Emerging AI- and Biomarker-Driven Precision Medicine in Autoimmune Rheumatic Diseases: From Diagnostics to Therapeutic Decision-Making
Abstract
1. Background
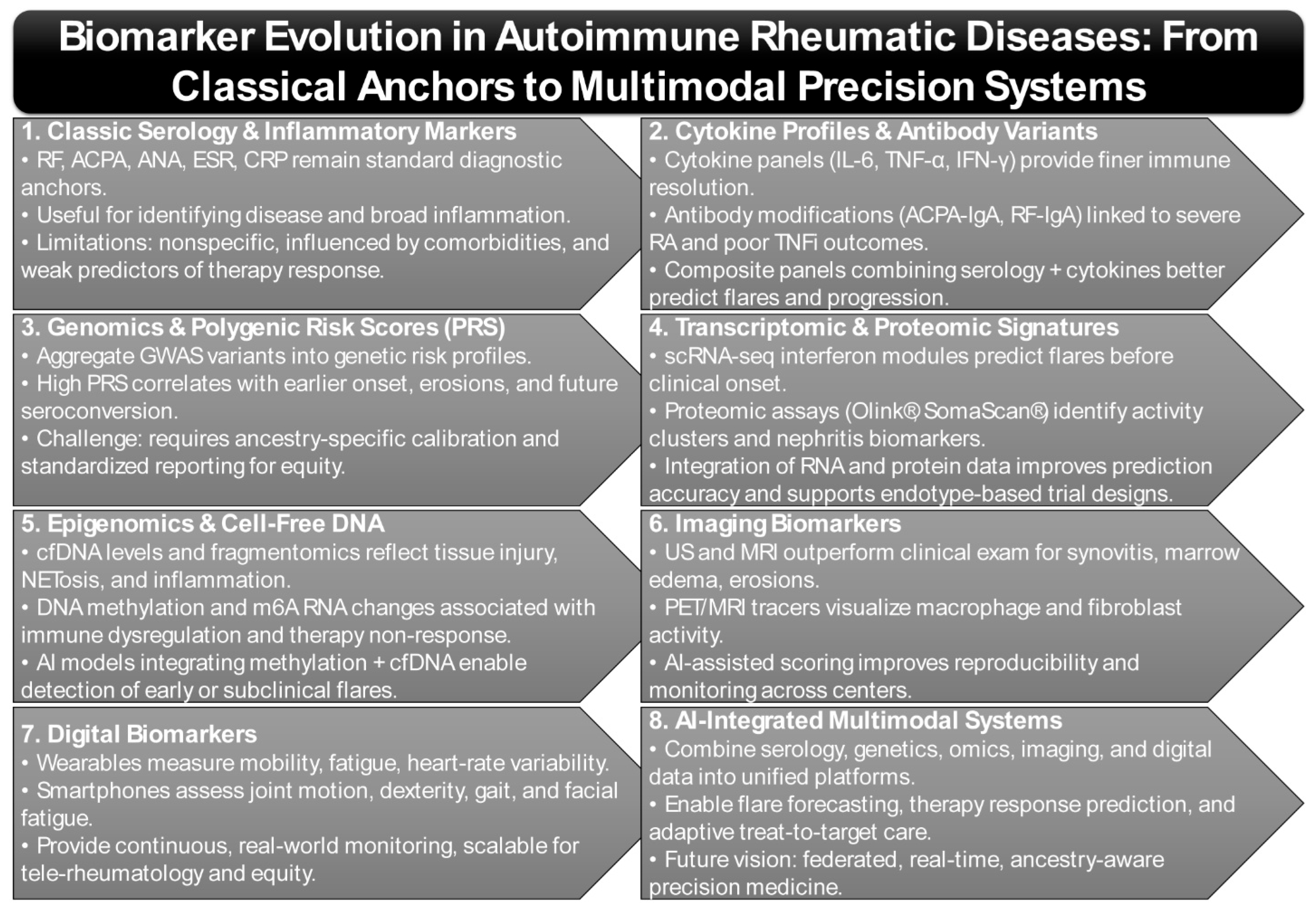
2. Biomarker Evolution in Autoimmune Rheumatic Diseases
2.1. Classic Biomarkers—Autoantibodies and Inflammatory Markers: Limitations and Drift
2.2. Genomics & Polygenic Risk
2.3. Transcriptomic & Proteomic Signatures
2.4. Epigenomic Alterations and Cell-Free DNA/Fragmentomics as Emerging Biomarkers
2.5. Imaging Biomarkers
2.6. Digital Biomarkers (Wearables/Smartphones)
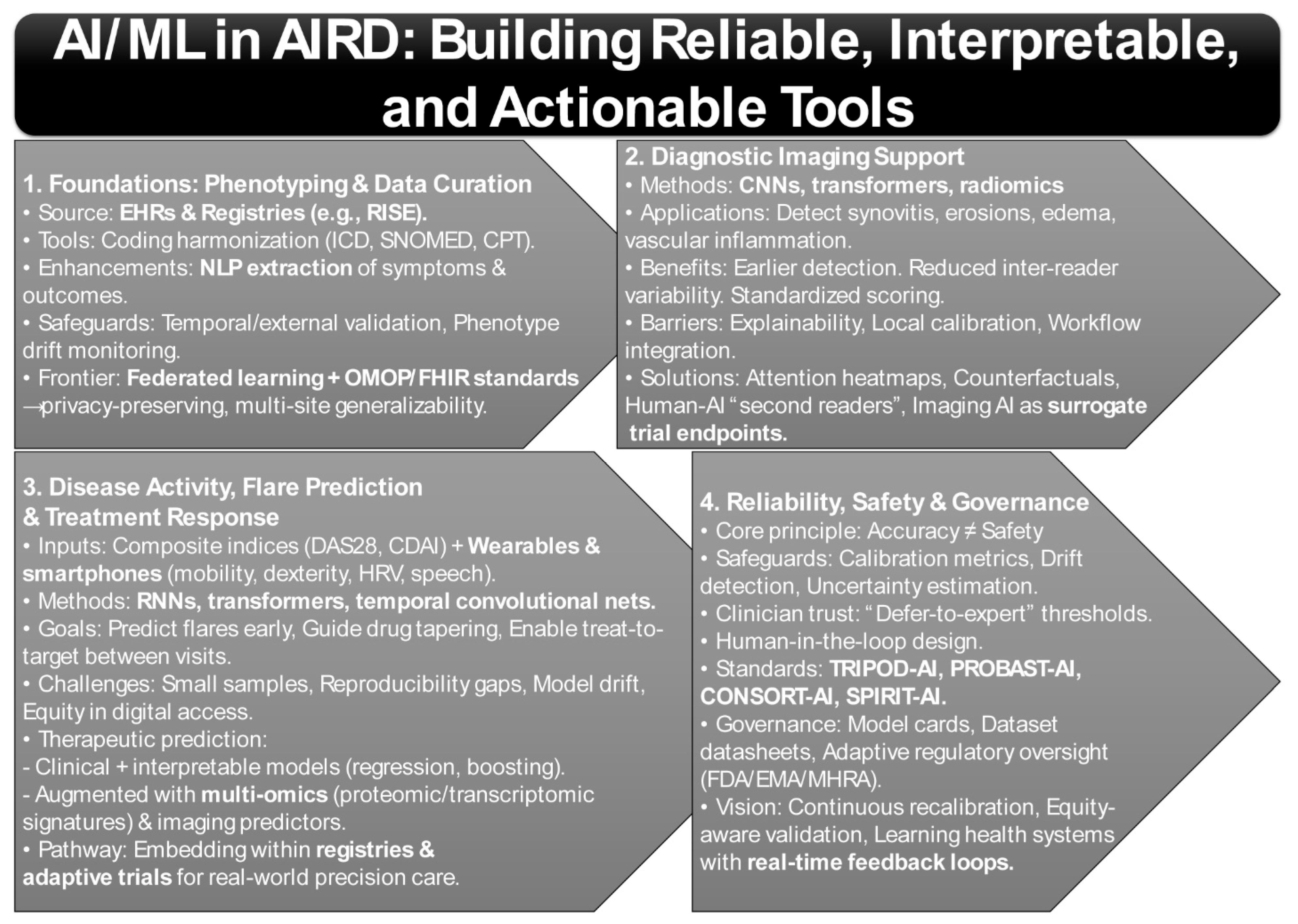
3. Harnessing AI and Machine Learning for Autoimmune Rheumatic Diseases
3.1. Phenotyping and EHR Curation
3.2. Diagnostic Imaging Support
3.3. Disease Activity, Flare Prediction, and Treatment Response
3.4. Reliability, Safety, and Governance
4. Redefining Autoimmune Rheumatic Disease Pathways: From Immune Signatures to AI-Enhanced Precision Medicine
4.1. Rheumatoid Arthritis (RA)
4.2. Systemic Lupus Erythematosus (SLE)
4.2.1. IFN Signature & Targeted Therapy
4.2.2. Digital Measures & Flare Prediction
4.3. Systemic Sclerosis (SSc)
4.4. Spondyloarthritis/Psoriatic Arthritis (SpA/PsA)
4.5. Other Conditions
4.5.1. Sjögren’s Disease (SjD)
4.5.2. Idiopathic Inflammatory Myopathies (IIM)
4.5.3. Vasculitides
5. Artificial Intelligence in Rheumatology: From Triage to Therapy Selection
5.1. AI-Enhanced Triage and Access
5.2. Imaging Decision Support
5.3. Predictive Tools for Therapy Selection
6. Data Infrastructures for AI in Rheumatology: Registries, Interoperability, and Federated Collaboration
6.1. Registries and EHR as Foundational Substrates
6.2. Interoperability and Common Data Models
6.3. Privacy-Preserving Collaboration Through Federated Learning
6.4. Pitfalls of Multisite Modeling and Mitigation Strategies
6.5. Case Illustration: Predicting RA Disease Activity Using RISE
6.6. Implementation Costs and Regulatory Readiness
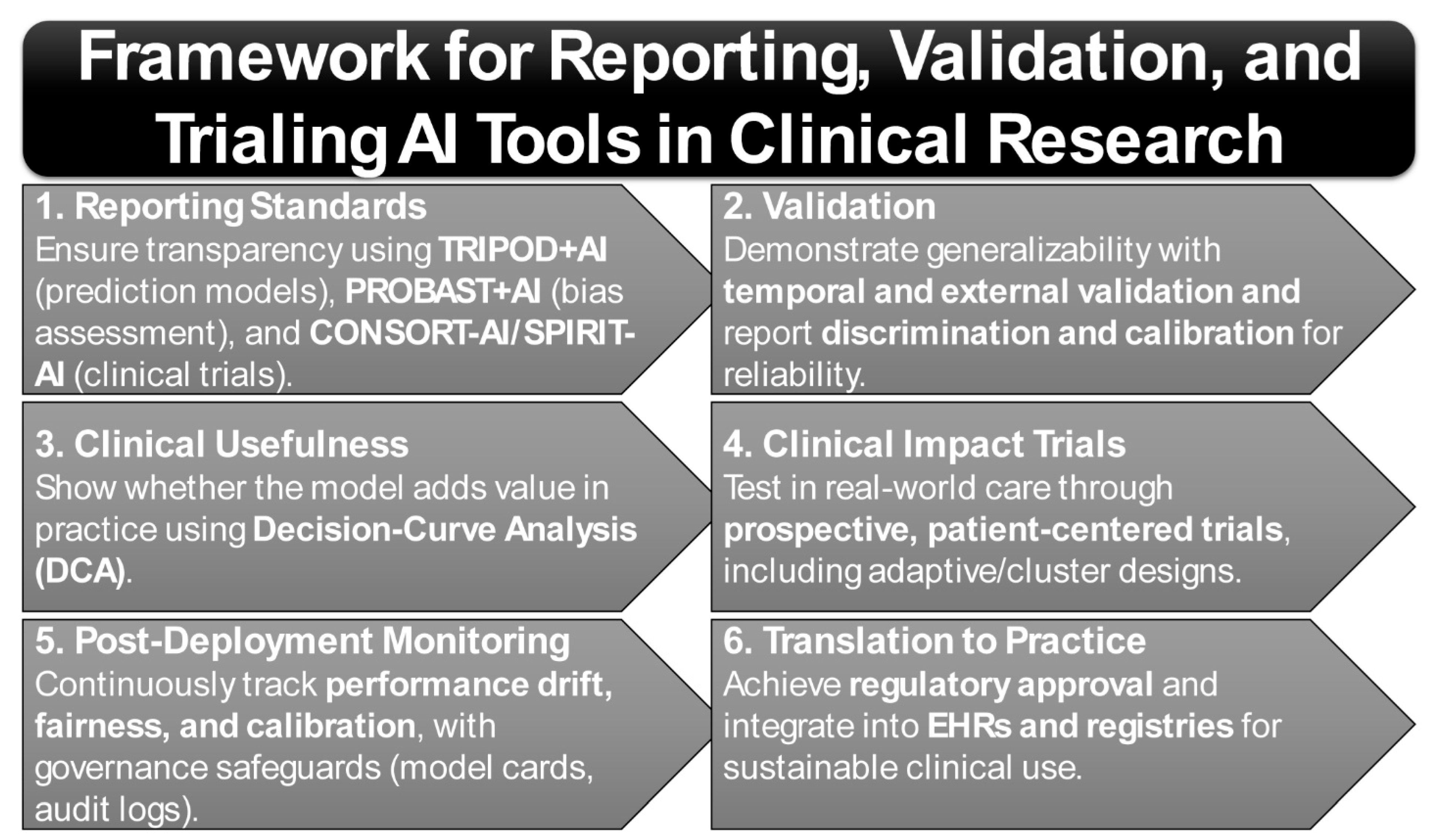
7. Standards and Study Designs for AI Prediction Models in Clinical Research
7.1. Core Reporting Standards for Prediction Models
7.2. Study Design Foundations: Reviewer Expectations and Best Practices
7.3. Methodological Appraisal and Evidence Grading
8. Equity and Portability in Polygenic Risk and AI Models: Addressing Ancestry Gaps and Bias in Precision Medicine
8.1. PRS Portability and Ancestry Gaps
8.2. Data Drift, Bias Audits, and Transparent Documentation
8.3. Governance and Safety by Design
9. Future Directions
9.1. Multimodal Fusion (Omics, Imaging, and Digital Phenotypes)
9.2. Mechanism-Aware Machine Learning to Guide Drug Targeting
9.3. Digital Twins, N-of-1 Trials, Adaptive Platforms, and Home Testing
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, H.; Okada, Y.; Nakayamada, S.; Miyazaki, Y.; Sonehara, K.; Namba, S.; Honda, S.; Shirai, Y.; Yamamoto, K.; Kubo, S.; et al. Extracting immunological and clinical heterogeneity across autoimmune rheumatic diseases by cohort-wide immunophenotyping. Ann. Rheum. Dis. 2024, 83, 242–252. [Google Scholar] [CrossRef]
- Al-Ewaidat, O.A.; Naffaa, M.M. Stroke risk in rheumatoid arthritis patients: Exploring connections and implications for patient care. Clin. Exp. Med. 2024, 24, 30. [Google Scholar] [CrossRef]
- Bilgin, E. Current application, possibilities, and challenges of artificial intelligence in the management of rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis. Ther. Adv. Musculoskelet. Dis. 2025, 17, 1759720X251343579. [Google Scholar] [CrossRef] [PubMed]
- Stafford, I.S.; Kellermann, M.; Mossotto, E.; Beattie, R.M.; MacArthur, B.D.; Ennis, S. A systematic review of the applications of artificial intelligence and machine learning in autoimmune diseases. NPJ Digit. Med. 2020, 3, 30. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Tsokos, G.C.; Lo, M.S.; Reis, P.C.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vasdev, V.; Patnaik, S.K.; Bhatt, S.; Singh, R.; Bhayana, A.; Hegde, A.; Kumar, A. The diagnostic utility of rheumatoid factor and anticitrullinated protein antibody for rheumatoid arthritis in the Indian population. Med. J. Armed Forces India 2022, 78, S69–S74. [Google Scholar] [CrossRef]
- Al-Ewaidat, O.A.; Naffaa, M.M. Deciphering Mechanisms, Prevention Strategies, Management Plans, Medications, and Research Techniques for Strokes in Systemic Lupus Erythematosus. Medicines 2024, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Fan, Y.; Zhao, X. Systemic lupus erythematosus: Updated insights on the pathogenesis, diagnosis, prevention and therapeutics. Signal Transduct. Target. Ther. 2025, 10, 102. [Google Scholar] [CrossRef]
- Findeisen, K.E.; Sewell, J.; Ostor, A.J.K. Biological Therapies for Rheumatoid Arthritis: An Overview for the Clinician. Biologics 2021, 15, 343–352. [Google Scholar] [CrossRef]
- Salaffi, F.; Carotti, M.; Di Carlo, M.; Ceccarelli, L.; Farah, S.; Poliseno, A.C.; Di Matteo, A.; Bandinelli, F.; Giovagnoni, A. Magnetic Resonance Imaging (MRI)-Based Semi-Quantitative Methods for Rheumatoid Arthritis: From Scoring to Measurement. J. Clin. Med. 2024, 13, 4137. [Google Scholar] [CrossRef]
- Parodis, I.; Lindblom, J.; Toro-Dominguez, D.; Beretta, L.; Borghi, M.O.; Castillo, J.; Carnero-Montoro, E.; Enman, Y.; Mohan, C.; Alarcon-Riquelme, M.E.; et al. Interferon and B-cell Signatures Inform Precision Medicine in Lupus Nephritis. Kidney Int. Rep. 2024, 9, 1817–1835. [Google Scholar] [CrossRef] [PubMed]
- Creagh, A.P.; Hamy, V.; Yuan, H.; Mertes, G.; Tomlinson, R.; Chen, W.H.; Williams, R.; Llop, C.; Yee, C.; Duh, M.S.; et al. Digital health technologies and machine learning augment patient reported outcomes to remotely characterise rheumatoid arthritis. NPJ Digit. Med. 2024, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Creagh, A.P.; Dondelinger, F.; Lipsmeier, F.; Lindemann, M.; De Vos, M. Longitudinal Trend Monitoring of Multiple Sclerosis Ambulation Using Smartphones. IEEE Open J. Eng. Med. Biol. 2022, 3, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Li, M.; Shang, S.; Cao, Y.; Shen, X.; Huang, C. Artificial intelligence in autoimmune diseases: A bibliometric exploration of the past two decades. Front. Immunol. 2025, 16, 1525462. [Google Scholar] [CrossRef]
- Sequi-Sabater, J.M.; Benavent, D. Artificial intelligence in rheumatology research: What is it good for? RMD Open 2025, 11, e004309. [Google Scholar] [CrossRef]
- Hammam, N.; Izadi, Z.; Li, J.; Evans, M.; Kay, J.; Shiboski, S.; Schmajuk, G.; Yazdany, J. The Relationship Between Electronic Health Record System and Performance on Quality Measures in the American College of Rheumatology’s Rheumatology Informatics System for Effectiveness (RISE) Registry: Observational Study. JMIR Med. Inform. 2021, 9, e31186. [Google Scholar] [CrossRef]
- Yazdany, J.; Bansback, N.; Clowse, M.; Collier, D.; Law, K.; Liao, K.P.; Michaud, K.; Morgan, E.M.; Oates, J.C.; Orozco, C.; et al. Rheumatology Informatics System for Effectiveness: A National Informatics-Enabled Registry for Quality Improvement. Arthritis Care Res. 2016, 68, 1866–1873. [Google Scholar] [CrossRef]
- Prot, V.; Aguilera, H.M.; Skallerud, B.; Persson, R.; Urheim, S. A method for non-invasive estimation of mitral valve annular regional strains. Comput. Biol. Med. 2025, 187, 109773. [Google Scholar] [CrossRef]
- Eden, R.; Chukwudi, I.; Bain, C.; Barbieri, S.; Callaway, L.; de Jersey, S.; George, Y.; Gorse, A.D.; Lawley, M.; Marendy, P.; et al. A scoping review of the governance of federated learning in healthcare. NPJ Digit. Med. 2025, 8, 427. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Damen, J.A.A.; Kaul, T.; Hooft, L.; Andaur Navarro, C.; Dhiman, P.; Beam, A.L.; Van Calster, B.; Celi, L.A.; Denaxas, S.; et al. PROBAST+AI: An updated quality, risk of bias, and applicability assessment tool for prediction models using regression or artificial intelligence methods. BMJ 2025, 388, e082505. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; van Smeden, M.; et al. TRIPOD + AI statement: Updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef]
- Ibrahim, H.; Liu, X.; Rivera, S.C.; Moher, D.; Chan, A.W.; Sydes, M.R.; Calvert, M.J.; Denniston, A.K. Reporting guidelines for clinical trials of artificial intelligence interventions: The SPIRIT-AI and CONSORT-AI guidelines. Trials 2021, 22, 11. [Google Scholar] [CrossRef]
- Ribeiro Junior, H.L.; Nepomuceno, F.; Pessoa, C.D.O. AI in clinical trials is missing from CONSORT and SPIRIT 2025 guidelines. Lancet 2025, 406, 25. [Google Scholar] [CrossRef]
- Hammam, N.; Evans, M.; Morgan, E.; Reimold, A.; Anastasiou, C.; Kay, J.L.; Yazdany, J.; Schmajuk, G. Treatment of Sarcoidosis in US Rheumatology Practices: Data from the American College of Rheumatology’s Rheumatology Informatics System for Effectiveness (RISE) Registry. Arthritis Care Res. 2022, 74, 371–376. [Google Scholar] [CrossRef]
- Guthridge, J.M.; Wagner, C.A.; James, J.A. The promise of precision medicine in rheumatology. Nat. Med. 2022, 28, 1363–1371. [Google Scholar] [CrossRef]
- Birtane, M.; Yavuz, S.; Tastekin, N. Laboratory evaluation in rheumatic diseases. World J. Methodol. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Martinez-Prat, L.; Nissen, M.J.; Lamacchia, C.; Bentow, C.; Cesana, L.; Roux-Lombard, P.; Gabay, C.; Mahler, M. Comparison of Serological Biomarkers in Rheumatoid Arthritis and Their Combination to Improve Diagnostic Performance. Front. Immunol. 2018, 9, 1113. [Google Scholar] [CrossRef]
- Steiner, G.; Toes, R.E.M. Autoantibodies in rheumatoid arthritis—Rheumatoid factor, anticitrullinated protein antibodies and beyond. Curr. Opin. Rheumatol. 2024, 36, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Brink, M.; Hansson, M.; Mathsson-Alm, L.; Wijayatunga, P.; Verheul, M.K.; Trouw, L.A.; Holmdahl, R.; Ronnelid, J.; Klareskog, L.; Rantapaa-Dahlqvist, S. Rheumatoid factor isotypes in relation to antibodies against citrullinated peptides and carbamylated proteins before the onset of rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Perera, J.; Delrosso, C.A.; Nerviani, A.; Pitzalis, C. Clinical Phenotypes, Serological Biomarkers, and Synovial Features Defining Seropositive and Seronegative Rheumatoid Arthritis: A Literature Review. Cells 2024, 13, 743. [Google Scholar] [CrossRef]
- Avouac, J.; Kay, J.; Choy, E. Personalised treatment of rheumatoid arthritis based on cytokine profiles and synovial tissue signatures: Potentials and challenges. Semin. Arthritis Rheum. 2025, 73, 152740. [Google Scholar] [CrossRef]
