Abstract
The axial skeleton, with the exception of spondyloarthropathy, is the most neglected aspect of rheumatology training and, as a result, perhaps the most complex. The clinical “problem” of back/neck pain could be considered the “orphan child” of medicine, and our perspective as rheumatologists is often sought for such entities. Sources of back/neck pain are myriad, and not all phenomena affecting the back are symptomatic. Perhaps the one that has most concerned rheumatologists is the cervical instability associated with rheumatoid arthritis. The current review examines intrinsic and extrinsic alterations in axial skeletal components, providing a guide to discriminating the causes (e.g., Scheuermann’s disease versus osteoporotic compression and the various forms of axial joint ankylosis) and the implications of vertebral endplate alterations. The specificity and sensitivity (limitations) of radiologic findings are reviewed, with a reminder that vertebral body osteophytes do not represent osteoarthritis and are therefore unlikely to explain back or neck complaints and that it is our clinical examination which will likely suggest symptom origin.
1. Introduction
The spine, that which identifies us as a member of the phylum Vertebrata, has been the most complex and neglected aspect of rheumatology training. Indeed, training programs have been required to “outsource” such experience/exposure. The Residency Review Committee (RRC) found the problem so egregious that they refused to award full accreditation to a major university rheumatology training program, requiring that they correct the deficit. I was a member of the community-based organization that was tasked with providing that mitigation. Inadequate exposure of rheumatology fellows to patients with back complaints was actually not an unexpected finding for an academic training program. The faculty established the criteria for admission to the clinics they supervised; said criteria were often dependent upon faculty interests in care for specific diseases and ennui with respect to “mundane” phenomena such as back pain.
This was at a time when a formal curriculum for rheumatology fellowship training had not yet been established. Experiences/exposures were determined by the “mix” of patients that happened to be present during a given trainee’s participation in the clinic and by department members’ research interests rather than assurances that all trainees were consistently exposed to the myriad of phenomena/problems pertinent to rheumatology in clinical practice. This was further complicated by attitudes, such as the conflation of fibromyalgia with psychogenic rheumatism and malingering, which some suggested were not “worthy” of appointment at a rheumatology clinic (e.g., [1]). Individuals with primary back complaints seldom make it through the “screening process” to access “valuable” clinic appointment slots, a problem now exacerbated by the long waiting lists for such opportunities.
While seemingly “uninteresting” to some of the directors of rheumatology training programs (and therefore “underrepresented” in clinics utilized for training), the subject is actually quite an appropriate issue for rheumatologists—given our wide-ranging perspective of and exposure to diseases and phenomena. After all, confident assessment transcends biomechanics, requiring an encyclopedic knowledge/understanding of diseases and phenomena which “impact” the back directly and indirectly, and what specialty in medicine incorporates that overview more extensively than rheumatology?
Back pain is a common complaint, and even if it does not “grant” routine access to rheumatology clinics, its occurrence among individuals with other disorders treated by those clinics demands that those of us involved in their care be conversant with the recognition of pain derivation and its mitigation. A national survey examining a three-month interval in 2002 noted the occurrence of back pain in one-fourth of respondents [2]. Given the shortfall in time permitted for patient interactions, there has been a tendency to defer to radiologic studies. Unfortunately, few deviations from the normal state (abnormalities) have specificity (compression fractures, osteomyelitis, neoplasia, and scoliosis are among the exceptions [3]). The challenge is assessing whether they have diagnostic implications or are simply incidental findings [4,5].
Not all back pain is related to the vertebral column, nor is all vertebral column pathology symptomatic, at least as far as producing pain. While fibromyalgia and allodynia may also be important considerations in the evaluation of back complaints [6], the current review is limited to phenomena directly affecting the axial skeletal components, that is, the vertebral bodies and their connections/relationships, and posterior vertebral elements, including facet joints, as well as sacroiliac disease.
Conversely, there is a critical pathology that is usually not associated with pain but which has draconian implications if not recognized in a timely manner. Cervical vertebral subluxation, especially at the atlanto-axial level is a rare but potentially disastrous complication of inflammatory arthritis, especially of the rheumatoid variety [7]. The normal conscious state is usually associated with sufficient cervical muscular constraints (normal muscle tone) to minimize subluxation. Radiologic X-ray views in full (but not forced) neck flexion and extension are often required for the visualization of this pathology. Such studies are essential with any interventions that reduce consciousness (e.g., anesthesia) or require cervical manipulation (e.g., positioning for procedures). Cervical stabilization collars and/or surgical “correction” may be required.
