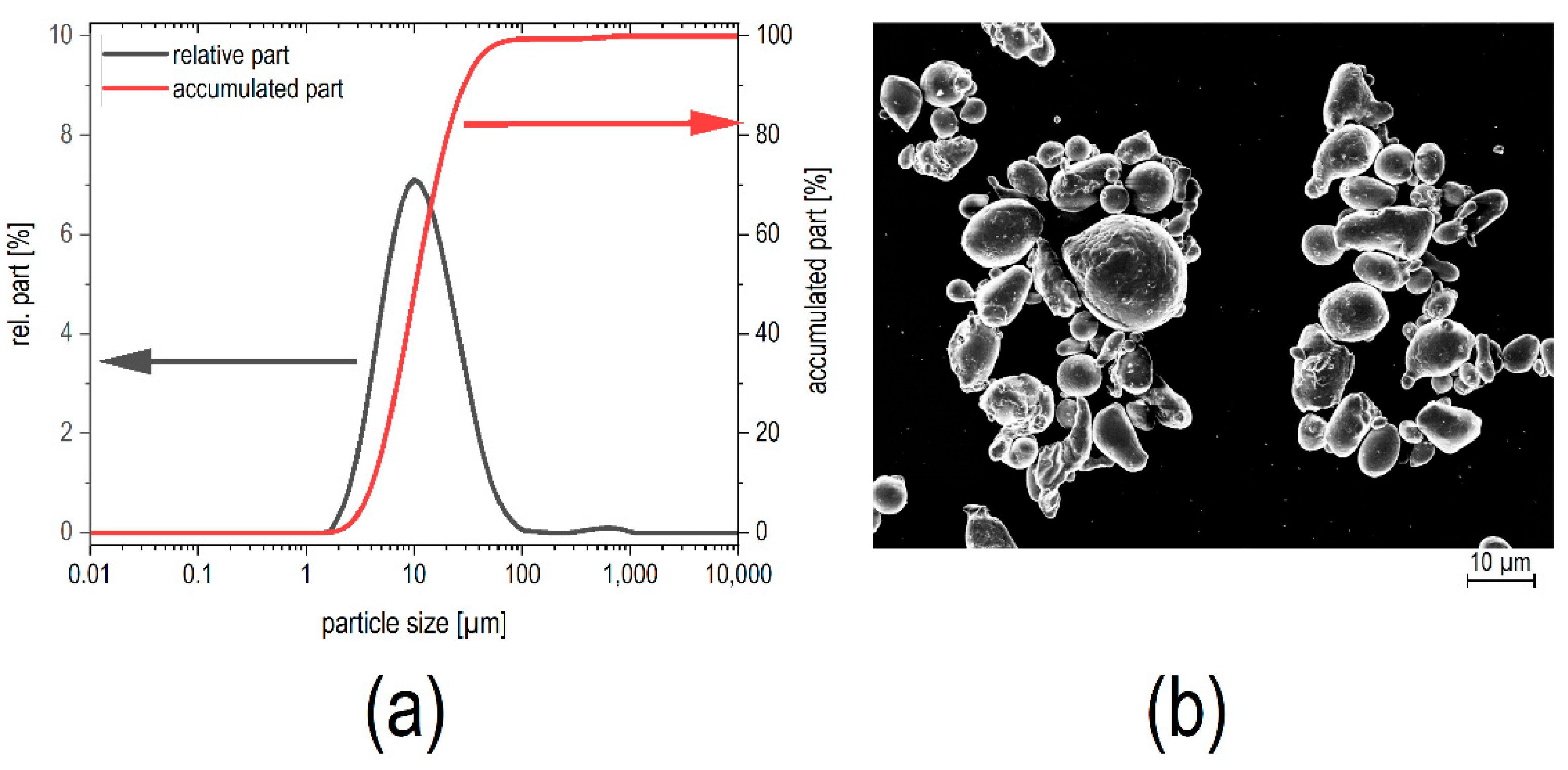
The morphological properties of the FeSiCr powder were studied. The particle size distribution as obtained from laser diffraction revealed a mean particle site of
d50 = 10.3 µm (
Figure 1a) with a broad distribution of sizes (
d10 = 4.1 µm,
d90 = 28.6 µm). The specific surface area was found to be
S = 0.3 m
2/g. This translates into a mean particle size of the primary particles of
dBET = 2.6 µm. SEM micrographs confirmed that the powder consisted of agglomerates with a size between 5 and 30 µm, built from smaller particles of around 1–3 µm in size.