- Rayner, F.; Hiu, S.; Melville, A.; Bigirumurame, T.; Anderson, A.; Dyke, B.; Kerrigan, S.; McGucken, A.; Prichard, J.; Shahrokhabadi, M.S.; et al. Clinical predictors of flare and drug-free remission in rheumatoid arthritis: Preliminary results from the prospective BIO-FLARE experimental medicine study. BMJ Open 2025, 15, e092478. [Google Scholar] [CrossRef]
- Huang, X.; Luu, L.D.W.; Jia, N.; Zhu, J.; Fu, J.; Xiao, F.; Liu, C.; Li, S.; Shu, G.; Hou, J.; et al. Multi-Platform Omics Analysis Reveals Molecular Signatures for Pathogenesis and Activity of Systemic Lupus Erythematosus. Front. Immunol. 2022, 13, 833699. [Google Scholar] [CrossRef]
- Huang, H.; Sun, X.; Zhang, Q.; Liu, C.; Cao, X.; Zhang, D.; Wang, G.; Pu, C. Combined serum IFN-gamma and IL-22 levels as predictive biomarkers for hepatocellular carcinoma risk: A clinical investigation. Biomed. Rep. 2025, 23, 149. [Google Scholar] [CrossRef]
- Pisetsky, D.S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Kutsuna, Y.J.; Aibara, N.; Hashizume, J.; Kawarabayashi, S.; Tamai, M.; Miyata, J.; Yoshifuji, H.; Miyamoto, H.; Sato, K.; Kodama, Y.; et al. Identification of immune complex antigens that are detected prior to early rheumatoid arthritis symptoms and increase with disease progression: Comprehensive serum immune complexome analysis to identify candidate disease biomarkers in health checkup cohort study. Clin. Immunol. 2025, 281, 110591. [Google Scholar] [CrossRef] [PubMed]
- Lerga-Jaso, J.; Terpolovsky, A.; Novkovic, B.; Osama, A.; Manson, C.; Bohn, S.; De Marino, A.; Kunitomi, M.; Yazdi, P.G. Optimization of multi-ancestry polygenic risk score disease prediction models. Sci. Rep. 2025, 15, 17495. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.S.; Chen, Y.J.; Hsiung, C.N.; Mao, C.L.; Wei, C.Y.; Chen, I.C.; Kao, C.M.; Hsiao, T.H.; Huang, W.N.; Chen, Y.H.; et al. Polygenic risk scores of rheumatoid arthritis associated with seropositivity and bone erosions in a Taiwanese population. Sci. Rep. 2025, 15, 25700. [Google Scholar] [CrossRef]
- Honda, S.; Ikari, K.; Yano, K.; Terao, C.; Tanaka, E.; Harigai, M.; Kochi, Y. Association of Polygenic Risk Scores with Radiographic Progression in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2022, 74, 791–800. [Google Scholar] [CrossRef]
- Ishigaki, K.; Sakaue, S.; Terao, C.; Luo, Y.; Sonehara, K.; Yamaguchi, K.; Amariuta, T.; Too, C.L.; Laufer, V.A.; Scott, I.C.; et al. Multi-ancestry genome-wide association analyses identify novel genetic mechanisms in rheumatoid arthritis. Nat. Genet. 2022, 54, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, O.; Fang, T.; Chen, M.; Zhang, X.; Cong, R.; Lu, D.; Zhang, R.; Jin, Q.; Wang, X. Phenome-wide causal proteomics enhance systemic lupus erythematosus flare prediction: A study in Asian populations. medRxiv 2024. [Google Scholar] [CrossRef]
- Paek, S.J.; Lee, H.S.; Lee, Y.J.; Bang, S.Y.; Kim, D.; Kang, B.K.; Park, D.J.; Joo, Y.B.; Kim, M.; Kim, H.; et al. Tracking clonal dynamics of CD8 T cells and immune dysregulation in progression of systemic lupus erythematosus with nephritis. Exp. Mol. Med. 2025, 57, 1700–1710. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, W.; Li, Y.; Deng, C.; Xuan, J.; Sun, Y.; He, Y. Bioinformatic analysis and experimental verification reveal expansion of monocyte subsets with an interferon signature in systemic lupus erythematosus patients. Arthritis Res. Ther. 2025, 27, 96. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Duan, C.; Xie, C.; Wang, H.; Li, Z.; Li, B.; Wang, T. Identification of key interferon-stimulated genes for indicating the condition of patients with systemic lupus erythematosus. Front. Immunol. 2022, 13, 962393. [Google Scholar] [CrossRef]
- Haslam, D.E.; Li, J.; Dillon, S.T.; Gu, X.; Cao, Y.; Zeleznik, O.A.; Sasamoto, N.; Zhang, X.; Eliassen, A.H.; Liang, L.; et al. Stability and reproducibility of proteomic profiles in epidemiological studies: Comparing the Olink and SOMAscan platforms. Proteomics 2022, 22, e2100170. [Google Scholar] [CrossRef]
- Shang, S.; Xia, J.; He, G.; Zheng, Y.; Zhang, J.; Lu, H.; Wang, H.; Li, W.; Li, Q.; Chen, X. Advances in precision medicine for lupus nephritis: Biomarker- and AI-driven diagnosis and treatment response prediction and targeted therapies. EBioMedicine 2025, 117, 105785. [Google Scholar] [CrossRef]
- Cervia-Hasler, C.; Bruningk, S.C.; Hoch, T.; Fan, B.; Muzio, G.; Thompson, R.C.; Ceglarek, L.; Meledin, R.; Westermann, P.; Emmenegger, M.; et al. Persistent complement dysregulation with signs of thromboinflammation in active Long Covid. Science 2024, 383, eadg7942. [Google Scholar] [CrossRef]
- Galozzi, P.; Basso, D.; Plebani, M.; Padoan, A. Artificial intelligence and laboratory data in rheumatic diseases. Clin. Chim. Acta 2023, 546, 117388. [Google Scholar] [CrossRef]
- Duvvuri, B.; Lood, C. Cell-Free DNA as a Biomarker in Autoimmune Rheumatic Diseases. Front. Immunol. 2019, 10, 502. [Google Scholar] [CrossRef]
- Dihlmann, S.; Kaduk, C.; Passek, K.H.; Spieler, A.; Bockler, D.; Peters, A.S. Exploring circulating cell-free DNA as a biomarker and as an inducer of AIM2-inflammasome-mediated inflammation in patients with abdominal aortic aneurysm. Sci. Rep. 2025, 15, 20196. [Google Scholar] [CrossRef] [PubMed]
- Jackson Chornenki, N.L.; Coke, R.; Kwong, A.C.; Dwivedi, D.J.; Xu, M.K.; McDonald, E.; Marshall, J.C.; Fox-Robichaud, A.E.; Charbonney, E.; Liaw, P.C. Comparison of the source and prognostic utility of cfDNA in trauma and sepsis. Intensive Care Med. Exp. 2019, 7, 29. [Google Scholar] [CrossRef]
- Liu, F.; Su, Y.; Liu, X.; Zhao, L.; Wu, Z.; Liu, Y.; Zhang, L. Cell-free DNA: A metabolic byproduct with diagnostic and prognostic potential in rheumatic disorders. Front. Pharmacol. 2025, 16, 1537934. [Google Scholar] [CrossRef]
- Lehmann, J.; Giaglis, S.; Kyburz, D.; Daoudlarian, D.; Walker, U.A. Plasma mtDNA as a possible contributor to and biomarker of inflammation in rheumatoid arthritis. Arthritis Res. Ther. 2024, 26, 97. [Google Scholar] [CrossRef]
- Kerachian, M.A.; Azghandi, M.; Mozaffari-Jovin, S.; Thierry, A.R. Guidelines for pre-analytical conditions for assessing the methylation of circulating cell-free DNA. Clin. Epigenetics 2021, 13, 193. [Google Scholar] [CrossRef]
- Peng, H.; Pan, M.; Zhou, Z.; Chen, C.; Xing, X.; Cheng, S.; Zhang, S.; Zheng, H.; Qian, K. The impact of preanalytical variables on the analysis of cell-free DNA from blood and urine samples. Front. Cell Dev. Biol. 2024, 12, 1385041. [Google Scholar] [CrossRef]
- Sathyanarayana, S.H.; Spracklin, S.B.; Deharvengt, S.J.; Green, D.C.; Instasi, M.D.; Gallagher, T.L.; Shah, P.S.; Tsongalis, G.J. Standardized Workflow and Analytical Validation of Cell-Free DNA Extraction for Liquid Biopsy Using a Magnetic Bead-Based Cartridge System. Cells 2025, 14, 1062. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Pan, M.; Shi, H.; Wang, L.; Bai, Y.; Ge, Q. Cell-Free DNA Fragmentomics: The Novel Promising Biomarker. Int. J. Mol. Sci. 2023, 24, 1503. [Google Scholar] [CrossRef] [PubMed]
- Fresneda Alarcon, M.; McLaren, Z.; Wright, H.L. Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O. Front. Immunol. 2021, 12, 649693. [Google Scholar] [CrossRef]
- Huang, Y.; Xue, Q.; Chang, J.; Wang, Y.; Cheng, C.; Xu, S.; Wang, X.; Miao, C. M6A methylation modification in autoimmune diseases, a promising treatment strategy based on epigenetics. Arthritis Res. Ther. 2023, 25, 189. [Google Scholar] [CrossRef]
- Wu, J.; Deng, L.J.; Xia, Y.R.; Leng, R.X.; Fan, Y.G.; Pan, H.F.; Ye, D.Q. Involvement of N6-methyladenosine modifications of long noncoding RNAs in systemic lupus erythematosus. Mol. Immunol. 2022, 143, 77–84. [Google Scholar] [CrossRef]
- Guo, D.; Liu, J.; Li, S.; Xu, P. Analysis of m6A regulators related immune characteristics in ankylosing spondylitis by integrated bioinformatics and computational strategies. Sci. Rep. 2024, 14, 2724. [Google Scholar] [CrossRef]
- Cheng, L.; Li, H.; Zhan, H.; Liu, Y.; Li, X.; Huang, Y.; Wang, L.; Zhang, F.; Li, Y. Alterations of m6A RNA methylation regulators contribute to autophagy and immune infiltration in primary Sjogren’s syndrome. Front. Immunol. 2022, 13, 949206. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Liu, X. Rheumatoid arthritis: Pathogenesis and therapeutic advances. MedComm 2024, 5, e509. [Google Scholar] [CrossRef] [PubMed]
- Wardowska, A. m6A RNA Methylation in Systemic Autoimmune Diseases-A New Target for Epigenetic-Based Therapy? Pharmaceuticals 2021, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mennea, P.D.; Chan, Y.K.E.; Cheng, Z.; Neofytou, M.C.; Surani, A.A.; Vijayaraghavan, A.; Ditter, E.J.; Bowers, R.; Eldridge, M.D.; et al. A standardized framework for robust fragmentomic feature extraction from cell-free DNA sequencing data. Genome Biol. 2025, 26, 141. [Google Scholar] [CrossRef]
- Xu, P.; Cai, J.; Gao, Y.; Rong, Z. MIRACLE: Multi-task Learning based Interpretable Regulation of Autoimmune Diseases through Common Latent Epigenetics. arXiv 2023. [Google Scholar] [CrossRef]
- Kim, S.; Zhang, L.; Qin, Y.; Bohn, R.I.C.; Park, H.J. Pathway information on methylation analysis using deep neural network (PROMINENT): An interpretable deep learning method with pathway prior for phenotype prediction using gene-level DNA methylation. Artif. Intell. Med. 2025, 170, 103236. [Google Scholar] [CrossRef]
- Lee, K.; Niku, S.; Koo, S.J.; Belezzuoli, E.; Guma, M. Molecular imaging for evaluation of synovitis associated with osteoarthritis: A narrative review. Arthritis Res. Ther. 2024, 26, 25. [Google Scholar] [CrossRef]
- Boeren, A.M.P.; Oei, E.H.G.; van der Helm-van, A.H.M. The value of MRI for detecting subclinical joint inflammation in clinically suspect arthralgia. RMD Open 2022, 8, e002128. [Google Scholar] [CrossRef]
- So, H.; Cheng, I.; Tam, L.S. The Role of Imaging in Predicting the Development of Rheumatoid Arthritis. Rheumatol. Immunol. Res. 2021, 2, 27–33. [Google Scholar] [CrossRef]
- Ogdie, A.; Coates, L.C.; Mease, P. Measuring Outcomes in Psoriatic Arthritis. Arthritis Care Res. 2020, 72 (Suppl. S10), 82–109. [Google Scholar] [CrossRef] [PubMed]
- Frenken, M.; Schleich, C.; Brinks, R.; Abrar, D.B.; Goertz, C.; Schneider, M.; Ostendorf, B.; Sewerin, P. The value of the simplified RAMRIS-5 in early RA patients under methotrexate therapy using high-field MRI. Arthritis Res. Ther. 2019, 21, 21. [Google Scholar] [CrossRef]
