Rare Case of Hemoglobin Lepore Trait in a Bangladeshi Patient with Polyarthritis and Fever: Case Description and Brief Literature Review
Abstract
1. Introduction
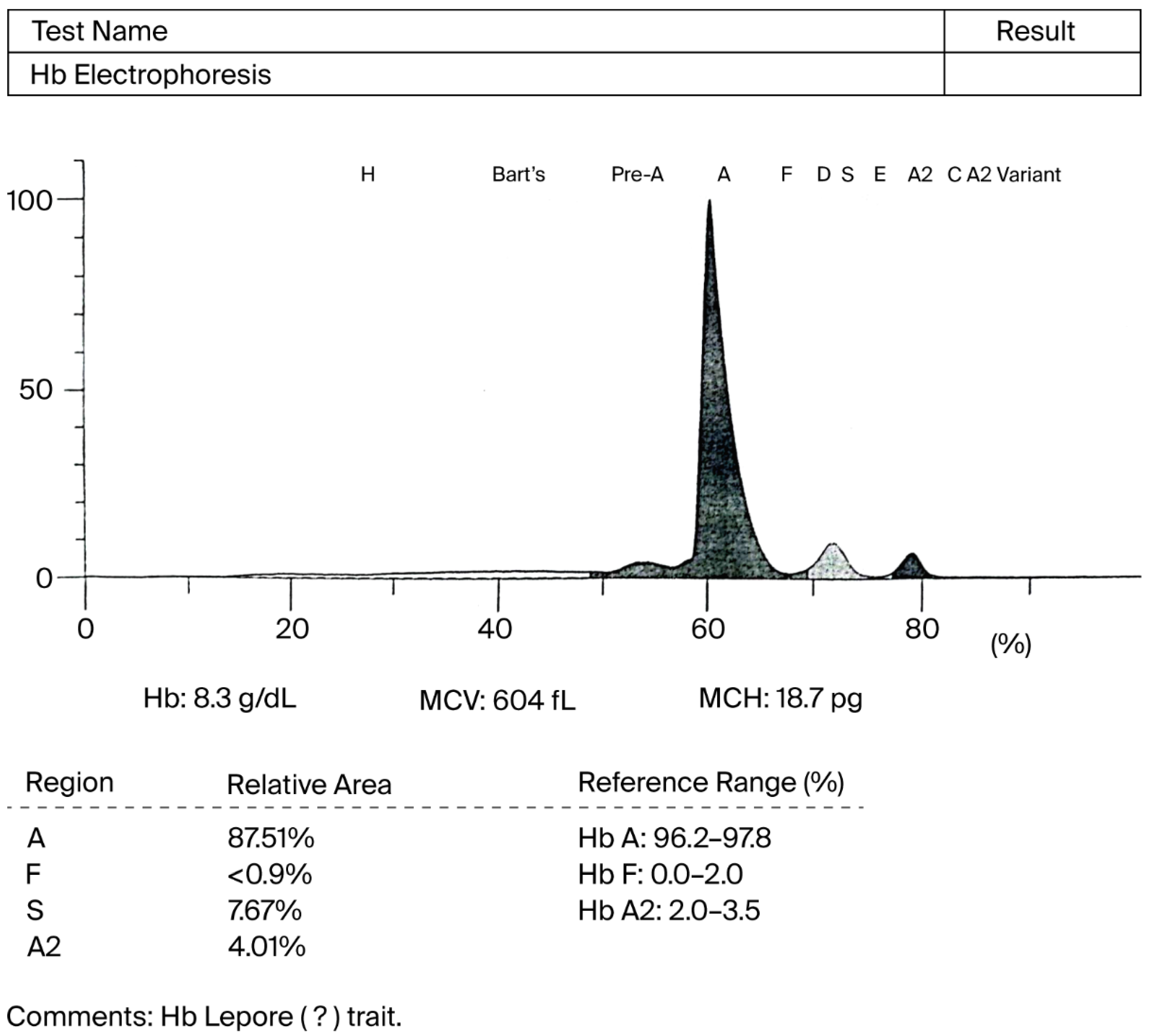
2. Case Presentation
Hospital Treatment and Evolution
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhusal, A.; Bhandari, S.; Seth, T.; Sah, R.P. Homozygous Lepore Syndrome: A Case Report. Ann. Med. Surg. 2022, 80, 104168. [Google Scholar] [CrossRef] [PubMed]
- Gerald, P.S.; Diamond, L.K. A new hereditary hemoglobinopathy (the Lepore trait) and its interaction with thalassemia trait. Blood 1958, 13, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Mohanty, S.; Acharya, S.; Das, S.; Behera, L. Clinicohematological Profile of Hemoglobin Lepore. Int. J. Pharm. Clin. Res. 2023, 15, 1152–1157. [Google Scholar]
- Bozkurt, G.; Baysal, E.; Gu, L.-H.; Huisman, T.H.J. Thalassemia intermedia in two patients with Hb Lepore-beta zero-thalassemia (Frameshift codon 8, -AA). Hemoglobin 1994, 18, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S. Haemoglobin Lepore and beta thalassaemia traits—Prenatal testing by both sequence analysis and MLPA for HBB gene: A case report. Indian J. Obstet. Gynecol. Res. 2024, 11, 112–115. [Google Scholar] [CrossRef]
- Huisman, T.H. Compound heterozygosity for Hb S and the hybrid HbS Lepore, P-Nilotic, and Kenya; comparison of hematological and hemoglobin composition data. Hemoglobin 1997, 21, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Viprakasit, V.; Pung-Amritt, P.; Suwanthon, L.; Clark, K.; Tanphaichtr, V.S. Complex interactions of deltabeta hybrid haemoglobin (Hb Lepore-Hollandia) Hb E (beta(26G-->A)) and alpha+ thalassaemia in a Thai family. Eur. J. Haematol. 2002, 68, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Pasangna, J.; George, E.; Nagaratnam, M. Haemoglobin Lepore in a Malay family: A case report. Malays. J. Pathol. 2005, 27, 33–37. [Google Scholar] [PubMed]
- Duma, H.; Efremov, G.; Sadikario, A.; Teodosijev, D.; Mladenovski, B.; Vlaski, R.; Andreeva, M. Study of nine families with haemoglobin-Lepore. Br. J. Haematol. 1968, 15, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Asees, M.Y.; Shrateh, O.N.; Jobran, A.W.M.; Assi, A.S. Rare occurrence of hemoglobin Lepore variant in a Palestinian patient: A case report and brief literature review. Ann. Med. Surg. 2023, 85, 5219–5222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zuo, L.; Li, D.; Li, J.; Tang, X.; Chen, G.; Zhou, J.; Lu, H.; Liao, C. Molecular epidemiology and hematologic characterization of δβ-thalassemia and hereditary persistence of fetal hemoglobin in 125,661 families of greater Guangzhou area, the metropolis of southern China. BMC Med. Genet. 