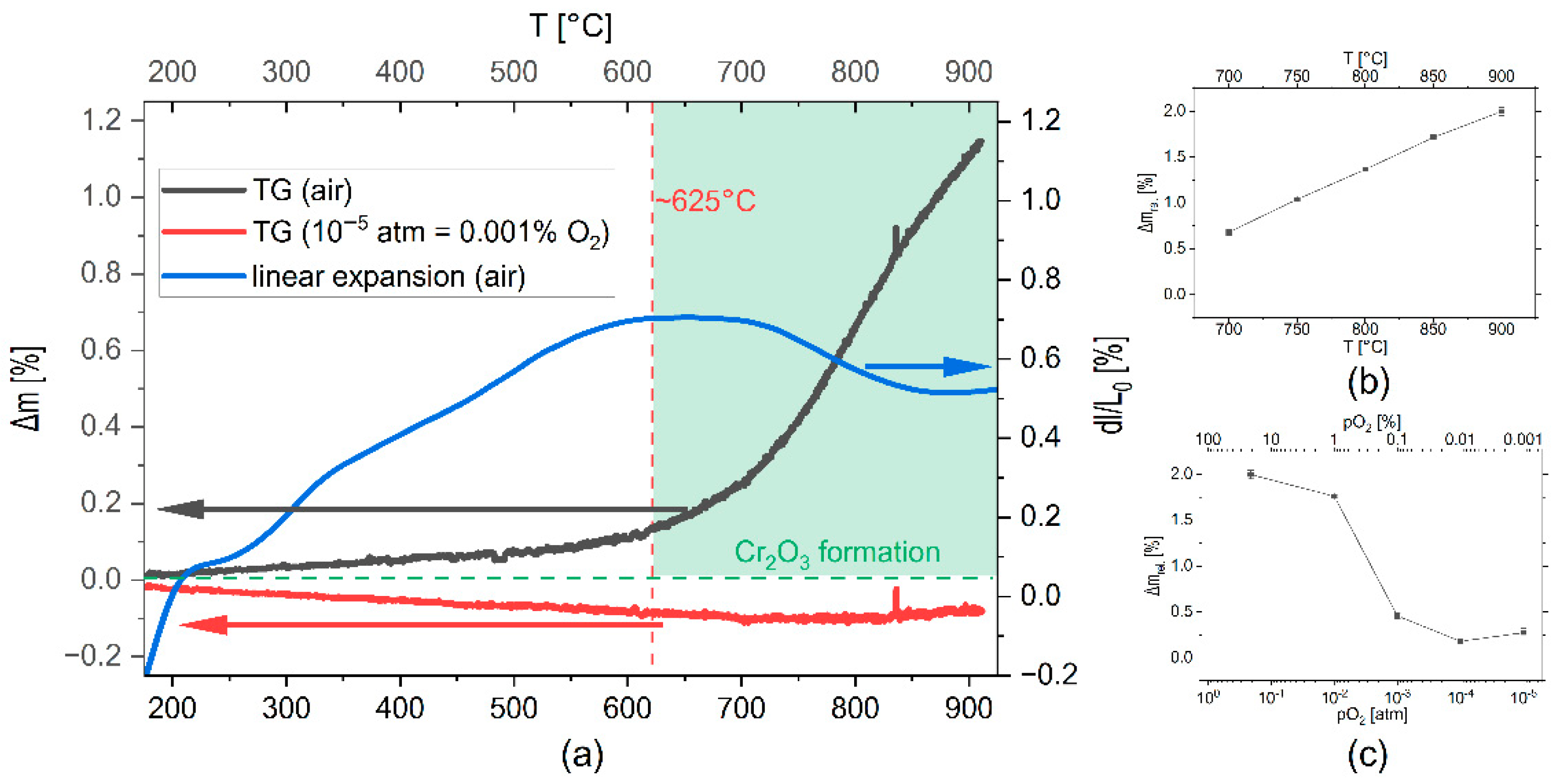
The oxidation behavior of the FeSiCr powder was investigated using thermal analysis. The mass gain of the powder as a function of temperature, as determined with thermogravimetry (TG), signaled the start of an oxidation process at about 300 °C in air (
Figure 2a), slowly increasing into a significant mass increase at about 600 °C, and reaching a mass gain of about 1.2% at 900 °C. Contrarily, no mass gain was observed in a gas atmosphere with
pO2 = 10
−5 atm. Simultaneously, the expansion of a powder pellet as a function of temperature in air atmosphere determined using a dilatometer indicated an almost constant expansion up to a maximum at about 625 °C, where the thermal expansion of the FeSiCr phase seemed to be overshadowed by another process leading to shrinkage at a higher temperature (
Figure 2a). The observed mass gain originated from partial oxidation of the alloy and the formation of a layer of Cr
2O
3 (as shown later). This tendency towards oxidation was also monitored by measuring the weight change of the samples upon annealing. Annealing for 1 h in air led to a mass gain increasing with temperature (
Figure 2b), reaching 2% at 900 °C. On the other hand, the observed mass gain decreased with decreasing partial pressure in the atmosphere during annealing for 1 h at 900 °C (
Figure 2c), indicating a significant lower tendency towards oxidation and Cr
2O
3 formation.