- Schleich, C.; Buchbender, C.; Sewerin, P.; Miese, F.; Aissa, J.; Brinks, R.; Schneider, M.; Antoch, G.; Ostendorf, B. Evaluation of a simplified version of the Rheumatoid Arthritis Magnetic Resonance Imaging Score (RAMRIS) comprising 5 joints (RAMRIS5). Clin. Exp. Rheumatol. 2015, 33, 209–215. [Google Scholar]
- Ben-Eltriki, M.; Rafiq, A.; Paul, A.; Prabhu, D.; Afolabi, M.O.S.; Baslhaw, R.; Neilson, C.J.; Driedger, M.; Mahmud, S.M.; Lacaze-Masmonteil, T.; et al. Adaptive designs in clinical trials: A systematic review-part I. BMC Med. Res. Methodol. 2024, 24, 229. [Google Scholar] [CrossRef]
- Kaizer, A.M.; Belli, H.M.; Ma, Z.; Nicklawsky, A.G.; Roberts, S.C.; Wild, J.; Wogu, A.F.; Xiao, M.; Sabo, R.T. Recent innovations in adaptive trial designs: A review of design opportunities in translational research. J. Clin. Transl. Sci. 2023, 7, e125. [Google Scholar] [CrossRef] [PubMed]
- Noversa de Sousa, R.; Tascilar, K.; Corte, G.; Atzinger, A.; Minopoulou, I.; Ohrndorf, S.; Waldner, M.; Schmidkonz, C.; Kuwert, T.; Knieling, F.; et al. Metabolic and molecular imaging in inflammatory arthritis. RMD Open 2024, 10, e003880. [Google Scholar] [CrossRef]
- MacKay, J.W.; Watkins, L.; Gold, G.; Kogan, F. [(18)F]NaF PET-MRI provides direct in-vivo evidence of the association between bone metabolic activity and adjacent synovitis in knee osteoarthritis: A cross-sectional study. Osteoarthr. Cartil. 2021, 29, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- MacRitchie, N.; Frleta-Gilchrist, M.; Sugiyama, A.; Lawton, T.; McInnes, I.B.; Maffia, P. Molecular imaging of inflammation—Current and emerging technologies for diagnosis and treatment. Pharmacol. Ther. 2020, 211, 107550. [Google Scholar] [CrossRef]
- Chandrupatla, D.; Molthoff, C.F.M.; Lammertsma, A.A.; van der Laken, C.J.; Jansen, G. The folate receptor beta as a macrophage-mediated imaging and therapeutic target in rheumatoid arthritis. Drug Deliv. Transl. Res. 2019, 9, 366–378. [Google Scholar] [CrossRef]
- Mori, Y.; Novruzov, E.; Schmitt, D.; Cardinale, J.; Watabe, T.; Choyke, P.L.; Alavi, A.; Haberkorn, U.; Giesel, F.L. Clinical applications of fibroblast activation protein inhibitor positron emission tomography (FAPI-PET). NPJ Imaging 2024, 2, 48. [Google Scholar] [CrossRef]
- Kastelik-Hryniewiecka, A.; Jewula, P.; Bakalorz, K.; Kramer-Marek, G.; Kuznik, N. Targeted PET/MRI Imaging Super Probes: A Critical Review of Opportunities and Challenges. Int. J. Nanomed. 2021, 16, 8465–8483. [Google Scholar] [CrossRef]
- Xu, L.; Bressem, K.; Adams, L.; Poddubnyy, D.; Proft, F. AI for imaging evaluation in rheumatology: Applications of radiomics and computer vision-current status, future prospects and potential challenges. Rheumatol. Adv. Pract. 2025, 9, rkae147. [Google Scholar] [CrossRef]
- Thesia, J.; Pandya, A. Wearable biosensors for autoimmune disorders. Prog. Mol. Biol. Transl. Sci. 2025, 215, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Hamy, V.; Llop, C.; Yee, C.W.; Garcia-Gancedo, L.; Maxwell, A.; Chen, W.H.; Tomlinson, R.; Bobbili, P.; Bendelac, J.; Landry, J.; et al. Patient-centric assessment of rheumatoid arthritis using a smartwatch and bespoke mobile app in a clinical setting. Sci. Rep. 2023, 13, 18311. [Google Scholar] [CrossRef]
- Wagner, S.R.; Gregersen, R.R.; Henriksen, L.; Hauge, E.M.; Keller, K.K. Smartphone Pedometer Sensor Application for Evaluating Disease Activity and Predicting Comorbidities in Patients with Rheumatoid Arthritis: A Validation Study. Sensors 2022, 22, 9396. [Google Scholar] [CrossRef]
- Reed, M.; Rampono, B.; Turner, W.; Harsanyi, A.; Lim, A.; Paramalingam, S.; Massasso, D.; Thakkar, V.; Mundae, M.; Rampono, E. A multicentre validation study of a smartphone application to screen hand arthritis. BMC Musculoskelet. Disord. 2022, 23, 433. [Google Scholar] [CrossRef] [PubMed]
- Venerito, V.; Manigold, T.; Capodiferro, M.; Markham, D.; Blanchard, M.; Iannone, F.; Hugle, T. Single-camera motion capture of finger joint mobility as a digital biomarker for disease activity in rheumatoid arthritis. Rheumatol. Adv. Pract. 2025, 9, rkae143. [Google Scholar] [CrossRef]
- Guo, L.; Chang, R.; Wang, J.; Narayanan, A.; Qian, P.; Leong, M.C.; Kundu, P.P.; Senthilkumar, S.; Garlapati, S.C.; Yong, E.C.K.; et al. Artificial intelligence-enhanced 3D gait analysis with a single consumer-grade camera. J. Biomech. 2025, 187, 112738. [Google Scholar] [CrossRef] [PubMed]
- Hadjileontiadis, L.J.; Charisis, V.; Hadjidimitriou, S.; Dias, S.B.; Apostolidis, G.; Dimaridis, G.; Kitsas, I.; Karlas, A.; Fasoula, N.A.; Levi-Schaffer, F.; et al. European advances in digital rheumatology: Explainable insights and personalized digital health tools for psoriatic arthritis. EClinicalMedicine 2025, 84, 103243. [Google Scholar] [CrossRef]
- Santosa, A.; Li, J.W.; Tan, T.C. Digital Health for Equitable Rheumatic Care: Integrating Real-World Experiences to Guide Policy Pathways. Healthcare 2025, 13, 438. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Chen, Y.; Luo, D.; Xu, K.; Zhang, L. Artificial intelligence for predicting treatment responses in autoimmune rheumatic diseases: Advancements, challenges, and future perspectives. Front. Immunol. 2024, 15, 1477130. [Google Scholar] [CrossRef]
- Zhao, J.; Li, L.; Li, J.; Zhang, L. Application of artificial intelligence in rheumatic disease: A bibliometric analysis. Clin. Exp. Med. 2024, 24, 196. [Google Scholar] [CrossRef]
- Nelson, A.E.; Arbeeva, L. Narrative Review of Machine Learning in Rheumatic and Musculoskeletal Diseases for Clinicians and Researchers: Biases, Goals, and Future Directions. J. Rheumatol. 2022, 49, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- McMaster, C.; Bird, A.; Liew, D.F.L.; Buchanan, R.R.; Owen, C.E.; Chapman, W.W.; Pires, D.E.V. Artificial Intelligence and Deep Learning for Rheumatologists. Arthritis Rheumatol. 2022, 74, 1893–1905. [Google Scholar] [CrossRef]
- Hurez, V.; Gauderat, G.; Soret, P.; Myers, R.; Dasika, K.; Sheehan, R.; Friedrich, C.; Reed, M.; Laigle, L.; Riquelme, M.A.; et al. Virtual patients inspired by multiomics predict the efficacy of an anti-IFNalpha mAb in cutaneous lupus. iScience 2025, 28, 111754. [Google Scholar] [CrossRef] [PubMed]
- Ebadi Jalal, M.; Emam, O.S.; Castillo-Olea, C.; Garcia-Zapirain, B.; Elmaghraby, A. Abnormality detection in nailfold capillary images using deep learning with EfficientNet and cascade transfer learning. Sci. Rep. 2025, 15, 2068. [Google Scholar] [CrossRef]
- Lledo-Ibanez, G.M.; Saez Comet, L.; Freire Dapena, M.; Mesa Navas, M.; Martin Cascon, M.; Guillen Del Castillo, A.; Simeon, C.P.; Martinez Robles, E.; Todoli Parra, J.; Varela, D.C.; et al. CAPI-Detect: Machine learning in capillaroscopy reveals new variables influencing diagnosis. Rheumatology 2025, 64, 3667–3675. [Google Scholar] [CrossRef]
- Knevel, R.; Liao, K.P. From real-world electronic health record data to real-world results using artificial intelligence. Ann. Rheum. Dis. 2023, 82, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Tonner, C.; Schmajuk, G.; Yazdany, J. A new era of quality measurement in rheumatology: Electronic clinical quality measures and national registries. Curr. Opin. Rheumatol. 2017, 29, 131–137. [Google Scholar] [CrossRef]
- Francisco, M.; Johansson, T.; Kazi, S. Overview of the American College of Rheumatology’s Electronic Health Record-Enabled Registry: The Rheumatology Informatics System for Effectiveness. Clin. Exp. Rheumatol. 2016, 34, S102–S104. [Google Scholar]
- Oatis, C.A.; Konnyu, K.J.; Franklin, P.D. Generating consistent longitudinal real-world data to support research: Lessons from physical therapists. ACR Open Rheumatol. 2022, 4, 771–774. [Google Scholar] [CrossRef]
- Izadi, Z.; Schmajuk, G.; Gianfrancesco, M.; Subash, M.; Evans, M.; Trupin, L.; Yazdany, J. Significant Gains in Rheumatoid Arthritis Quality Measures Among RISE Registry Practices. Arthritis Care Res. 2022, 74, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei Hosseini, S.A.; Kazemzadeh, R.; Foster, B.J.; Arpali, E.; Susal, C. New Tools for Data Harmonization and Their Potential Applications in Organ Transplantation. Transplantation 2024, 108, 2306–2317. [Google Scholar] [CrossRef]
- Carbonaro, A.; Giorgetti, L.; Ridolfi, L.; Pasolini, R.; Pagliarani, A.; Cavallucci, M.; Andalo, A.; Gaudio, L.D.; De Angelis, P.; Vespignani, R.; et al. From raw data to research-ready: A FHIR-based transformation pipeline in a real-world oncology setting. Comput. Biol. Med. 2025, 197, 111051. [Google Scholar] [CrossRef]
- Omar, M.; Naffaa, M.E.; Glicksberg, B.S.; Reuveni, H.; Nadkarni, G.N.; Klang, E. Advancing rheumatology with natural language processing: Insights and prospects from a systematic review. Rheumatol. Adv. Pract. 2024, 8, rkae120. [Google Scholar] [CrossRef] [PubMed]
- Benavent, D.; Madrid-Garcia, A. Large language models and rheumatology: Are we there yet? Rheumatol. Adv. Pract. 2025, 9, rkae119. [Google Scholar] [CrossRef]
- Humbert-Droz, M.; Izadi, Z.; Schmajuk, G.; Gianfrancesco, M.; Baker, M.C.; Yazdany, J.; Tamang, S. Development of a Natural Language Processing System for Extracting Rheumatoid Arthritis Outcomes from Clinical Notes Using the National Rheumatology Informatics System for Effectiveness Registry. Arthritis Care Res. 2023, 75, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, A.; Sada, Y.H.; Nowakowski, S.; Guffey, D.; Zhu, H.; Yarlagadda, S.R.; Li, A.; Razjouyan, J. A Multi-Institutional Natural Language Processing Pipeline to Extract Performance Status from Electronic Health Records. Cancer Control 2024, 31, 10732748241279518. [Google Scholar] [CrossRef]
- Zhang, F.; Kreuter, D.; Chen, Y.; Dittmer, S.; Tull, S.; Shadbahr, T.; Schut, M.; Asselbergs, F.; Kar, S.; Sivapalaratnam, S.; et al. Recent methodological advances in federated learning for healthcare. Patterns 2024, 5, 101006. [Google Scholar] [CrossRef]
- Austin, J.A.; Lobo, E.H.; Samadbeik, M.; Engstrom, T.; Philip, R.; Pole, J.D.; Sullivan, C.M. Decades in the Making: The Evolution of Digital Health Research Infrastructure Through Synthetic Data, Common Data Models, and Federated Learning. J. Med. Internet Res. 2024, 26, e58637. [Google Scholar] [CrossRef]
- Stoel, B. Use of artificial intelligence in imaging in rheumatology—Current status and future perspectives. RMD Open 2020, 6, e001063. [Google Scholar] [CrossRef]