2. Shape Variation
The normal shapes of the various vertebral sections are highly reproducible, with pattern deviation identifying pathologic states. The normal complement may be altered, as well as “positioning” among those sections. Lumbarization of the first sacral vertebra may be associated with its own full-sized disc, lumbar-type facets, and even the anomalous articulation of its transverse process with the adjacent vertebra [8,9]. The sacralization of L5 involves the elongation and broadening of its transverse processes, which may even fuse with the sacrum. There are no standardized methods for their identification [8,10], although Castellvi et al. [11] divided them into dysplastic, incomplete, complete, or mixed. Such anomalous vertebrae have been reported in 9.9–18.1% and even up to 36% of patients, with lumbarization and sacralization, reported in 3–7% and 1.7–14% of patients, respectively [12,13,14,15,16]. Prevalence assessments apparently differ in relation to “differences in individual diagnostic and classification criteria, observer error, imaging techniques, and confounding factors of the population being studied” [8], p. 231.
Referred to as lumbarization of sacral vertebrae and sacralization of lumbar vertebrae, it is unclear if they are actually symptomatic (or at least that they actually are associated with pain production at least in the absence of diarthrodial joint involvement [8,9,17,18,19,20,21,22]), and this will not be discussed further.
Similarly, it is unclear whether either the pseudo-articulation between L5 transverse processes with the sacrum and Baastrup’s phenomena of the spinous process “kissing” or the formation of pseudoarthroses (found in 41% of asymptomatic individuals) actually are sources of pain [3]. The disruption of vertebral pedicles (spondylolysis) is usually asymptomatic [16,17], even in the presence of a positive bone scan [23]. Baastrup’s disease/phenomena is present in 81% of individuals aged 90 years old and over, but it has also been noted in 41% of asymptomatic individuals [21]. Spinal stenosis is also commonly reported, but diagnostic criteria have not been standardized, meaning that its significance in the absence of clinical symptoms is also unclear [24,25,26,27].
Angulation, wherein lateral viewing reveals independent variation in anterior and posterior vertebral body heights, is typically noted in osteoporosis cases. The tendency for central vertebral body depression facilitates distinguishing the related compression fractures from the apophyseal process responsible for the wedge shape noted in Scheuermann’s disease, originally termed osteochondritis juvenilis dorsi [28,29]. The vertebral endplate phenomena, referred to as apophysitis or osteochondrosis, results in a peculiar failure of anterior vertebral body minor growth centers, in which an increase in vertebral height is compromised at the expense of increased growth in the anterior aspect (recognized upon viewing vertebrae from a lateral perspective [30,31,32,33,34]). Diagnosing Scheuermann’s disease is challenging, as illustrated by the fact that its incidence reportedly ranges from 0.4% to 8% [29,32,35,36,37,38]. Sorenson [39] suggested hyper-kyphosis greater than 40°, irregular upper and lower vertebral endplates, and loss of disk space height as the criteria for its recognition. Alternatively, diagnosis has been predicated on wedging of 5° or more in three consecutive vertebrae in the absence of evidence of traumatic, congenital, or infectious disease. Anterior extension was noted by Scoles et al. [29] in 94% of Scheuermann’s disease cases (absent in controls, even with only one or two wedged). While posterior height was only slightly greater in Scheuermann’s disease, apophysis did not contribute to its increase [18,40]. Masharawi and Rothschild [41] suggested that the relationship of mid-vertebral height to anterior and posterior vertebral heights provides a more reproducible standard for its recognition. The expansion of vertebrae may suggest Paget’s disease [42]. This may present as sclerosis and a thickening of the margins of the vertebral body (giving it a “picture frame” appearance) or general trabecular coarsening, or it may be diffuse, producing a dense ivory-like vertebra [33,43,44].
Scheuermann’s disease sometimes afflicts only one or two vertebrae or may result in significant kyphosis. All kyphosis in adults is structural [34], with Scheuermann’s disease responsible for 1–8% of hyper-kyphosis [34,38,45]. Contrary to muscular anomaly-related kyphosis, Scheuermann’s disease is unchanged by forward bending. Kyphosis and possibly scoliosis may also be the product of arrested development related to the failure of major vertebral growth centers. This may produce hemi- or even butterfly vertebrae. Posterior vertebral elements may not fuse to vertebral bodies or may be the result of fractures (e.g., spondylolisthesis). Kyphosis and scoliosis are otherwise beyond the scope of the present discussion, in view of the controversy as to when related symptomatology includes pain.