We also monitored the temperature and oxygen partial pressure during annealing in the furnace next to the sample position. The obtained in situ annealing protocols are shown in
Figure 3. For annealing at various temperatures in air, the observed temperature—time protocols indicated a regular annealing process; the
pO2 was close to the expected value of 0.21 atm (
Figure 3a). However, during annealing at various oxygen partial pressures, a different picture emerged: the
pO2 remained stable and constant in air atmosphere, but with nominally decreasing oxygen partial pressure, the
pO2 signal from the oxygen sensor decreased below the nominal
pO2 value already during the heating period, showing much lower values of
pO2 during the dwell time of the anneals. For example, at nominal
pO2 = 10
−5 atm, the
pO2 close to the sample position rapidly decreased upon heating and reached values as low as 10
−16 atm during heating at 900 °C. This behavior demonstrated that FeSiCr soaked up all available oxygen in the gas atmosphere with reduced oxygen concentration, thus locally generating very reducing atmospheric conditions.
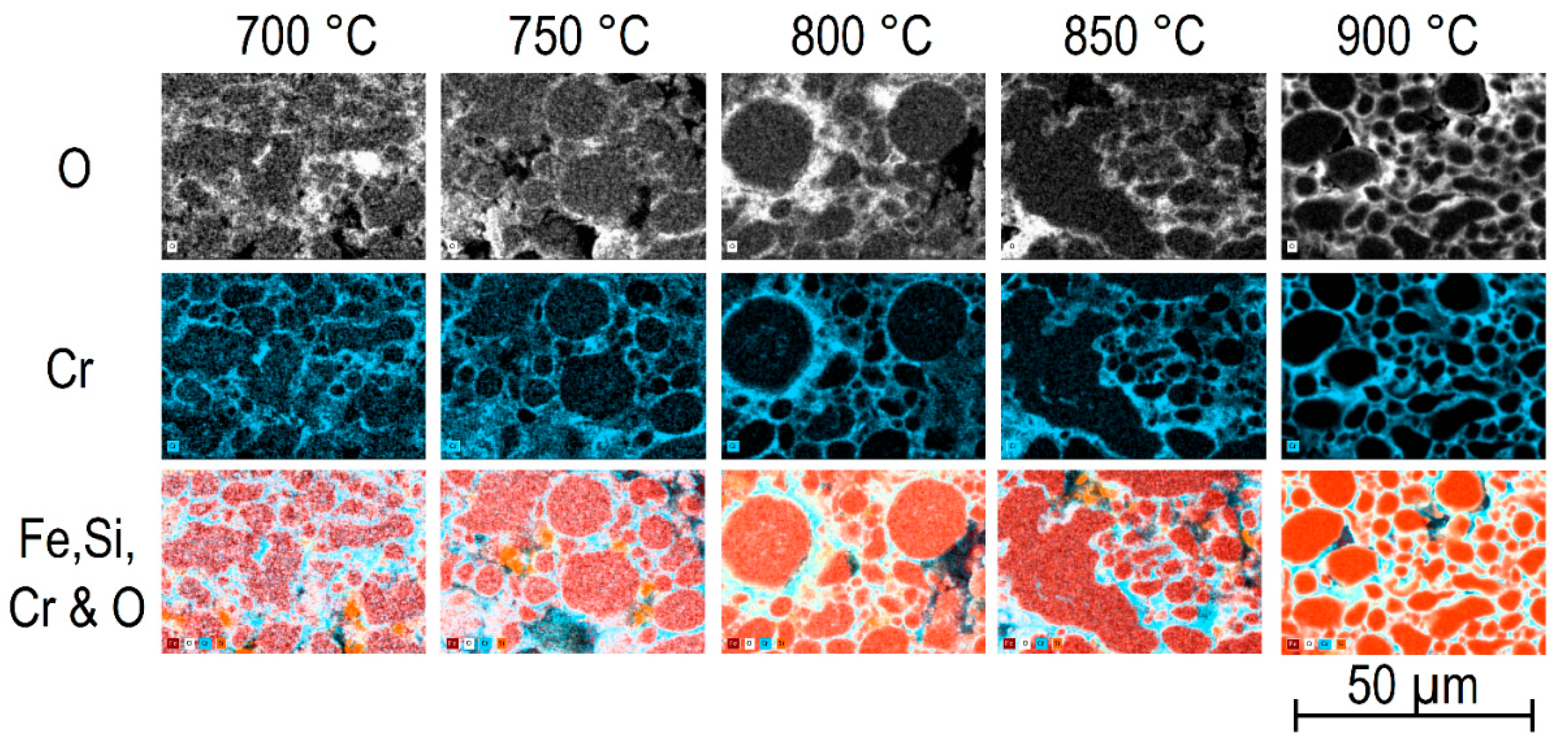
The samples obtained after annealing at different temperatures in air, or at different