- Bird, A.; Oakden-Rayner, L.; McMaster, C.; Smith, L.A.; Zeng, M.; Wechalekar, M.D.; Ray, S.; Proudman, S.; Palmer, L.J. Artificial intelligence and the future of radiographic scoring in rheumatoid arthritis: A viewpoint. Arthritis Res. Ther. 2022, 24, 268. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, S.; Zwagemaker, A.F.; Huo, A.; Li, Y.J.; Zhou, F.; Hilliard, P.; Squire, S.; Bouskill, V.; Mohanta, A.; et al. A semiquantitative color Doppler ultrasound scoring system for evaluation of synovitis in joints of patients with blood-induced arthropathy. Insights Imaging 2021, 12, 132. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallieres, M.; Abdalah, M.A.; Aerts, H.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Oettl, F.C.; Zsidai, B.; Oeding, J.F.; Hirschmann, M.T.; Feldt, R.; Fendrich, D.; Kraeutler, M.J.; Winkler, P.W.; Szaro, P.; Samuelsson, K.; et al. Artificial intelligence-assisted analysis of musculoskeletal imaging-A narrative review of the current state of machine learning models. Knee Surg. Sports Traumatol. Arthrosc. 2025, 33, 3032–3038. [Google Scholar] [CrossRef]
- Salvi, M.; Seoni, S.; Campagner, A.; Gertych, A.; Acharya, U.R.; Molinari, F.; Cabitza, F. Explainability and uncertainty: Two sides of the same coin for enhancing the interpretability of deep learning models in healthcare. Int. J. Med. Inform. 2025, 197, 105846. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Y.; Wang, Q.; Shi, J.; Wang, H.; Ye, Z.; Xue, P.; Qiao, Y. Impact of human and artificial intelligence collaboration on workload reduction in medical image interpretation. NPJ Digit. Med. 2024, 7, 349. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Roemer, F.W.; Crema, M.D.; Jarraya, M.; Mobasheri, A.; Hayashi, D. Strategic application of imaging in DMOAD clinical trials: Focus on eligibility, drug delivery, and semiquantitative assessment of structural progression. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231165558. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, M.M.A.; Westgeest, A.A.A.; Schwarting, A.; Jacobs, J.W.G.; Heller, C.; van Laar, J.M.; Lafeber, F.; Tekstra, J.; Triantafyllias, K.; Welsing, P.M.J. Development and Validation of Rheumatoid Arthritis Disease Activity Indices Including HandScan (Optical Spectral Transmission) Scores. Arthritis Care Res. 2022, 74, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J. Importance of Time-Integrated Cumulative Parameters for Radiographic Progression Prediction of Rheumatoid Arthritis. J. Rheum. Dis. 2022, 29, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Gumber, L.; Rayner, F.; Bigirumurame, T.; Dyke, B.; Melville, A.; Kerrigan, S.; McGucken, A.; Naamane, N.; Prichard, J.; Buckley, C.D.; et al. Patient-reported outcomes as early warning signs of flare following drug cessation in rheumatoid arthritis. RMD Open 2025, 11, e005442. [Google Scholar] [CrossRef]
- Gandrup, J.; Selby, D.A.; van der Veer, S.N.; McBeth, J.; Dixon, W.G. Using patient-reported data from a smartphone app to capture and characterize real-time patient-reported flares in rheumatoid arthritis. Rheumatol. Adv. Pract. 2022, 6, rkac021. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Nowroozi, A.; Rezaei, N. Artificial Intelligence in Rheumatoid Arthritis: Current Status and Future Perspectives: A State-of-the-Art Review. Rheumatol. Ther. 2022, 9, 1249–1304. [Google Scholar] [CrossRef]
- Gul, H.; Di Matteo, A.; Anioke, I.; Shuweidhi, F.; Mankia, K.; Ponchel, F.; Emery, P. Predicting Flare in Patients with Rheumatoid Arthritis in Biologic Induced Remission, on Tapering, and on Stable Therapy. ACR Open Rheumatol. 2024, 6, 294–303. [Google Scholar] [CrossRef]
- Patharkar, A.; Cai, F.; Al-Hindawi, F.; Wu, T. Predictive modeling of biomedical temporal data in healthcare applications: Review and future directions. Front. Physiol. 2024, 15, 1386760. [Google Scholar] [CrossRef]
- Richardson, S.; Lawrence, K.; Schoenthaler, A.M.; Mann, D. A framework for digital health equity. NPJ Digit. Med. 2022, 5, 119. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.E.; Dorn, C.; Park, D.J.; Matheny, M.E. Emerging algorithmic bias: Fairness drift as the next dimension of model maintenance and sustainability. J. Am. Med. Inform. Assoc. 2025, 32, 845–854. [Google Scholar] [CrossRef]
- Temmoku, J.; Migita, K.; Yoshida, S.; Matsumoto, H.; Fujita, Y.; Matsuoka, N.; Yashiro-Furuya, M.; Asano, T.; Sato, S.; Suzuki, E.; et al. Real-world comparative effectiveness of bDMARDs and JAK inhibitors in elderly patients with rheumatoid arthritis. Medicine 2022, 101, e31161. [Google Scholar] [CrossRef]
- Eberhard, A.; Di Giuseppe, D.; Askling, J.; Bergman, S.; Bower, H.; Chatzidionysiou, K.; Forsblad-d’Elia, H.; Kastbom, A.; Olofsson, T.; Frisell, T.; et al. Effectiveness of JAK Inhibitors Compared with Biologic Disease-Modifying Antirheumatic Drugs on Pain Reduction in Rheumatoid Arthritis: Results from a Nationwide Swedish Cohort Study. Arthritis Rheumatol. 2025, 77, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Efthimiou, O.; Seo, M.; Chalkou, K.; Debray, T.; Egger, M.; Salanti, G. Developing clinical prediction models: A step-by-step guide. BMJ 2024, 386, e078276. [Google Scholar] [CrossRef]
- Liu, D.; Yu, G.; Yuan, N.; Nie, D. The efficacy and safety of biologic or targeted synthetic DMARDs in rheumatoid arthritis treatment: One year of review 2024. Allergol. Immunopathol. 2025, 53, 140–162. [Google Scholar] [CrossRef]
- Favalli, E.G.; Maioli, G.; Caporali, R. Biologics or Janus Kinase Inhibitors in Rheumatoid Arthritis Patients Who are Insufficient Responders to Conventional Anti-Rheumatic Drugs. Drugs 2024, 84, 877–894. [Google Scholar] [CrossRef]
- Wang, S.S.; Lewis, M.J.; Pitzalis, C. DNA Methylation Signatures of Response to Conventional Synthetic and Biologic Disease-Modifying Antirheumatic Drugs (DMARDs) in Rheumatoid Arthritis. Biomedicines 2023, 11, 1987. [Google Scholar] [CrossRef]
- Bhasin, S.; Cheung, P.P. The Role of Power Doppler Ultrasonography as Disease Activity Marker in Rheumatoid Arthritis. Dis. Markers 2015, 2015, 325909. [Google Scholar] [CrossRef] [PubMed]
- Subash, M.; Liu, L.H.; DeQuattro, K.; Choden, S.; Jacobsohn, L.; Katz, P.; Bajaj, P.; Barton, J.L.; Bartels, C.; Bermas, B.; et al. The Development of the Rheumatology Informatics System for Effectiveness Learning Collaborative for Improving Patient-Reported Outcome Collection and Patient-Centered Communication in Adult Rheumatology. ACR Open Rheumatol. 2021, 3, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, A.; Adams, L.; Barrett, M.; Bechtel, C.; Brennan, P.; Butte, A.; Faulkner, J.; Fontaine, E.; Friedhoff, S.; Halamka, J.; et al. The Promise of Digital Health: Then, Now, and the Future. NAM Perspect. 2022. [Google Scholar] [CrossRef]
- Subasri, V.; Krishnan, A.; Kore, A.; Dhalla, A.; Pandya, D.; Wang, B.; Malkin, D.; Razak, F.; Verma, A.A.; Goldenberg, A.; et al. Detecting and Remediating Harmful Data Shifts for the Responsible Deployment of Clinical AI Models. JAMA Netw. Open 2025, 8, e2513685. [Google Scholar] [CrossRef]
- Chen, R.J.; Wang, J.J.; Williamson, D.F.K.; Chen, T.Y.; Lipkova, J.; Lu, M.Y.; Sahai, S.; Mahmood, F. Algorithmic fairness in artificial intelligence for medicine and healthcare. Nat. Biomed. Eng. 2023, 7, 719–742. [Google Scholar] [CrossRef] [PubMed]
- Kore, A.; Abbasi Bavil, E.; Subasri, V.; Abdalla, M.; Fine, B.; Dolatabadi, E.; Abdalla, M. Empirical data drift detection experiments on real-world medical imaging data. Nat. Commun. 2024, 15, 1887. [Google Scholar] [CrossRef]
- Liu, L.H.; Choden, S.; Yazdany, J. Quality improvement initiatives in rheumatology: An integrative review of the last 5 years. Curr. Opin. Rheumatol. 2019, 31, 98–108. [Google Scholar] [CrossRef]
- Liu, T.; Gu, Y.; Chen, H.; Zhang, Y.; Zheng, L.; Huang, X.; Xu, Y.; Wen, C.; Chen, M.; Lin, J.; et al. A foundational triage system for improving accuracy in moderate acuity level emergency classifications. Commun. Med. 2025, 5, 322. [Google Scholar] [CrossRef]
- Portela, A.; Banga, J.R.; Matabuena, M. Conformal prediction for uncertainty quantification in dynamic biological systems. PLoS Comput. Biol. 2025, 21, e1013098. [Google Scholar] [CrossRef]
- Chen, D.; He, E.; Pace, K.; Chekay, M.; Raman, S. Concordance with SPIRIT-AI guidelines in reporting of randomized controlled trial protocols investigating artificial intelligence in oncology: A systematic review. Oncologist 2025, 30, oyaf112. [Google Scholar] [CrossRef]
- Bodnari, A.; Travis, J. Scaling enterprise AI in healthcare: The role of governance in risk mitigation frameworks. NPJ Digit. Med. 2025, 8, 272. [Google Scholar] [CrossRef]
- Palaniappan, K.; Lin, E.Y.T.; Vogel, S. Global Regulatory Frameworks for the Use of Artificial Intelligence (AI) in the Healthcare Services Sector. Healthcare 2024, 12, 562. [Google Scholar] [CrossRef]
- Martin, A.R.; Kanai, M.; Kamatani, Y.; Okada, Y.; Neale, B.M.; Daly, M.J. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 2019, 51, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Brooks, J.R.; Alford, C.C.; Chang, C.S.; Mueller, N.M.; Umscheid, C.A.; Bierman, A.S. Awareness of Racial and Ethnic Bias and Potential Solutions to Address Bias with Use of Health Care Algorithms. JAMA Health Forum 2023, 4, e231197. [Google Scholar] [CrossRef] [PubMed]
- Norori, N.; Hu, Q.; Aellen, F.M.; Faraci, F.D.; Tzovara, A. Addressing bias in big data and AI for health care: A call for open science. Patterns 2021, 2, 100347. [Google Scholar] [CrossRef] [PubMed]
- Cope, A.P.; Jasenecova, M.; Vasconcelos, J.C.; Filer, A.; Raza, K.; Qureshi, S.; D’Agostino, M.A.; McInnes, I.B.; Isaacs, J.D.; Pratt, A.G.; et al. Abatacept in individuals at high risk of rheumatoid arthritis (APIPPRA): A randomised, double-blind, multicentre, parallel, placebo-controlled, phase 2b clinical trial. Lancet 2024, 403, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Rech, J.; Tascilar, K.; Hagen, M.; Kleyer, A.; Manger, B.; Schoenau, V.; Hueber, A.J.; Kleinert, S.; Baraliakos, X.; Braun, J.; et al. Abatacept inhibits inflammation and onset of rheumatoid arthritis in individuals at high risk (ARIAA): A randomised, international, multicentre, double-blind, placebo-controlled trial. Lancet 2024, 403, 850–859. [Google Scholar] [CrossRef]
- Jin, S.; Zhao, J.; Li, M.; Zeng, X. New insights into the pathogenesis and management of rheumatoid arthritis. Chronic Dis. Transl. Med. 2022, 8, 256–263. [Google Scholar] [CrossRef]