Osteoporosis is not the only cause of what appears to be compression. This can result from trauma and avascular necrosis. The latter is especially common in sickle cell disease [46]. The alterations in the latter tend to be centrally located, imbuing the vertebrae with an “H” shape as opposed to the fish vertebra-like shape that is more common in osteoporosis [47]. Other causes of compressed or even flatted vertebrae include pyogenic and tubercular osteomyelitis, brucellosis, neoplasia (e.g., metastases), and even fungal infection [48,49]. Brucellosis is associated with a unique anterior excavation just proximal to the apophysis, forming a groove [50,51]. Avascular necrosis is often recognized because of internal vertebral “clefts” of resorbed dead bone—recognizable upon X-ray. Similarly, neoplasia in the form of benign (e.g., hemangioma) and malignant (e.g., osteosarcoma) bone tumors, multiple myeloma, and leukemia may be recognized upon X-ray, along with histiocytosis (variably characterized as a neoplasia and as inflammatory or immunologic in derivation [13,52,53,54]).
3. Vertebral Endplates
The significance of vertebral endplate abnormalities has been quite controversial. They have been noted in 56% of male and 30% of female college teachers [37]. Interobserver reporting is problematic, as there is less than 50% agreement on the nature of endplate malterations (e.g., defect, erosion, fracture, Schmorl’s nodes) or even their existence [55]. Schmorl’s nodes are not strongly associated with back pain [56,57,58]. Zehra et al. [57] found endplate defects in 67.5% of individuals aged 51.6 +/− 8.5 years of age, noting and association between their depth and individual age. Samartzis et al. [59] divided them into patterns based on size, shape, and edge characteristics, an approach that is currently under investigation and without evidence of the relationship between the presence or absence of pain. Endplate irregularity and Schmorl’s nodes have been noted in Scheuermann’s disease but have not been correlated with pain complaints [34,60,61].
Lateral and anterior vertebral body defects (Figure 1) may be caused by pyogenic, tubercular, and fungal infections, as well as by metastatic disease or by pressure from gouty tophi or from aortic pulsations related to an aneurysm [62,63,64,65].
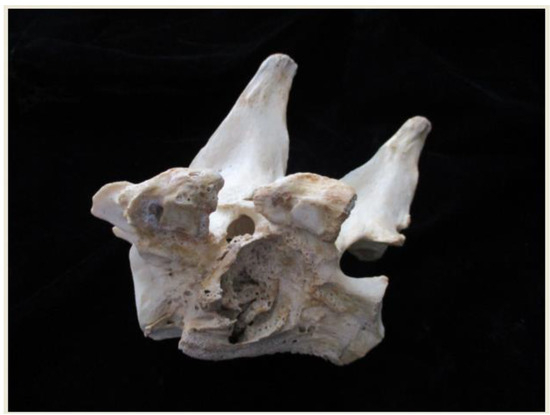
Figure 1.
Lateral view of two California sea lion Zalophus californicus vertebrae (fused via infectious spondylitis). Note the vertebral destruction and filigree surface of the surrounding periosteal reaction.
4. Disc Disease
Disc disease is perhaps one of the most controversial aspects in the field, as it is common, the attribution of symptoms is unclear, and many individuals are asymptomatic [66,67,68]. Adams and Dolan divided disc degeneration into two phenotypes. One is characterized by vertebral endplate lesions, which is a significant heritable component [69]. Paradoxically, it is characterized by circumferential anulus fibrosus tears, in contrast to the second phenotype, which has been identified as anulus-driven with radial tears. The latter seems to be associated with repetitive bending and lifting. It is unclear if these are actually distinct or even distinguishable.