pO2 values at 900 °C, were investigated using electron microscopy. EDX maps of samples annealed in air between 700 and 900 °C indicated the formation of a Cr
2O
3 layer at the surface of the alloy particles (
Figure 4). The O- and Cr-maps showed a layer of Cr and O connecting the larger alloy particles, with the thickness of the Cr
2O
3 layer increasing with annealing temperature (
Figure 4). This tendency towards oxidation and the oxide layer formation agrees well with the results from thermogravimetry (TG,
Figure 2a) and the mass change during annealing (
Figure 2b), indicating an enhancement of mass with annealing temperature.
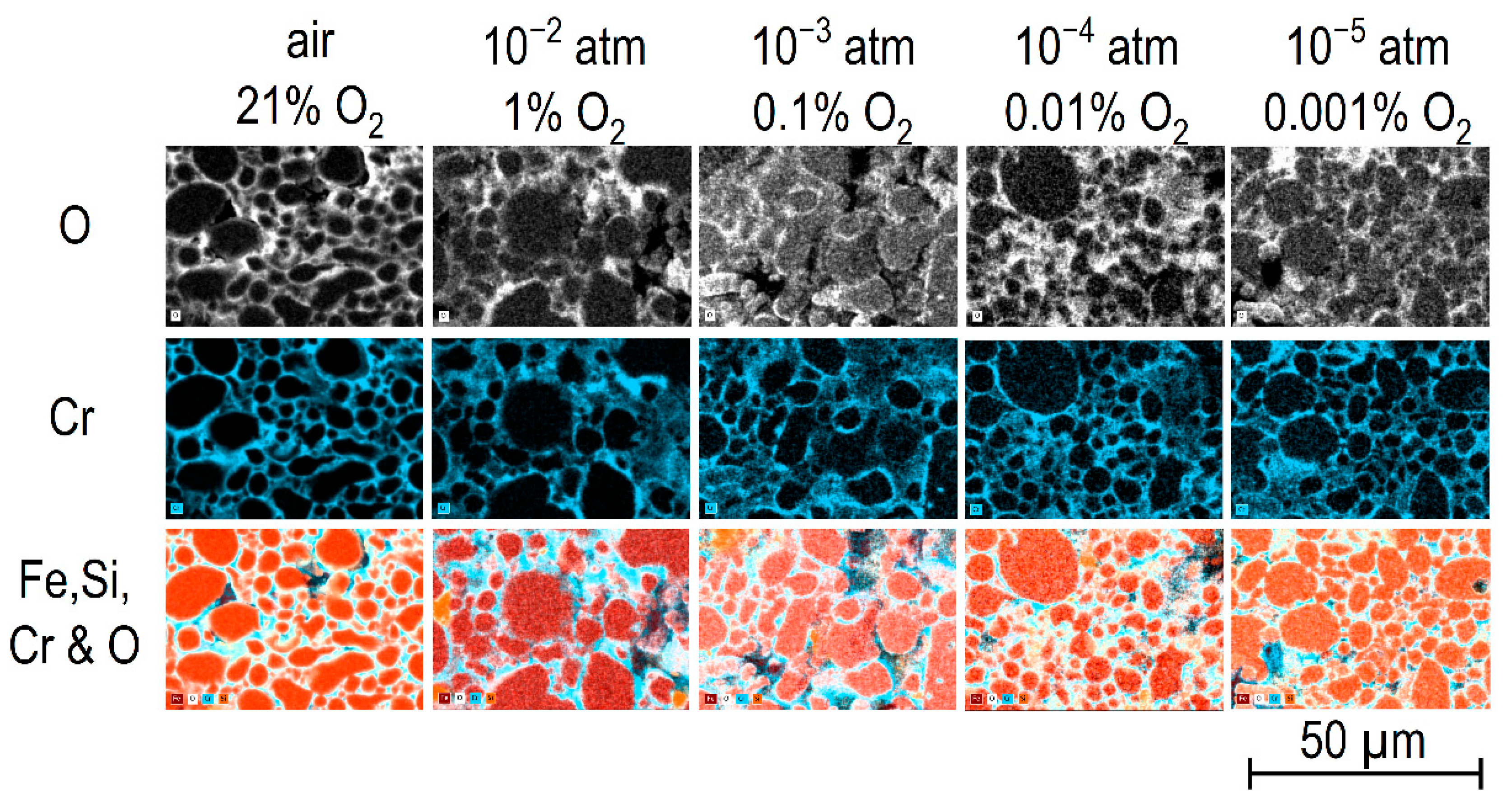
Annealing of the samples at 900 °C at different reduced oxygen partial pressures from 0.21 atm down to 10
−5 atm was mirrored in less intense oxidation and Cr
2O
3 surface layer formation as compared to air (
Figure 5). Correspondingly, the TG curve recorded during annealing in nitrogen with
pO2 = 10
−5 atm did not reveal any mass uptake (
Figure 2a), and the mass change of the samples during a 1 h anneal at 900 °C in that gas atmosphere was very small (
Figure 2c). These findings indicate that the formation of a Cr
2O
3 surface layer was less pronounced under decreasing
pO2 in the surrounding gas atmosphere.
In addition, the element concentrations of Fe, Si, and Cr in the FeSiCr particles were determined using EDX (measurement of five point-scans in the particle centers). It is interesting to note, that after annealing in air, the Cr concentration within the alloy particles decreased with increasing annealing temperature, whereas the Fe concentration increased, and the Si content remained more or less unchanged (
Figure 6a). This observation might be interpreted as the increasing diffusion of Cr out of the alloy particles with increasing annealing temperature, as the formation of the Cr
2O
3 surface layer requires the diffusion of Cr from the bulk of the particles to the surface. After annealing at 900 °C, the Cr concentration in the alloy particles decreased to about 1 wt% as compared to about 4% in the initial alloy. Consequently, the Fe concentration in the alloy particles increased. If the samples were annealed at 900 °C in reduced
pO2, the Cr concentration in the alloy slightly increased with decreasing
pO2, indicating the reduced formation of a chromia surface layer and, hence, increased Cr content in the alloy particles (
Figure 6b).