- McDonald, S.M.; Felfeliyan, B.; Hassan, A.; Kupper, J.C.; El-Hajj, R.; Wichuk, S.; Aneja, A.; Kwok, C.; Zhang, C.X.Y.; Jans, L.; et al. Evaluating potential for AI automation of quantitative and semi-quantitative MRI scoring in arthritis, especially at the knee: A systematic literature review. Skeletal Radiol. 2025, 54, 2339–2349. [Google Scholar] [CrossRef]
- Mao, Y.; Imahori, K.; Fang, W.; Sugimori, H.; Kiuch, S.; Sutherland, K.; Kamishima, T. Artificial Intelligence Quantification of Enhanced Synovium Throughout the Entire Hand in Rheumatoid Arthritis on Dynamic Contrast-Enhanced MRI. J. Magn. Reson. Imaging 2025, 61, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Nicoara, A.I.; Sas, L.M.; Bita, C.E.; Dinescu, S.C.; Vreju, F.A. Implementation of artificial intelligence models in magnetic resonance imaging with focus on diagnosis of rheumatoid arthritis and axial spondyloarthritis: Narrative review. Front. Med. 2023, 10, 1280266. [Google Scholar] [CrossRef]
- Schlereth, M.; Mutlu, M.Y.; Utz, J.; Bayat, S.; Heimann, T.; Qiu, J.; Ehring, C.; Liu, C.; Uder, M.; Kleyer, A.; et al. Deep learning-based classification of erosion, synovitis and osteitis in hand MRI of patients with inflammatory arthritis. RMD Open 2024, 10, e004273. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sporn, K.; Prabhakar, V.; Alnemri, A.; Khanna, A.; Paladugu, P.; Gowda, C.; Clarkson, L.; Zaman, N.; Tavakkoli, A. Computational and Imaging Approaches for Precision Characterization of Bone, Cartilage, and Synovial Biomolecules. J. Pers. Med. 2025, 15, 298. [Google Scholar] [CrossRef] [PubMed]
- Currie, G.; Rohren, E. The deep radiomic analytics pipeline. Vet. Radiol. Ultrasound 2022, 63 (Suppl. S1), 889–896. [Google Scholar] [CrossRef]
- Ou, Y.; Ambalathankandy, P.; Furuya, R.; Kawada, S.; Zeng, T.; An, Y.; Kamishima, T.; Tamura, K.; Ikebe, M. A Sub-Pixel Accurate Quantification of Joint Space Narrowing Progression in Rheumatoid Arthritis. IEEE J. Biomed. Health Inform. 2023, 27, 53–64. [Google Scholar] [CrossRef]
- Ichikawa, S.; Kamishima, T.; Sutherland, K.; Okubo, T.; Katayama, K. Radiographic quantifications of joint space narrowing progression by computer-based approach using temporal subtraction in rheumatoid wrist. Br. J. Radiol. 2016, 89, 20150403. [Google Scholar] [CrossRef]
- Ou, J.; Zhang, J.; Alswadeh, M.; Zhu, Z.; Tang, J.; Sang, H.; Lu, K. Advancing osteoarthritis research: The role of AI in clinical, imaging and omics fields. Bone Res. 2025, 13, 48. [Google Scholar] [CrossRef]
- Woelfle, T.; Bourguignon, L.; Lorscheider, J.; Kappos, L.; Naegelin, Y.; Jutzeler, C.R. Wearable Sensor Technologies to Assess Motor Functions in People with Multiple Sclerosis: Systematic Scoping Review and Perspective. J. Med. Internet Res. 2023, 25, e44428. [Google Scholar] [CrossRef]
- Lee, S.; Kang, S.; Eun, Y.; Won, H.H.; Kim, H.; Lee, J.; Koh, E.M.; Cha, H.S. Machine learning-based prediction model for responses of bDMARDs in patients with rheumatoid arthritis and ankylosing spondylitis. Arthritis Res. Ther. 2021, 23, 254. [Google Scholar] [CrossRef]
- Tao, W.; Concepcion, A.N.; Vianen, M.; Marijnissen, A.C.A.; Lafeber, F.; Radstake, T.; Pandit, A. Multiomics and Machine Learning Accurately Predict Clinical Response to Adalimumab and Etanercept Therapy in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 212–222. [Google Scholar] [CrossRef]
- Benavent, D.; Carmona, L.; Garcia Llorente, J.F.; Montoro, M.; Ramirez, S.; Oton, T.; Loza, E.; Gomez-Centeno, A. Artificial intelligence to predict treatment response in rheumatoid arthritis and spondyloarthritis: A scoping review. Rheumatol. Int. 2025, 45, 91. [Google Scholar] [CrossRef]
- Hameed, M.; Exarchou, S.; Eberhard, A.; Sharma, A.; Bergstrom, U.; Cagnotto, G.; Einarsson, J.T.; Turesson, C. Predictors at diagnosis for start of biologic disease-modifying antirheumatic drugs in patients with early rheumatoid arthritis: A cohort study. BMJ Open 2024, 14, e076131. [Google Scholar] [CrossRef]
- Postal, M.; Vivaldo, J.F.; Fernandez-Ruiz, R.; Paredes, J.L.; Appenzeller, S.; Niewold, T.B. Type I interferon in the pathogenesis of systemic lupus erythematosus. Curr. Opin. Immunol. 2020, 67, 87–94. [Google Scholar] [CrossRef]
- Miyachi, K.; Iwamoto, T.; Kojima, S.; Ida, T.; Suzuki, J.; Yamamoto, T.; Mimura, N.; Sugiyama, T.; Tanaka, S.; Furuta, S.; et al. Relationship of systemic type I interferon activity with clinical phenotypes, disease activity, and damage accrual in systemic lupus erythematosus in treatment-naive patients: A retrospective longitudinal analysis. Arthritis Res. Ther. 2023, 25, 26. [Google Scholar] [CrossRef] [PubMed]
- Kaan, E.D.; Brunekreef, T.E.; Drylewicz, J.; van den Hoogen, L.L.; van der Linden, M.; Leavis, H.L.; van Laar, J.M.; van der Vlist, M.; Otten, H.G.; Limper, M. Association of autoantibodies with the IFN signature and NETosis in patients with systemic lupus erythematosus. J. Transl. Autoimmun. 2024, 9, 100246. [Google Scholar] [CrossRef] [PubMed]
- Felten, R.; Scher, F.; Sagez, F.; Chasset, F.; Arnaud, L. Spotlight on anifrolumab and its potential for the treatment of moderate-to-severe systemic lupus erythematosus: Evidence to date. Drug Des. Devel Ther. 2019, 13, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Khamashta, M.; Merrill, J.T.; Werth, V.P.; Kalunian, K.; Brohawn, P.; Illei, G.G.; Drappa, J.; Wang, L.; Yoo, S.; et al. Anifrolumab, an Anti-Interferon-alpha Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017, 69, 376–386. [Google Scholar] [CrossRef]
- Baker, T.; Sharifian, H.; Newcombe, P.J.; Gavin, P.G.; Lazarus, M.N.; Ramaswamy, M.; White, W.I.; Ferrari, N.; Muthas, D.; Tummala, R.; et al. Type I interferon blockade with anifrolumab in patients with systemic lupus erythematosus modulates key immunopathological pathways in a gene expression and proteomic analysis of two phase 3 trials. Ann. Rheum. Dis. 2024, 83, 1018–1027. [Google Scholar] [CrossRef]
- Cleanthous, S.; Strzok, S.; Haier, B.; Cano, S.; Morel, T. The Patient Experience of Fatigue in Systemic Lupus Erythematosus: A Conceptual Model. Rheumatol. Ther. 2022, 9, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.; Asaduzzaman, A.; Noamani, B.; Fortin, P.R.; Gladman, D.D.; Touma, Z.; Urowitz, M.B.; Wither, J. The baseline interferon signature predicts disease severity over the subsequent 5 years in systemic lupus erythematosus. Arthritis Res. Ther. 2021, 23, 29. [Google Scholar] [CrossRef]
- Ruscitti, P.; Allanore, Y.; Baldini, C.; Barilaro, G.; Bartoloni Bocci, E.; Bearzi, P.; Bellis, E.; Berardicurti, O.; Biaggi, A.; Bombardieri, M.; et al. Tailoring the treatment of inflammatory rheumatic diseases by a better stratification and characterization of the clinical patient heterogeneity. Findings from a systematic literature review and experts’ consensus. Autoimmun. Rev. 2024, 23, 103581. [Google Scholar] [CrossRef]
- Oliveira, J.J.; Karrar, S.; Rainbow, D.B.; Pinder, C.L.; Clarke, P.; Rubio Garcia, A.; Al-Assar, O.; Burling, K.; Morris, S.; Stratton, R.; et al. The plasma biomarker soluble SIGLEC-1 is associated with the type I interferon transcriptional signature, ethnic background and renal disease in systemic lupus erythematosus. Arthritis Res. Ther. 2018, 20, 152. [Google Scholar] [CrossRef] [PubMed]
- Perng, Y.C.; Lenschow, D.J. ISG15 in antiviral immunity and beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, J. IFN-gamma, should not be ignored in SLE. Front. Immunol. 2022, 13, 954706. [Google Scholar] [CrossRef]
- Gomez-Banuelos, E.; Goldman, D.W.; Andrade, V.; Darrah, E.; Petri, M.; Andrade, F. Uncoupling interferons and the interferon signature explains clinical and transcriptional subsets in SLE. Cell Rep. Med. 2024, 5, 101569. [Google Scholar] [CrossRef]
- Jupe, E.R.; Lushington, G.H.; Purushothaman, M.; Pautasso, F.; Armstrong, G.; Sorathia, A.; Crawley, J.; Nadipelli, V.R.; Rubin, B.; Newhardt, R.; et al. Tracking of Systemic Lupus Erythematosus (SLE) Longitudinally Using Biosensor and Patient-Reported Data: A Report on the Fully Decentralized Mobile Study to Measure and Predict Lupus Disease Activity Using Digital Signals-The OASIS Study. BioTech 2023, 12, 62. [Google Scholar] [CrossRef]
- Li, Y.; Yao, L.; Lee, Y.A.; Huang, Y.; Merkel, P.A.; Vina, E.; Yeh, Y.Y.; Li, Y.; Allen, J.M.; Bian, J.; et al. A fair machine learning model to predict flares of systemic lupus erythematosus. JAMIA Open 2025, 8, ooaf072. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, Y.; Song, Y.; Wu, K.; Chen, T.; Zhang, Y.; Jia, W.; Zhang, H.T.; Liang, D.D.; Yang, J.; et al. Deep learning model to predict lupus nephritis renal flare based on dynamic multivariable time-series data. BMJ Open 2024, 14, e071821. [Google Scholar] [CrossRef] [PubMed]
- Kamudoni, P.; Lyden, K.; Gunther, O.; Jaitely, V.; Araujo, T.D.; Spies, E.; Park, J.; Thomas, E.; Buie, J.; Blankenship, J.M.; et al. Identifying meaningful aspects of health and concepts of interest for assessment in systemic lupus erythematosus: Implications for digital clinical measure development. J. Patient Rep. Outcomes 2024, 8, 154. [Google Scholar] [CrossRef]
- Brzezinska, O.E.; Rychlicki-Kicior, K.A.; Makowska, J.S. Automatic assessment of nailfold capillaroscopy software: A pilot study. Reumatologia 2024, 62, 346–350. [Google Scholar] [CrossRef]
- Emam, O.S.; Ebadi Jalal, M.; Garcia-Zapirain, B.; Elmaghraby, A.S. Artificial Intelligence Algorithms in Nailfold Capillaroscopy Image Analysis: A Systematic Review. medRxiv 2024. [Google Scholar] [CrossRef]
- Adams, L.C.; Bressem, K.K.; Poddubnyy, D. Artificial intelligence and machine learning in axial spondyloarthritis. Curr. Opin. Rheumatol. 2024, 36, 267–273. [Google Scholar] [CrossRef]
- Pons, M.; Georgiadis, S.; Hetland, M.L.; Ahmadzay, Z.F.; Rasmussen, S.; Christiansen, S.N.; Di Giuseppe, D.; Wallman, J.K.; Pavelka, K.; Zavada, J.; et al. Predictors of Secukinumab Treatment Response and Continuation in Axial Spondyloarthritis: Results from the EuroSpA Research Collaboration Network. J. Rheumatol. 2025, 52, 572–582. [Google Scholar] [CrossRef]
- Dalix, E.; Marcelli, C.; Bejan-Angoulvant, T.; Finckh, A.; Rancon, F.; Akrour, M.; De Araujo, L.; Presles, E.; Marotte, H.; ROC-SpA study group. Rotation or change of biotherapy after TNF blocker treatment failure for axial spondyloarthritis: The ROC-SpA study, a randomised controlled study protocol. BMJ Open 2024, 14, e087872. [Google Scholar] [CrossRef]