5. Bridging/Fusion/Ankylosis
Assaults on the organismal stability provided by the vertebral column sometimes stimulate a restorative response. This may take the form of extrinsic ligamentous ossification or new bone formation in intrinsic connecting structures (i.e., the anulus fibrosus, longitudinal ligaments, or directly through the intervertebral disc). The former identifies the group of disorders designated as spondyloarthropathy, which includes ankylosing spondylitis, the arthritis associated with the skin disease psoriasis and inflammatory bowel disease, reactive arthritis (formerly referred to as Reiter’s syndrome, a name that has subsequently entered obsolescence after his recognition as a war criminal), and an undifferentiated form of arthritis [53,65]. Uniform smooth ossification of the anulus fibrosus derived directly from endplate edges is characteristic of the arthritis associated with inflammatory bowel disease and ankylosing spondylitis (Figure 2), while bulky bridging, often inserting beyond the endplates, is more characteristic of the other forms [53,65]. Spondyloarthropathy must be distinguished from the uniform, generally left-sided paravertebral ligament ossification (referred to as diffuse idiopathic skeletal hyperostosis, i.e., DISH) [70]. Usually idiopathic, DISH is also a known complication ingesting excessive doses of vitamin A/retinoic acid, but it can also present as reactive bone associated with spondyloarthropathy [71,72,73]. Ligamentous ossification may also be found with fractures (traumatic, osteoporosis, or infection-related), although the latter more commonly directly involves the vertebral body [33,74]. The infection-related destruction of vertebral endplates and associated disc destruction often stimulates reactive new bone formation, which bridges adjacent vertebrae. The resultant ankylosis from any of the above entities may be so extensive that vertebrae appear coalesced. Such must be distinguished from congenital/homeobox fusions. The length of the latter is shortened compared to that of vertebrae ankylosed by spondyloarthropathy and DISH because intervertebral discs (the “spacers”) do not develop [53]. However, the expansion of one component of fused vertebra is highly suggestive of an infectious process [65,75,76].
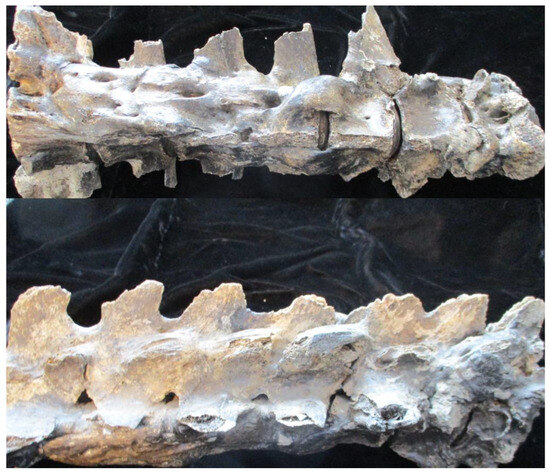
Figure 2.
Anterior and lateral view of Pleistocene horse Equus scotti vertebrae. Note the uniform smooth ossification of the anulus fibrosus.
6. Spondylosis Deformans
Spondylosis deformans is the name now applied to vertebral body osteophytes [77]. They are not a sign of osteoarthritis, and in fact, they appear asymptomatic, unless one presses on blood vessels or nerve tracts. This contrasts with facet (zygapophyseal) joint osteophytes, which do identify osteoarthritis [78]. The phenomena referred to as spondylosis deformans appears to be the result of traction osteophytes projecting perpendicular to the long axis of the vertebral body. While anulus fibrosus disruption and disk prolapse appear to be important mechanisms for the development of the latter, the phenomena are generally asymptomatic unless they impinge on neural structures [79,80].
7. Facet Joints
The posterior components of vertebrae deserve scrutiny. Cohen and Raja [81] suggest that facet disease is responsible for 15% of lower back pain complaints. Zygapophyseal (facet) joint osteophytes may be symptomatic, as their erosion may be related to spondyloarthropathy and calcium pyrophosphate deposition disease (CPPD) [82]. While Murtagh [83] suggested that facet osteophytes (osteoarthritis) are responsible for more than 40 percent of lower back pain, facet osteoarthritis is present in 8 to 14% of asymptomatic individuals [84]. Techniques for documenting facet joint-derived pain have been controversial [85,86,87]. It is unclear whether pain reduction via the injection of anesthetic agents can be confidently attributed to facet disease, given the associated infiltration of surrounding tissues, even if performed under fluoroscopic conditions [81,85,88]. Thus, the possible alternative diagnosis of muscle strains and even fibromyalgia cannot be dismissed. The presence of tender areas or trigger point-reproducible pain suggests the latter.