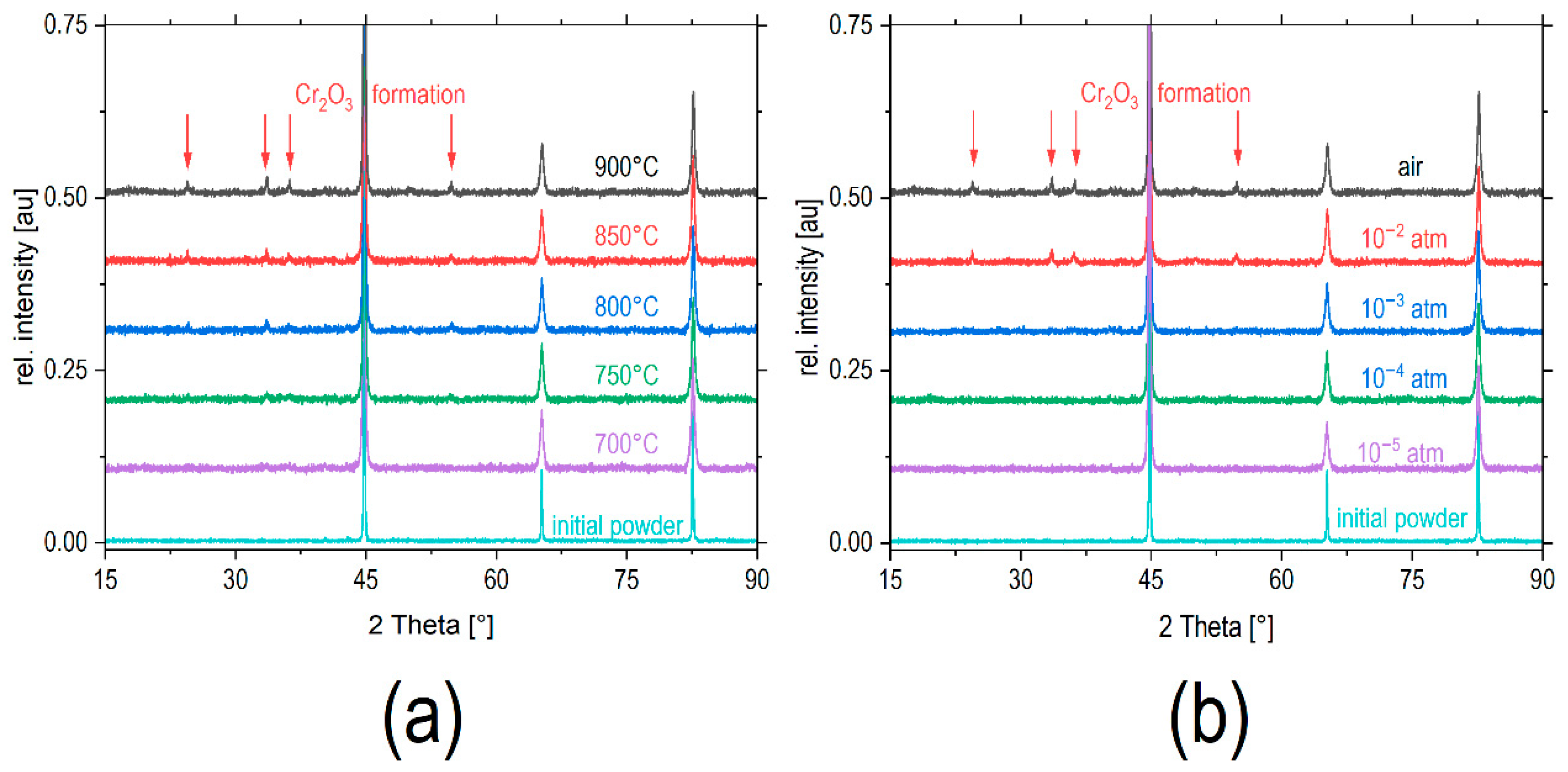
The results of an XRD study confirm the tendency towards oxidation upon annealing in air (
Figure 7a). The initial alloy powder exhibited peaks of the alloy with a body-centered lattice typical of α-Fe [
13]. Peaks of a corundum-type structured Cr
2O
3 phase [
14] started to appear at 750 °C with their intensity increasing with the annealing temperature. After annealing at 900 °C, the main peaks of chromia clearly appeared. The concentration of the formed Cr
2O
3 phase was determined using Rietveld refinements, and the results are shown in
Figure 8a. The chromia content was practically zero in the initial powder and increased to 12.2(3) % after annealing at 900 °C. Simultaneously, the lattice parameter
a0 of the body-centered cubic alloy phase decreased from
a0 = 2.8601(1) Å of the initial alloy to
a0 = 2.8580(1) Å for the 900 °C annealed sample. The decrease in the cubic lattice parameter reflects the change in the alloy composition, as Cr tended to diffuse to the alloy particle surface to be oxidized into Cr
2O
3; therefore, the alloy contained less Cr and more Fe with increasing annealing temperature (
Figure 6a). It has been demonstrated that the cubic lattice parameter in the body-centered Fe–Cr alloy system increases with the Cr content, even more than expected according to linear Vegard’s rule [
15,
16].