- Cozzi, G.; Scagnellato, L.; Lorenzin, M.; Collesei, A.; Oliviero, F.; Damasco, A.; Cosma, C.; Basso, D.; Doria, A.; Ramonda, R. Predictors of response to bDMARDs and tsDMARDs in psoriatic arthritis: A pilot study on the role of musculoskeletal ultrasound. Front. Med. 2024, 11, 1482894. [Google Scholar] [CrossRef]
- Tillett, W.; Ogdie, A.; Passey, A.; Gorecki, P. Impact of psoriatic arthritis and comorbidities on ustekinumab outcomes in psoriasis: A retrospective, observational BADBIR cohort study. RMD Open 2023, 9, e002533. [Google Scholar] [CrossRef] [PubMed]
- Kunzler, T.; Bamert, M.; Sprott, H. Factors predicting treatment response to biological and targeted synthetic disease-modifying antirheumatic drugs in psoriatic arthritis—A systematic review and meta-analysis. Clin. Rheumatol. 2024, 43, 3723–3746. [Google Scholar] [CrossRef]
- Guo, H.; Gao, J.; Gong, L.; Wang, Y. Multi-omics analysis reveals novel causal pathways in psoriasis pathogenesis. J. Transl. Med. 2025, 23, 100. [Google Scholar] [CrossRef]
- Shi, Z.; Ding, Y.; Dong, X.; Li, G.; Li, B.; Hou, J.; Xue, L. The diagnostic value and clinical relevance of salivary gland ultrasound in patients with highly suspected Sjogren’s Disease: A prospective monocentric study. Arthritis Res. Ther. 2025, 27, 175. [Google Scholar] [CrossRef]
- Yang, J.; Park, Y.; Lee, J.J.; Kim, W.U.; Park, S.H.; Kwok, S.K. Clinical value of salivary gland ultrasonography in evaluating secretory function, disease activity, and lymphoma risk factors in primary Sjogren’s syndrome. Clin. Rheumatol. 2025, 44, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- De Vita, S.; Isola, M.; Baldini, C.; Goules, A.V.; Chatzis, L.G.; Quartuccio, L.; Zabotti, A.; Giovannini, I.; Donati, V.; Ferro, F.; et al. Predicting lymphoma in Sjogren’s syndrome and the pathogenetic role of parotid microenvironment through precise parotid swelling recording. Rheumatology 2023, 62, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Umapathy, V.R.; Natarajan, P.M.; Swamikannu, B. Review Insights on Salivary Proteomics Biomarkers in Oral Cancer Detection and Diagnosis. Molecules 2023, 28, 5283. [Google Scholar] [CrossRef] [PubMed]
- Sembler-Moller, M.L.; Belstrom, D.; Locht, H.; Pedersen, A.M.L. Proteomics of saliva, plasma, and salivary gland tissue in Sjogren’s syndrome and non-Sjogren patients identify novel biomarker candidates. J. Proteomics 2020, 225, 103877. [Google Scholar] [CrossRef]
- Hu, S.; Gao, K.; Pollard, R.; Arellano-Garcia, M.; Zhou, H.; Zhang, L.; Elashoff, D.; Kallenberg, C.G.; Vissink, A.; Wong, D.T. Preclinical validation of salivary biomarkers for primary Sjogren’s syndrome. Arthritis Care Res. 2010, 62, 1633–1638. [Google Scholar] [CrossRef]
- Bonroy, C.; Piette, Y.; Allenbach, Y.; Bossuyt, X.; Damoiseaux, J. Positioning of myositis-specific and associated autoantibody (MSA/MAA) testing in disease criteria and routine diagnostic work-up. J. Transl. Autoimmun. 2022, 5, 100148. [Google Scholar] [CrossRef]
- McLeish, E.; Slater, N.; Mastaglia, F.L.; Needham, M.; Coudert, J.D. From data to diagnosis: How machine learning is revolutionizing biomarker discovery in idiopathic inflammatory myopathies. Brief. Bioinform. 2023, 25, bbad514. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Du, Y.; Wang, L.; Wang, Q.; Wu, H.; Liu, L.; Xue, J. Mortality risk in patients with anti-MDA5 dermatomyositis is related to rapidly progressive interstitial lung disease and anti-Ro52 antibody. Arthritis Res. Ther. 2023, 25, 127. [Google Scholar] [CrossRef] [PubMed]
- Nagawa, K.; Suzuki, M.; Yamamoto, Y.; Inoue, K.; Kozawa, E.; Mimura, T.; Nakamura, K.; Nagata, M.; Niitsu, M. Texture analysis of muscle MRI: Machine learning-based classifications in idiopathic inflammatory myopathies. Sci. Rep. 2021, 11, 9821. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, S.; Hou, B.; Santini, F.; Yuan, L.; Guo, Y.; Zhu, J.; Hilbert, T.; Kober, T.; Zhang, Y.; et al. Assessment of idiopathic inflammatory myopathy using a deep learning method for muscle T2 mapping segmentation. Eur. Radiol. 2023, 33, 2350–2357. [Google Scholar] [CrossRef]
- Danieli, M.G.; Brunetto, S.; Gammeri, L.; Palmeri, D.; Claudi, I.; Shoenfeld, Y.; Gangemi, S. Machine learning application in autoimmune diseases: State of art and future prospectives. Autoimmun. Rev. 2024, 23, 103496. [Google Scholar] [CrossRef]
- Moingeon, P. Artificial intelligence-driven drug development against autoimmune diseases. Trends Pharmacol. Sci. 2023, 44, 411–424. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Xu, H.; Li, Z.; Feng, F.; Zhang, J.; Xu, Z.; Ni, H.; Guo, Y.; Li, Y. Malignancy in Idiopathic Inflammatory Myopathies: Recent Insights. Clin. Rev. Allergy Immunol. 2025, 68, 83. [Google Scholar] [CrossRef]
- van der Geest, K.S.M.; Treglia, G.; Glaudemans, A.; Brouwer, E.; Sandovici, M.; Jamar, F.; Gheysens, O.; Slart, R. Diagnostic value of [18F]FDG-PET/CT for treatment monitoring in large vessel vasculitis: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3886–3902. [Google Scholar] [CrossRef]
- Wilk, B.; Wisenberg, G.; Dharmakumar, R.; Thiessen, J.D.; Goldhawk, D.E.; Prato, F.S. Hybrid PET/MR imaging in myocardial inflammation post-myocardial infarction. J. Nucl. Cardiol. 2020, 27, 2083–2099. [Google Scholar] [CrossRef]
- Brilland, B.; Riou, J.; Quemeneur, T.; Vandenbussche, C.; Merillon, N.; Boizard-Moracchini, A.; Roy, M.; Despre, M.; Piccoli, G.B.; Djema, A.; et al. Identification of Renal Transcripts Associated with Kidney Function and Prognosis in ANCA-Associated Vasculitis. J. Am. Soc. Nephrol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Han, S.; Li, L.; Fu, Y.; Zhou, D. Interferon-Stimulated Genes: Novel Targets in Renal Pathogenesis. Kidney Dis. 2025, 11, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.; Agbareia, R.; Naffaa, M.E.; Watad, A.; Glicksberg, B.S.; Nadkarni, G.N.; Klang, E. Applications of Artificial Intelligence in Vasculitides: A Systematic Review. ACR Open Rheumatol. 2025, 7, e70016. [Google Scholar] [CrossRef]
- Maarseveen, T.D.; Glas, H.K.; Veris-van Dieren, J.; van den Akker, E.; Knevel, R. Improving musculoskeletal care with AI enhanced triage through data driven screening of referral letters. NPJ Digit. Med. 2025, 8, 98. [Google Scholar] [CrossRef]
- Knitza, J.; Janousek, L.; Kluge, F.; von der Decken, C.B.; Kleinert, S.; Vorbruggen, W.; Kleyer, A.; Simon, D.; Hueber, A.J.; Muehlensiepen, F.; et al. Machine learning-based improvement of an online rheumatology referral and triage system. Front. Med. 2022, 9, 954056. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, J.; Li, J.; Yao, J.; Zhao, H. Biomarkers of rheumatoid arthritis-associated interstitial lung disease: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1455346. [Google Scholar] [CrossRef]
- Frederiksen, B.A.; Hammer, H.B.; Terslev, L.; Ammitzboll-Danielsen, M.; Savarimuthu, T.R.; Weber, A.B.H.; Just, S.A. Automated ultrasound system ARTHUR V.2.0 with AI analysis DIANA V.2.0 matches expert rheumatologist in hand joint assessment of rheumatoid arthritis patients. RMD Open 2025, 11, 005805. [Google Scholar] [CrossRef] [PubMed]
- Nigro, A. Fast-track capillaroscopic progression in systemic sclerosis: A case-based review of active pattern emerging within 3 months of raynaud’s phenomenon onset. Rheumatol. Int. 2025, 45, 203. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, D. Revolutionizing Medical Imaging: The Transformative Role of Artificial Intelligence in Diagnostics and Treatment. Diagnostics 2025, 15, 1557. [Google Scholar] [CrossRef]
- Kocak, B.; Ponsiglione, A.; Stanzione, A.; Bluethgen, C.; Santinha, J.; Ugga, L.; Huisman, M.; Klontzas, M.E.; Cannella, R.; Cuocolo, R. Bias in artificial intelligence for medical imaging: Fundamentals, detection, avoidance, mitigation, challenges, ethics, and prospects. Diagn. Interv. Radiol. 2025, 31, 75–88. [Google Scholar] [CrossRef]
- Hasanzadeh, F.; Josephson, C.B.; Waters, G.; Adedinsewo, D.; Azizi, Z.; White, J.A. Bias recognition and mitigation strategies in artificial intelligence healthcare applications. NPJ Digit. Med. 2025, 8, 154. [Google Scholar] [CrossRef]
- Koo, B.S.; Eun, S.; Shin, K.; Yoon, H.; Hong, C.; Kim, D.H.; Hong, S.; Kim, Y.G.; Lee, C.K.; Yoo, B.; et al. Machine learning model for identifying important clinical features for predicting remission in patients with rheumatoid arthritis treated with biologics. Arthritis Res. Ther. 2021, 23, 178. [Google Scholar] [CrossRef]
- Bellocchi, C.; Favalli, E.G.; Maioli, G.; Agape, E.; Rossato, M.; Paini, M.; Severino, A.; Vigone, B.; Biggioggero, M.; Trombetta, E.; et al. Whole-Blood RNA Sequencing Profiling of Patients with Rheumatoid Arthritis Treated with Tofacitinib. ACR Open Rheumatol. 2025, 7, e11761. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Jung, O.; Hennighausen, L. JAK inhibitors dampen activation of interferon-stimulated transcription of ACE2 isoforms in human airway epithelial cells. Commun. Biol. 2021, 4, 654. [Google Scholar] [CrossRef]
- Bergero, M.A.; Martinez, P.; Modina, P.; Hosman, R.; Villamil, W.; Gudino, R.; David, C.; Costa, L. Artificial intelligence model for predicting early biochemical recurrence of prostate cancer after robotic-assisted radical prostatectomy. Sci. Rep. 2025, 15, 30822. [Google Scholar] [CrossRef]
- Chen, M.M.; Rosenkrantz, A.B.; Nicola, G.N.; Silva, E., 3rd; McGinty, G.; Manchikanti, L.; Hirsch, J.A. The Qualified Clinical Data Registry: A Pathway to Success Within MACRA. AJNR Am. J. Neuroradiol. 2017, 38, 1292–1296. [Google Scholar] [CrossRef]
- Kersey, E.; Li, J.; Adler-Milstein, J.; Yazdany, J.; Shiboski, S.; Schmajuk, G. Association of Qualified Clinical Data Registry Clinician Dashboard Engagement with Performance on Quality-of-Care Measures: Cross-Sectional Analysis. J. Med. Internet Res. 2025, 27, e72709. [Google Scholar] [CrossRef]
- Tabari, P.; Costagliola, G.; De Rosa, M.; Boeker, M. State-of-the-Art Fast Healthcare Interoperability Resources (FHIR)-Based Data Model and Structure Implementations: Systematic Scoping Review. JMIR Med. Inform. 2024, 12, e58445. [Google Scholar] [CrossRef]
- Marfoglia, A.; Nardini, F.; Arcobelli, V.A.; Moscato, S.; Mellone, S.; Carbonaro, A. Towards real-world clinical data standardization: A modular FHIR-driven transformation pipeline to enhance semantic interoperability in healthcare. Comput. Biol. Med. 2025, 187, 109745. [Google Scholar] [CrossRef]