8. Sacroiliac Joint
The most caudal aspect of the axial skeleton is the sacrum and its articulation with the ilium. That joint has featured prominently in directing attention to the possibility of spondyloarthropathy [89,90]. Spondyloarthropathy-related sacroiliac erosions must be distinguished from those produced by infectious processes (e.g., tuberculosis) and from developmental phenomena. Ankylosis/fusion may be attributed to spondyloarthropathy if the bridging occurs in the lower two-thirds of the joint, the region that defines the diarthrodial/synovial-line portion [53]. However, radiologic techniques—for example, both standard and computed tomography (CT)—actually lack specificity, as demonstrated by a false positive rate as high as 86%, even with 15 degree angulated views, which apparently have 100% sensitivity [91,92,93]. Magnetic resonance imaging (MRI) findings are suggestive of sacroiliac involvement, which, unfortunately, is also found quite commonly in normal healthy individuals [5,94,95]. A clinical finding may be helpful: individuals with spondyloarthropathy often have reduced forward flexion in the back. The Schöber sign derives from measuring the distance between a line drawn from the iliac crest and a point 10 cm cephalad when the individual is standing upright. A second measurement is made between those two points when the individual bends at the waist. Expansion to less than 13 cm (3 cm greater than the original distance) indicates the compromised flexion abilities common among individuals with spondyloarthropathy [96,97,98].
9. Therapeutic Approach
Phenomena affecting the axial skeleton may be asymptomatic, severely debilitating, unworthy of attention, only worthy of an observation, or may be worthy of critical intervention. How is that assessment made? Back pain with activity that can be improved by rest may be related to a specific activity, one which can be avoided or modified, thus resolving the problem. Persistence in spite of bed rest (note that two days of absolute bed rest often resolves back pain) suggests that an extensive evaluation is warranted. Please note that the bed rest has to be absolute; the individual must only arise for toilet use! The exacerbation of back pain by spinal hyperextension is highly suggestive of facet joint involvement, for which injection therapy may be considered.
If back pain improves with activity, it is likely inflammatory in derivation [99,100,101]. Respiration-associated back extension exercises often relieve pain. However, the total avoidance of flexion activities is essential, as any flexion activity overpowers the benefit of extension exercises [40]. The presence of fever and/or elevated sedimentation rate (ESR) or C-reactive protein (CRP) suggests an inflammatory process, but that does not distinguish between spondyloarthropathy and infection. The absence of fever or ESR/CRP elevation does not preclude spondyloarthropathy from being the source of the back pain.
10. Denouement
Some of the inherent limitations in the technology used in the diagnosis of back pain complaints have been reviewed, emphasizing the preeminent position of clinical experience, represented by the broad spectrum that characterizes the results of rheumatology training and practice in its diagnosis. One might question the prevalence of the various phenomena/diseases that produce back pain. Many phenomena/diseases that produce back pain are more common than spondyloarthropathy, and failing to diagnose these other phenomena/diseases would be embarrassing for a rheumatologist. Aneurysm-, infection-, gout-, and neoplasia-related back pain may be rare [102,103] but will remain undiagnosed for too long if their possibilities of occurrence are not considered. One will not make diagnoses that are not considered, even subconsciously. A mentor once shared with me his experience in a mall. He heard hoofbeats, expecting to see a horse. The source turned out to be a zebra that had gotten lose from a small menagerie. If he had been unaware of the difference, the source might not have been identified. This is the case with back pain. Without a broad background, diagnoses will likely be misapplied and treatable entities will be overlooked—to the disadvantage of the afflicted.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
I appreciate the honor of being Editor-in-Chief of Rheumato.
Conflicts of Interest
The author declares no conflict of interest.
References
- Wolfe, F. Fibromyalgianess. Arthritis Care Res. 2009, 61, 715–716. [Google Scholar] [CrossRef] [PubMed]
- Hrudey, W.P. Overdiagnosis and overtreatment of low back pain: Long-term effects. J. Occup. Rehab. 1991, 1, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.; Weinstein, J.N. Low back pain. N. Engl. J. Med. 2001, 344, 363–370. [Google Scholar] [CrossRef]
- Chou, R.; Qaseem, A.; Snow, V.; Casey, D.; Cross, J.T., Jr.; Shekelle, P.; Owens, D.K.; Clinical Efficacy Assessment Subcommittee of the American College of Physicians and the American College of Physicians/American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: A joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann. Intern. Med. 2007, 147, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Balza, R.; Palmer, W.E. Symptom-imaging correlation in lumbar spine pain. Skelet. Radiol. 2023, 52, 1901–1909. [Google Scholar] [CrossRef]
- Rothschild, B.M. Fibromyalgia: An explanation for the aches and pains of the 90’s. Contemp. Med. 1991, 17, 9–14. [Google Scholar]
- Garfin, S.R. Rheumatoid Arthritis of the Cervical Spine. 2022. Available online: https://emedicine.medscape.com/article/1266195-overview (accessed on 1 July 2023).