If the samples were annealed at 900 °C in various oxygen partial pressures, then the tendency towards the formation of Cr
2O
3, as observed in air, was significantly reduced. After annealing in gas atmospheres with lower
pO2, the intensity of the Cr
2O
3 peaks decreased (
Figure 7b). Rietveld refinements confirmed a decrease in the Cr
2O
3 concentration in the annealed samples and reached about 8(1) wt% at
pO2 = 10
−5 atm (
Figure 8b). At the same time, the cubic alloy lattice parameter increased with decreasing
pO2, indicating an increase in the Cr content in the alloy and, consequently, less Cr
2O
3 surface layer formation (
Figure 8b).
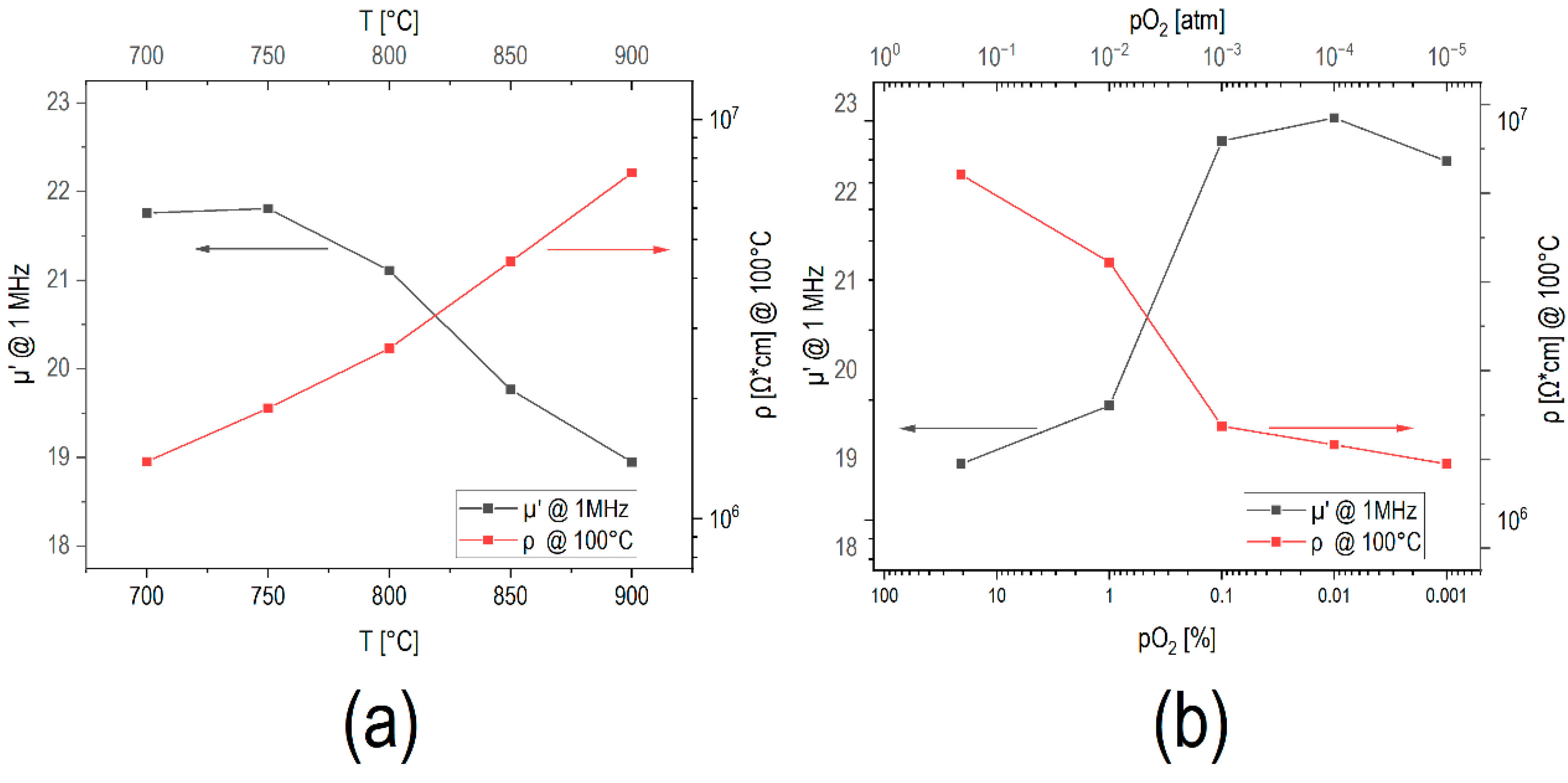
The formation of insulating Cr
2O
3 layers at the surface of the alloy particles directly affected the electrical resistivity. The increasing Cr
2O
3 layer thickness formed with increasing annealing temperature in air led to an increase in resistivity by about one order of magnitude (
Figure 9a). Similar results were reported by Hsiang et al. [
4]. On the other hand, a decrease in
pO2 at 900 °C tended to slow down the formation of this chromia surface layer (
Figure 8b). Consequently, a reduction of resistivity by about one order of magnitude was observed upon changing the
pO2 from air to 10
−5 atm (
Figure 9b).