- Rossander, A.; Lindskold, L.; Ranerup, A.; Karlsson, D. A State-of-the Art Review of SNOMED CT Terminology Binding and Recommendations for Practice and Research. Methods Inf. Med. 2021, 60, e76–e88. [Google Scholar] [CrossRef] [PubMed]
- Bakken, S. Standards and frameworks. J. Am. Med. Inform. Assoc. 2024, 31, 1629–1630. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, J.; Zhou, J.; Hu, G. Twenty-Five Years of Evolution and Hurdles in Electronic Health Records and Interoperability in Medical Research: Comprehensive Review. J. Med. Internet Res. 2025, 27, e59024. [Google Scholar] [CrossRef]
- Gulden, C.; Macho, P.; Reinecke, I.; Strantz, C.; Prokosch, H.U.; Blasini, R. recruIT: A cloud-native clinical trial recruitment support system based on Health Level 7 Fast Healthcare Interoperability Resources (HL7 FHIR) and the Observational Medical Outcomes Partnership Common Data Model (OMOP CDM). Comput. Biol. Med. 2024, 174, 108411. [Google Scholar] [CrossRef]
- Kim, D.; Oh, K.; Lee, Y.; Woo, H. Overview of fair federated learning for fairness and privacy preservation. Expert Syst. Appl. 2025, 293, 128568. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, X.; Liu, K.; Liu, Z.; Chen, W.; Liu, B.; Ngai, E.W.; Hu, Y. A personalized federated learning approach to enhance joint modeling for heterogeneous medical institutions. Digit. Health 2025, 11, 20552076251360861. [Google Scholar] [CrossRef] [PubMed]
- Dayan, I.; Roth, H.R.; Zhong, A.; Harouni, A.; Gentili, A.; Abidin, A.Z.; Liu, A.; Costa, A.B.; Wood, B.J.; Tsai, C.S.; et al. Federated learning for predicting clinical outcomes in patients with COVID-19. Nat. Med. 2021, 27, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Solitano, V.; Ahuja, D.; Lee, H.H.; Gaikwad, R.; Yeh, K.H.; Facciorusso, A.; Singh, A.G.; Ma, C.; Ananthakrishnan, A.N.; Yuan, Y.; et al. Comparative Safety of JAK Inhibitors vs TNF Antagonists in Immune-Mediated Inflammatory Diseases: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2025, 8, e2531204. [Google Scholar] [CrossRef]
- Li, H.; Zang, C.; Xu, Z.; Pan, W.; Rajendran, S.; Chen, Y.; Wang, F. Federated target trial emulation using distributed observational data for treatment effect estimation. NPJ Digit. Med. 2025, 8, 387. [Google Scholar] [CrossRef]
- Matta, S.; Lamard, M.; Zhang, P.; Le Guilcher, A.; Borderie, L.; Cochener, B.; Quellec, G. A systematic review of generalization research in medical image classification. Comput. Biol. Med. 2024, 183, 109256. [Google Scholar] [CrossRef]
- Le, J.P.; Shashikumar, S.P.; Malhotra, A.; Nemati, S.; Wardi, G. Making the Improbable Possible: Generalizing Models Designed for a Syndrome-Based, Heterogeneous Patient Landscape. Crit. Care Clin. 2023, 39, 751–768. [Google Scholar] [CrossRef]
- Zhu, H.; Bai, J.; Li, N.; Li, X.; Liu, D.; Buckeridge, D.L.; Li, Y. FedWeight: Mitigating covariate shift of federated learning on electronic health records data through patients re-weighting. NPJ Digit. Med. 2025, 8, 286. [Google Scholar] [CrossRef]
- Wu, Q.; Reps, J.M.; Li, L.; Zhang, B.; Lu, Y.; Tong, J.; Zhang, D.; Lumley, T.; Brand, M.T.; Van Zandt, M.; et al. COLA-GLM: Collaborative one-shot and lossless algorithms of generalized linear models for decentralized observational healthcare data. NPJ Digit. Med. 2025, 8, 442. [Google Scholar] [CrossRef]
- Zhang, F.; Zhai, D.; Bai, G.; Jiang, J.; Ye, Q.; Ji, X.; Liu, X. Towards fairness-aware and privacy-preserving enhanced collaborative learning for healthcare. Nat. Commun. 2025, 16, 2852. [Google Scholar] [CrossRef]
- Curtis, J.R.; Su, Y.; Black, S.; Xu, S.; Langholff, W.; Bingham, C.O.; Kafka, S.; Xie, F. Machine Learning Applied to Patient-Reported Outcomes to Classify Physician-Derived Measures of Rheumatoid Arthritis Disease Activity. ACR Open Rheumatol. 2022, 4, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Kienast, M.; Medawar, E.; Reinelt, J.; Merola, A.; Klopfenstein, S.A.I.; Flint, A.R.; Heeren, P.; Poncette, A.S.; Balzer, F.; et al. A Standardized Clinical Data Harmonization Pipeline for Scalable AI Application Deployment (FHIR-DHP): Validation and Usability Study. JMIR Med. Inform. 2023, 11, e43847. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Pfaff, E.; Prud’hommeaux, E.; Booth, D.; Sharma, D.K.; Huo, N.; Yu, Y.; Zong, N.; Ruddy, K.J.; Chute, C.G.; et al. FHIR-Ontop-OMOP: Building clinical knowledge graphs in FHIR RDF with the OMOP Common data Model. J. Biomed. Inform. 2022, 134, 104201. [Google Scholar] [CrossRef]
- El Arab, R.A.; Al Moosa, O.A. Systematic review of cost effectiveness and budget impact of artificial intelligence in healthcare. NPJ Digit. Med. 2025, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Scipion, C.E.A.; Manchester, M.A.; Federman, A.; Wang, Y.; Arias, J.J. Barriers to and facilitators of clinician acceptance and use of artificial intelligence in healthcare settings: A scoping review. BMJ Open 2025, 15, e092624. [Google Scholar] [CrossRef]
- Wei, Q.; Pan, S.; Liu, X.; Hong, M.; Nong, C.; Zhang, W. The integration of AI in nursing: Addressing current applications, challenges, and future directions. Front. Med. 2025, 12, 1545420. [Google Scholar] [CrossRef]
- Nowell, W.B.; Curtis, J.R. Remote Therapeutic Monitoring in Rheumatic and Musculoskeletal Diseases: Opportunities and Implementation. Med. Res. Arch. 2023, 11, 3957. [Google Scholar] [CrossRef]
- FDA. Artificial Intelligence and Machine Learning Software as a Medical Device (SaMD); FDA: Silver Spring, MD, USA, 2024. [Google Scholar]
- EMA. Reflection Paper on the Use of Artificial Intelligence (AI) in the Medicinal Product Lifecycle (EMA/CHMP/CVMP/83833/2023); EMA: Amsterdam, NL, USA, 2024. [Google Scholar]
- Hopewell, S.; Chan, A.W.; Collins, G.S.; Hrobjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 explanation and elaboration: Updated guideline for reporting randomised trials. BMJ 2025, 389, e081124. [Google Scholar] [CrossRef]
- Collins, G.S.; Dhiman, P.; Ma, J.; Schlussel, M.M.; Archer, L.; Van Calster, B.; Harrell, F.E., Jr.; Martin, G.P.; Moons, K.G.M.; van Smeden, M.; et al. Evaluation of clinical prediction models (part 1): From development to external validation. BMJ 2024, 384, e074819. [Google Scholar] [CrossRef]
- de Hond, A.A.H.; Shah, V.B.; Kant, I.M.J.; Van Calster, B.; Steyerberg, E.W.; Hernandez-Boussard, T. Perspectives on validation of clinical predictive algorithms. NPJ Digit. Med. 2023, 6, 86. [Google Scholar] [CrossRef]
- Piovani, D.; Sokou, R.; Tsantes, A.G.; Vitello, A.S.; Bonovas, S. Optimizing Clinical Decision Making with Decision Curve Analysis: Insights for Clinical Investigators. Healthcare 2023, 11, 2244. [Google Scholar] [CrossRef]
- Vickers, A.J.; Holland, F. Decision curve analysis to evaluate the clinical benefit of prediction models. Spine J. 2021, 21, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.F.; Brown, M.D.; Zhu, K.; Janes, H. Assessing the Clinical Impact of Risk Prediction Models with Decision Curves: Guidance for Correct Interpretation and Appropriate Use. J. Clin. Oncol. 2016, 34, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Tam, T.Y.C.; Sivarajkumar, S.; Kapoor, S.; Stolyar, A.V.; Polanska, K.; McCarthy, K.R.; Osterhoudt, H.; Wu, X.; Visweswaran, S.; Fu, S.; et al. A framework for human evaluation of large language models in healthcare derived from literature review. NPJ Digit. Med. 2024, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Venerito, V. Artificial intelligence in rheumatology: Days of a future past. Rheumatol. Adv. Pract. 2025, 9, rkaf022. [Google Scholar] [CrossRef]
- Chopra, H.; Annu; Shin, D.K.; Munjal, K.; Priyanka; Dhama, K.; Emran, T.B. Revolutionizing clinical trials: The role of AI in accelerating medical breakthroughs. Int. J. Surg. 2023, 109, 4211–4220. [Google Scholar] [CrossRef]
- Dolin, P.; Li, W.; Dasarathy, G.; Berisha, V. Statistically Valid Post-Deployment Monitoring Should Be Standard for AI-Based Digital Health. arXiv 2025. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Y.; Zhou, T.; Tong, D.; Ma, J.; Li, J. A survey of artificial intelligence in rheumatoid arthritis. Rheumatol. Immunol. Res. 2023, 4, 69–77. [Google Scholar] [CrossRef]
- Moreno-Grau, S.; Vernekar, M.; Lopez-Pineda, A.; Mas-Montserrat, D.; Barrabes, M.; Quinto-Cortes, C.D.; Moatamed, B.; Lee, M.T.M.; Yu, Z.; Numakura, K.; et al. Polygenic risk score portability for common diseases across genetically diverse populations. Hum. Genomics 2024, 18, 93. [Google Scholar] [CrossRef]
- Miao, J.; Guo, H.; Song, G.; Zhao, Z.; Hou, L.; Lu, Q. Quantifying portable genetic effects and improving cross-ancestry genetic prediction with GWAS summary statistics. Nat. Commun. 2023, 14, 832. [Google Scholar] [CrossRef]
- Roschewitz, M.; Khara, G.; Yearsley, J.; Sharma, N.; James, J.J.; Ambrozay, E.; Heroux, A.; Kecskemethy, P.; Rijken, T.; Glocker, B. Automatic correction of performance drift under acquisition shift in medical image classification. Nat. Commun. 2023, 14, 6608. [Google Scholar] [CrossRef]
- Lambert, B.; Forbes, F.; Doyle, S.; Dehaene, H.; Dojat, M. Trustworthy clinical AI solutions: A unified review of uncertainty quantification in Deep Learning models for medical image analysis. Artif. Intell. Med. 2024, 150, 102830. [Google Scholar] [CrossRef]
- Gilbert, S.; Adler, R.; Holoyad, T.; Weicken, E. Could transparent model cards with layered accessible information drive trust and safety in health AI? NPJ Digit. Med. 2025, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Chinta, S.V.; Wang, Z.; Palikhe, A.; Zhang, X.; Kashif, A.; Smith, M.A.; Liu, J.; Zhang, W. AI-driven healthcare: Fairness in AI healthcare: A survey. PLoS Digit. Health 2025, 4, e0000864. [Google Scholar] [CrossRef] [PubMed]
- Gallifant, J.; Kistler, E.A.; Nakayama, L.F.; Zera, C.; Kripalani, S.; Ntatin, A.; Fernandez, L.; Bates, D.; Dankwa-Mullan, I.; Celi, L.A. Disparity dashboards: An evaluation of the literature and framework for health equity improvement. Lancet Digit. Health 2023, 5, e831–e839. [Google Scholar] [CrossRef]
- Alderman, J.E.; Palmer, J.; Laws, E.; McCradden, M.D.; Ordish, J.; Ghassemi, M.; Pfohl, S.R.; Rostamzadeh, N.; Cole-Lewis, H.; Glocker, B.; et al. Tackling algorithmic bias and promoting transparency in health datasets: The STANDING Together consensus recommendations. Lancet Digit. Health 2025, 7, e64–e88. [Google Scholar] [CrossRef] [PubMed]