- French, H.D.; Somasundaram, A.J.; Schaefer, N.R.; Laherty, R.W. Lumbosacral Transitional Vertebrae and Its Prevalence in the Australian Population. Glob. Spine J. 2014, 4, 229–232. [Google Scholar] [CrossRef]
- Konin, G.; Walz, D. Lumbosacral Transitional Vertebrae: Classification, Imaging Findings, and Clinical Relevance. Am. J. Neuroradiol. 2010, 31, 1778–1786. [Google Scholar] [CrossRef]
- Jenkins, A.L., III; O’Donnell, J.; Chung, R.J.; Jenkins, S.; Hawks, C.; Lazarus, D.; McCaffrey, T.; Terai, H.; Harvie, C. Redefining the Classification for Bertolotti Syndrome: Anatomical Findings in Lumbosacral Transitional Vertebrae Guide Treatment Selection. World Neurosurg. 2023, 175, e303–e313. [Google Scholar] [CrossRef]
- Castellvi, A.E.; Goldstein, L.A.; Chan, D.P. Lumbosacral Transitional Vertebrae and Their Relationship With Lumbar Extradural Defects. Spine 1984, 9, 493–495. [Google Scholar] [CrossRef]
- Nardo, L.; Alizai, H.; Virayavanich, W.; Liu, F.; Hernandez, A.; Lynch, J.A.; Nevitt, M.C.; McCulloch, C.E.; Lane, N.E.; Link, T.M. Lumbosacral Transitional Vertebrae: Association with Low Back Pain. Radiology 2012, 265, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Tini, P.G.; Wieser, C.; Zinn, W.M. The transitional vertebra of the lumbosacral spine: Its radiological classification, incidence, prevalence, and clinical significance. Rheumatol. Rehabil. 1977, 16, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Bron, J.L.; Van Royen, B.J.; Wuisman, P.I.J.M. The clinical significance of lumbosacral transitional anomalies. Acta Orthop. Belg. 2007, 73, 687–695. [Google Scholar] [PubMed]
- Steinberg, E.; Luger, E.; Arbel, R.; Menachem, A.; Dekel, S. A Comparative Roentgenographic Analysis of the Lumbar Spine in Male Army Recruits with and without Lower Back Pain. Clin. Radiol. 2003, 58, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Hald, H.J.; Danz, B.; Schwab, R.; Burmeister, K.; Bähren, W. Radiographically demonstrable spinal changes in asymptomatic young men. Rofo 1995, 163, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Elster, A.D. Bertolotti’s syndrome revisited. Transitional vertebrae of the lumbar spine. Spine 1989, 14, 1373–1377. [Google Scholar] [CrossRef]
- Song, J.; Betzler, B.K.; Kaliya-Perumal, A.-K.; Oh, J.Y.-L. Prevalence of Lumbosacral Transition Vertebrae in Symptomatic Adults and the Levels of Degeneration in the Suprajacent Disc. Surgeries 2023, 4, 120–126. [Google Scholar] [CrossRef]
- Taskaynatan, M.A.; Izci, Y.; Ozgul, A.; Hazneci, B.; Dursun, H.; Kalyon, T.A. Clinical Significance of Congenital Lumbosacral Malformations in Young Male Population With Prolonged Low Back Pain. Spine 2005, 30, E210–E213. [Google Scholar] [CrossRef]
- Stinchfield, F.E.; Sinton, W.A. Clinical significance of the transitional lumbosacral vertebra: Relationship to back pain, disk diseases, and sciatica. JAMA 1995, 157, 1107–1109. [Google Scholar] [CrossRef]
- Kwong, Y.; Rao, N.; Latief, K. MDCT findings in Baastrup disease: Disease or normal feature of the aging spine? Am. J. Roentgenol. 2011, 196, 1156–1159. [Google Scholar] [CrossRef]