2020, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Kausar, A.; Old, J.M.; Henderson, S.J.; Gallienne, A.E. Characterization of Hb Lepore variants in the UK population. Hemoglobin 2015, 39, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Waye, J.S.; Eng, B.; Patterson, M.; Chui, D.H.; Chang, L.S.; Cogionis, B.; Poon, A.O.; Olivieri, N.F. Hb E/Hb LeporeHollandia in a family from Bangladesh. Am. J. Hematol. 1994, 47, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Khan, W.A.; Das, S.A.; Banu, B. Homozygous Hemoglobin Lepore: A Rare Condition Seen in a Bangladeshi Family. Mymensingh Med. J. 2021, 30, 1172–1176. [Google Scholar] [PubMed]
| Test Name | Result | Unit | Reference Range (Adult Male) |
|---|---|---|---|
| Hemoglobin | 7.9 | gm/dL | 12.5–17.0 |
| ESR (Westergren) | 106 | mm/1st hr | 0–10 |
| Total WBC | 24,160 | /cumm | 4000–11,000 |
| Neutrophils | 86 | % | 40–70 |
| Lymphocytes | 08 | % | 20–40 |
| Monocytes | 04 | % | 02–08 |
| Eosinophils | 02 | % | 01–06 |
| Basophils | 00 | % | 00–01 |
| Total Eosinophils | 483 | /cumm | 50–450 |
| Total RBC | 4.33 | m/μL | 4.5–6.5 |
| HCT/PCV | 25.2 | % | 40–54 |
| MCV | 58.1 | fL | 76–94 |
| MCH | 18.1 | pg | 27–32 |
| MCHC | 31.2 | g/dL | 29–34 |
| RDW-CV | 14.7 | % | 11.0–16.0 |
| RDW-SD | 34.4 | fl | 35–56 |
| PDW | 20.4 | fL | 35–56 |
| Total Platelet Count | 369,000 | /cumm | 150,000–450,000 |
| MPV | 9.8 | fL | 7.0–11.0 |
| PCT | 0.36 | % | 0.1–0.2 |
| P-LCR | 39.0 | % | 3.0–43.0 |
| P-LCC | 144 | 103/uL | 44.0–140.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferdous, N.; Islam, M.N.; Mustakim, A.T.; Rasker, J.J. Rare Case of Hemoglobin Lepore Trait in a Bangladeshi Patient with Polyarthritis and Fever: Case Description and Brief Literature Review. Rheumato 2025, 5, 16. https://doi.org/10.3390/rheumato5040016
Ferdous N, Islam MN, Mustakim AT, Rasker JJ. Rare Case of Hemoglobin Lepore Trait in a Bangladeshi Patient with Polyarthritis and Fever: Case Description and Brief Literature Review. Rheumato. 2025; 5(4):16. https://doi.org/10.3390/rheumato5040016
Chicago/Turabian StyleFerdous, Nira, Md. Nazrul Islam, Abu Talha Mustakim, and Johannes J. Rasker. 2025. "Rare Case of Hemoglobin Lepore Trait in a Bangladeshi Patient with Polyarthritis and Fever: Case Description and Brief Literature Review" Rheumato 5, no. 4: 16. https://doi.org/10.3390/rheumato5040016
APA StyleFerdous, N., Islam, M. N., Mustakim, A. T., & Rasker, J. J. (2025). Rare Case of Hemoglobin Lepore Trait in a Bangladeshi Patient with Polyarthritis and Fever: Case Description and Brief Literature Review. Rheumato, 5(4), 16. https://doi.org/10.3390/rheumato5040016