The permeability at 1 MHz is shown as function of the annealing temperature in
Figure 9, with the corresponding permeability spectra in
Figure 10. Samples annealed at 700 °C exhibited a permeability of about µ′ = 22. It has been demonstrated that higher compaction pressure results in a larger final density of the pressed and annealed samples [
4]. Compacting with 800 MPa pressure was shown to yield a permeability of µ′ = 34, and subsequent annealing at 750 °C was found to increase the permeability to about µ′ = 38 only [
4]. This indicates that plastic deformation of the alloy particles seemed to be the major mechanism for the densification of the samples, whereas sintering and grain growth seemed to play a minor role only. This is consistent with the observed small shrinkage of the samples during annealing and with the almost zero shrinkage in the dilatometric curve (
Figure 2a).
The permeability spectra showed slight variations in the permeability with frequency up to about 30 MHz, followed by a stronger decay in the hundreds of MHz region up to a resonance frequency at about 1 GHz (
Figure 10). FeSiCr samples with higher permeability of µ′ = 44 as presented in ref. [
2] exhibited their resonance frequency at a lower frequency of about 100 MHz in agreement with Snoek’s law.
The permeability at 1 MHz occurred at about µ′ = 22 for samples sintered in air at 700 °C and 750 °C and started to decrease from µ′ = 21 after annealing at 800 °C down to µ′ = 18.5 for samples annealed at 900 °C (
Figure 9a). A similar behavior with a decrease in permeability after annealing at
T > 800 °C was reported in ref. [
4]. This reduction in permeability originates from the insulating Cr
2O
3 layer at the grain surfaces of the alloy particles. This layer seems to hinder domain wall motion and therefore lowers the permeability. Bulk chromium oxide Cr
2O
3 itself is an antiferromagnetic material. After nano-structuring into ultra-thin layers or as nanosized particles, it exhibits a weak ferromagnetism that increases with decreasing particle size [
17,
18]. However, it is expected that the (less magnetic) chromia surface layer weakens the exchange across particles and contributes to the permeability reduction. For samples annealed at 900 °C at different
pO2 values, the permeability increased from µ′ = 18.5 after annealing in air at 900 °C to about µ′ = 22.5 for samples annealed at
pO2 = 10
−5 atm (
Figure 9b). Such increased permeability is consistent with the observations of less Cr
2O
3 formation under more reducing conditions.
FeSiCr alloys represent an interesting family of magnetic materials used for the fabrication of multilayer powder inductors. In this study, we compared the effect of the annealing temperature and oxygen partial pressure as parameters to tailor the oxidation state of the alloy particle surfaces and the corresponding performance of the material. It has been known that increasing annealing temperatures enhance the formation of a Cr2O3 oxide surface layer and, hence, the resistivity of the alloy, but also reduce permeability. It has been documented here that a reduction of the oxygen partial pressure during annealing at 900 °C is an option to reduce and slow down the formation of a chromia layer of sufficient thickness and, simultaneously, avoid the reduction of permeability upon annealing at 900 °C. This offers new paths for firing multilayer inductors with enhanced performance including high insulation resistance and permeability, or inductance.