- Leone, A.; Cianfoni, A.; Cerase, A.; Magarelli, N.; Bonomo, L. Lumbar spondylosis: A review. Skeletal Radiol. 2011, 40, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, N.; Wilkinson, R.H.; Emans, J.; Treves, S.; Micheli, L.; Papanicolaou, R.W.N.; Pennell, R.; Maurer, A.; Bonakdarpour, A.; Gehweiler, J.; et al. Bone scintigraphy and radiography in young athletes with low back pain. Am. J. Roentgenol. 1985, 145, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Andreisek, G.; Imhof, M.; Wertli, M.; Winklhofer, S.; Pfirrmann, C.W.A.; Hodler, J.; Steurer, J.; for the Lumbar Spinal Stenosis Outcome Study Working Group Zurich. A Systematic Review of Semiquantitative and Qualitative Radiologic Criteria for the Diagnosis of Lumbar Spinal Stenosis. Am. J. Roentgenol. 2013, 201, W735–W746. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, Y.; Yoshimura, N.; Muraki, S.; Yamada, H.; Nagata, K.; Hashizume, H.; Takiguchi, N.; Minamide, A.; Oka, H.; Kawaguchi, H.; et al. Associations between radiographic lumbar spinal stenosis and clinical symptoms in the general population: The Wakayama Spine Study. Osteoarthr. Cartil. 2013, 21, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Giannadakis, C.; Rao, V.; Jakola, A.S.; Nerland, U.; Nygaard, Ø.P.; Solberg, T.K.; Gulati, S.; Solheim, O. Is there an association between radiological severity of lumbar spinal stenosis and disability, pain, or surgical outcome? A multicenter observational study. Spine 2016, 41, E78–E83. [Google Scholar] [CrossRef]
- Zeifang, F.; Schiltenwolf, M.; Abel, R.; Moradi, B. Gait analysis does not correlate with clinical and MR imaging parameters in patients with symptomatic lumbar spinal stenosis. BMC Musculoskelet. Disord. 2008, 9, 89. [Google Scholar] [CrossRef]
- Scheuermann, H.W. The classic: Kyphosis dorsalis juvenilis. Ugeskr Laeger 1920, 82, 385–393. [Google Scholar] [CrossRef]
- Scoles, P.V.; Latimer, B.M.; Digiovanni, B.F.; Vargo, E.; Bauza, S.; Jellema, L.M. Vertebral alterations in Scheuermann’s kyphosis. Spine 1991, 16, 509–515. [Google Scholar] [CrossRef]
- Digiovanni, B.F.; Scoles, P.V.; Latimer, B.M. Anterior extension of the thoracic vertebral bodies in Scheuermann’s kyphosis. An anatomic study. Spine 1989, 14, 712–716. [Google Scholar] [CrossRef]
- Fried, K. Die Dysspondylien: Ein Beitrag zum M. Scheuermann. Radiologe 1982, 22, 412–418. [Google Scholar]
- Mallet, J.; REY, J.C.; Raimbeau, G.; Senly, G. Maladiede Scheuermann. Dysrophie rachidienne de croissance. Chir. Orthopédique 1984, 34, 29–39. [Google Scholar]
- Ristolainen, L.; Kettunen, J.A.; Heliövaara, M.; Kujala, U.M.; Heinonen, A.; Schlenzka, D. Untreated Scheuermann’s disease: A 37-year follow-up study. Eur. Spine J. 2011, 21, 819–824. [Google Scholar] [CrossRef]
- Zaina, F.; Atanasio, S.; Ferraro, C.; Fusco, C.; Negrini, A.; Romano, M.; Negrini, S. Review of rehabilitation and orthopedic conservative approach to sagittal plane diseases during growth: Hyperkyphosis, junctional kyphosis, and Scheuermann disease. Eur. J. Phys. Rehab. Med. 2009, 45, 595–603. [Google Scholar]
- Armbrecht, G.; Felsenberg, D.; Ganswindt, M.; Lunt, M.; Kaptoge, S.K.; Abendroth, K.; Aroso, A.; Banzer, D.; Bhalla, A.K.; Dequeker, J.; et al. Vertebral Scheuermann’s disease in Europe: Prevalence, geographic variation and radiological correlates in men and women aged 50 and over. Osteoporosis Int. 2015, 26, 2509–2519. [Google Scholar] [CrossRef] [PubMed]
- Fisk, J.W.; Baigent, M.L.; Hill, P.D. Scheuermann’s disease. Clinical and radiological survey of 17 and 18 year olds. Am. J. Phys. Med. 1984, 63, 18–30. [Google Scholar] [PubMed]
- Murray, P.M.; Weinstein, S.L.; Spratt, K.F. The natural history and long-term follow-up of Scheuermann kyphosis. J. Bone Jt. Surg. 1993, 75, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Wenger, D.R.; Frick, S.I. Scheuermann kyphosis. Spine 1989, 24, 2630–2639. [Google Scholar] [CrossRef]
- Sorenson, K.H. Scheuermann’s Juvenile Kyphosis: Clinical Appearances, Radiography, Aetiology, and Prognosis. Munksgaard: Copenhagen, Denmark, 1964. [Google Scholar]