- Teikari, P.; Jarrell, M.; Azh, M.; Pesola, H. The Architecture of Trust: A Framework for AI-Augmented Real Estate Valuation in the Era of Structured Data. arXiv 2025. [Google Scholar] [CrossRef]
- Davis, S.E.; Embi, P.J.; Matheny, M.E. Sustainable deployment of clinical prediction tools-a 360 degrees approach to model maintenance. J. Am. Med. Inform. Assoc. 2024, 31, 1195–1198. [Google Scholar] [CrossRef]
- Rosenthal, J.T.; Beecy, A.; Sabuncu, M.R. Rethinking clinical trials for medical AI with dynamic deployments of adaptive systems. NPJ Digit. Med. 2025, 8, 252. [Google Scholar] [CrossRef]
- Zerrouk, N.; Auge, F.; Niarakis, A. Building a modular and multi-cellular virtual twin of the synovial joint in Rheumatoid Arthritis. NPJ Digit. Med. 2024, 7, 379. [Google Scholar] [CrossRef] [PubMed]
| Disease | Key Biomarkers/Targets | AI/Digital Innovations | Clinical Impact | Key Limitations/Gaps |
|---|---|---|---|---|
| Rheumatoid Arthritis (RA) | Pre-RA prevention with abatacept (APIPPRA, ARIAA); autoantibodies (ACPA, RF); MRI-detected subclinical inflammation | Deep learning for US/MRI synovitis segmentation; sub-pixel JSN quantification; smartphone-based fist closure (MeFISTO) as a functional biomarker; ML models combining multi-omics + imaging | Demonstrates feasibility of disease interception; scalable imaging and digital biomarkers; early steps toward individualized drug response prediction | Long-term durability of prevention unknown; small imaging datasets; lack of external validation; heterogeneity in ML pipelines |
| Systemic Lupus Erythematosus (SLE) | Type I IFN gene signature; SIGLEC-1 expression; proteomic biomarkers (SAA1, B4GALT5, etc.) | IFN-signature guided therapy with anifrolumab; wearables + PROs (OASIS study); EHR-based flare prediction (FLAME); deep learning for lupus nephritis flares; proteomic + ML flare models | Establishes IFN signature as both predictive and monitoring biomarker; digital phenotyping enables early flare detection | Variable organ-specific response; inconsistent LN outcomes; digital tools often under-validated; flare definitions heterogeneous |
| Systemic Sclerosis (SSc) | Microvascular patterns (giant capillaries, hemorrhages, density loss) on nailfold capillaroscopy | AI-assisted NFC classification: ResNet, EfficientNet, CAPI-Detect; large, annotated NFC datasets; pattern staging (early/active/late) | Enhances reproducibility and early diagnosis; potential for risk stratification (e.g., pulmonary hypertension, ulcers) | Few longitudinal outcome studies; lack of standardized acquisition protocols; external validation limited |
| Spondyloarthritis (SpA) | HLA-B27, MRI sacroiliac inflammation, PROs, PK parameters | Registry-based ML models (EuroSpA secukinumab cohort); ROC-SpA trial testing PK-guided prediction | Supports treatment persistence and real-world prediction; PK may inform therapeutic drug monitoring | Heterogeneous endpoints; small sample sizes; lack of standardized composite outcomes |
| Psoriatic Arthritis (PsA) | Disease activity, comorbidities, sonographic inflammation | US-based short-interval predictors (MIJET/2MIJET); early discrimination of JAKi vs. TNFi/ILi responses | Demonstrates feasibility of early imaging response markers; pragmatic outcome (drug retention) | Small pilot cohorts; scarce validated molecular predictors; multi-domain disease complicates modeling |
| Sjögren’s Disease (SjD) | SGUS scores (OMERACT, Hočevar); salivary/tear proteomics; expanded autoantibodies | Standardized SGUS linked to lymphoma risk; proteomic pipelines integrating saliva, plasma, tissue | Non-invasive early diagnosis and risk stratification; complements biopsy | Need for longitudinal validation; risk of over-screening; proteomic candidates require replication |
| Idiopathic Inflammatory Myopathies (IIM) | Myositis-specific autoantibodies (MSAs); MRI muscle edema; multi-omics panels | ML clustering integrating MSAs + MRI + omics; radiomics-based antibody group prediction | Improved subtype stratification; potential guidance for ILD or therapy selection | Mostly retrospective, single-center; translation to outcomes (e.g., steroid-sparing) unproven |
| Vasculitides | CRP, ANCA patterns, type I IFN signatures; renal 12-gene transcriptomic panel | PET-CT radiomics/ML distinguishing GCA vs. atherosclerosis; transcriptomics predicting kidney failure in AAV | Enables precision risk stratification (renal outcomes, vascular inflammation) | Early-stage; need for harmonized endpoints; prospective trials embedding predictors into care are lacking |
| Domain | Data/ Input | Model Types | Validation Status | Clinical Maturity | Key Challenges | Next Translational Step |
|---|---|---|---|---|---|---|
| Triage & Access | Referral letters (NLP), structured intake | NLP (transformers, boosting) | External-site validation [212] | High—first in line for deployment | Calibration drift, subgroup fairness, governance rules | Registry/workflow embedding, prospective drift monitoring |
| Imaging Decision Support | MRI, US, NVC images | CNNs, end-to-end pipelines | Multicenter feasibility (MRI/US/NVC); reproducibility demonstrated [83,98,112] | Moderate—reader-assist tools maturing | Scanner/vendor heterogeneity, lack of prospective trials | Workflow-embedded prospective evaluation; standardized reporting |
| Therapy Selection | Clinical data, serology, omics, imaging | Gradient boosting, multimodal ML, RNA-seq signatures | Mostly retrospective; limited external/temporal validation [132,165] | Early—promising but not trial-ready | Heterogeneity, lack of harmonized endpoints, no impact trials | Registry pilots; decision-curve analysis; prospective impact studies |
| Risk Stratification (Adjacent: RA-ILD) | Biomarkers (KL-6), imaging, clinical data | XGBoost, ensemble ML | Cohort-level validation [214] | Moderate—translational potential for early screening | Generalizability, integration into care pathway |
| Infrastructure Pillar | Strengths | Limitations | Clinical Applications |
|---|---|---|---|
| Registries & EHR (e.g., RISE) | National-scale QCDR; supports quality improvement and reimbursement; real-world evidence of improved care quality. | Dependent on practice adoption and data quality; disease coverage is still limited (e.g., lupus measures emerging). | Quality benchmarking, CMS Quality Payment Program reporting, registry-based research. |
| Interoperability (OMOP/FHIR) | FHIR enables clinical data exchange; OMOP supports multi-site analytics; hybrid architectures proven feasible. | Standards alone are insufficient; require metadata, governance, and ontology alignment (SNOMED CT, LOINC). | Multi-site analytics, phenotyping, clinical trial recruitment, harmonized real-world evidence generation. |
| Privacy-Preserving Collaboration (Federated Learning) | Allows multi-site model training without centralizing patient data; governance frameworks emerging; feasible in diverse clinical tasks. | Technically complex; potential for bias and fairness issues; resource-intensive implementation. | Comparative effectiveness research (e.g., RA biologics), collaborative risk prediction across sites. |
| Multisite Modeling Pitfalls & Mitigations | Recognition of covariate shift, site bias, and acquisition drift; new methods (FedWeight, COLA-GLMM) improve calibration and cross-site validity. | Residual generalization challenges: continuous monitoring and retraining required. | Development of fairer, more robust models; deployment with embedded recalibration triggers. |
| Case Example: RA Disease Activity Prediction | Demonstrated feasibility using EHR + PRO features; established templates for computable disease-activity endpoints. | Early studies lacked robust external validation; not yet embedded in clinical dashboards. | Risk-stratified dashboards for RA management; integration of prediction models into quality improvement cycles. |
| Domain | Key Challenges | Risks if Unaddressed | Mitigation Strategies | Minimum Reporting Set |
|---|---|---|---|---|
| PRS Portability & Ancestry Gaps | Loss of accuracy across ancestries due to allele-frequency and LD differences; poor calibration across sex, age, and SES strata. | Worsening health disparities; misleading risk predictions; inequitable clinical recommendations. | Multi-ancestry GWAS; ancestry-aware modeling; ancestry/site-specific recalibration; subgroup reporting of performance. | Ancestry-stratified R2/AUC; calibration curves; decision-curve analysis by subgroup. |
| Data Drift & Bias Audits | Model degradation due to covariate, prior-probability, acquisition, and concept drift; hidden biases in datasets. | Silent failure of AI models; unfair treatment allocation; erosion of clinical trust. | Drift detectors with temporal validation; scheduled recalibration; bias audits; integration of bias dashboards in registries. | Performance stratified by sex, age, ancestry, SES proxies, and site; bias dashboard outputs. |
| Governance & Safety by Design | Ensuring continuous safety across lifecycles: bias detection, calibration monitoring, clinician-defer thresholds, and accountability. | Unsafe deployment; lack of transparency; patient harm; regulatory non-compliance. | Pre-deployment bias assessment; live calibration monitoring; safety circuit-breakers; audit trails; periodic re-validation and changelogs. | Intended-use statement; subgroup performance reports; update logs with versioning; governance protocols. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ewaidat, O.A.; Naffaa, M.M. Emerging AI- and Biomarker-Driven Precision Medicine in Autoimmune Rheumatic Diseases: From Diagnostics to Therapeutic Decision-Making. Rheumato 2025, 5, 17. https://doi.org/10.3390/rheumato5040017
Al-Ewaidat OA, Naffaa MM. Emerging AI- and Biomarker-Driven Precision Medicine in Autoimmune Rheumatic Diseases: From Diagnostics to Therapeutic Decision-Making. Rheumato. 2025; 5(4):17. https://doi.org/10.3390/rheumato5040017
Chicago/Turabian StyleAl-Ewaidat, Ola A., and Moawiah M. Naffaa. 2025. "Emerging AI- and Biomarker-Driven Precision Medicine in Autoimmune Rheumatic Diseases: From Diagnostics to Therapeutic Decision-Making" Rheumato 5, no. 4: 17. https://doi.org/10.3390/rheumato5040017
APA StyleAl-Ewaidat, O. A., & Naffaa, M. M. (2025). Emerging AI- and Biomarker-Driven Precision Medicine in Autoimmune Rheumatic Diseases: From Diagnostics to Therapeutic Decision-Making. Rheumato, 5(4), 17. https://doi.org/10.3390/rheumato5040017