- Bick, E.M.; Copel, J.W. Ring apophysis of human vertebra: Contribution to human ontogeny. J. Bone Jt. Surg. 1951, 33, 783–787. [Google Scholar] [CrossRef]
- Masharawi, Y.; Rothschild, B.; Peled, N.; Hershkovitz, I. A Simple Radiological Method for Recognizing Osteoporotic Thoracic Vertebral Compression Fractures and Distinguishing Them From Scheuermann Disease. Spine 2009, 34, 1995–1999. [Google Scholar] [CrossRef]
- Dell’Atti, C.; Cassar-Pullicino, V.N.; Lalam, R.K.; Tins, B.J.; Tyrrell, P.N.M. The spine in Paget’s disease. Skelet. Radiol. 2007, 36, 609–626. [Google Scholar] [CrossRef]
- Mirra, J.M.; Brien, E.W.; Tehranzadeh, J. Paget’s disease of bone: Review with emphasis on radiologic features, part II. Skelet. Radiol. 1995, 24, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Murphey, M.D.; Motamedi, K.; Mulligan, M.E.; Resnik, C.S.; Gannon, F.H. Radiologic spectrum of Paget disease of bone and its complications with pathologic correlation. RadioGraphics 2002, 22, 1191–1216. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.G. Scheurmann disease. J. Bone Jt. Surg. 1990, 72, 940–945. [Google Scholar] [CrossRef]
- Kartikueyan, R.; Chowdhury, S.R.; Krishnan, P.; Das, S. Characteristic vertebral imaging in sickle cell disease. J. Neurosci. Rural Pract. 2017, 8, 270–271. [Google Scholar] [CrossRef] [PubMed]
- Ntagiopoulos, P.G.; Moutzouris, D.A.; Manetas, S. The “fish-vertebra” sign. Emerg. Med. J. 2007, 24, 674–675. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Mosele, N.; Gnassi, A.; Baracco, R.; Rodà, M.G.; Cerchiaro, M.; Ruggieri, P. Vertebra Plana: A Narrative Clinical and Imaging Overview among Possible Differential Diagnoses. Diagnostics 2023, 13, 1438. [Google Scholar] [CrossRef] [PubMed]
- Baky, F.; Milbrandt, T.A.; Arndt, C.; Houdek, M.T.; Larson, A.N. Vertebra Plana in Children May Result from Etiologies Other Than Eosinophilic Granuloma. Clin. Orthop. Relat. Res. 2020, 478, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, B.; Haeusler, M. Possible vertebral brucellosis infection in a Neanderthal. Sci. Rep. 2021, 11, 19846. [Google Scholar] [CrossRef]
- Zhao, Y.-T.; Yang, J.-S.; Liu, T.-J.; He, L.-M.; Hao, D.-J. Sclerosing vertebra in the spine: Typical sign of spinal brucellosis. Spine J. 2015, 15, 550–551. [Google Scholar] [CrossRef]
- Ciffdemir, M.; Maya, M.; Selcuk, E.; Yalniz, E. Tumors of the spine. World J. Orthop. 2016, 7, 109–116. [Google Scholar] [CrossRef]
- Resnick, D. Diagnosis of Bone and Joint Disorders; Saunders: Philadelphia, PA, USA, 2002. [Google Scholar]
- Rothschild, B.M.; Tanke, D.; Rühli, F.; Pokhojaev, A.; May, H. Suggested Case of Langerhans Cell Histiocytosis in a Cretaceous dinosaur. Sci. Rep. 2020, 10, 2203. [Google Scholar] [CrossRef] [PubMed]
- Zehra, U.; Bow, C.; Lotz, J.C.; Williams, F.M.K.; Rajasekaran, S.; Karppinen, J.; Luk, K.D.K.; Battiê, M.C.; Samartzis, D. Structural vertebral endplate nomenclature and etiology: A study by the ISSLS Spinal Phenotype Focus Group. Eur. Spine J. 2018, 27, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Zehra, U.; Cheung, J.P.Y.; Bow, C.; Lu, W.; Samartzis, D. Multidimensional vertebral endplate defects are associated with disc degeneration, modic changes, facet joint abnormalities, and pain. J. Orthop. Res. 2018, 37, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Miyazaki, T.; Ohnari, H.; Takino, T.; Tomita, K. Schmorl’s nodes and low back pain: Analysis of magnetic resonance imaging findings in symptomatic and asymptomatic individuals. Eur. Spine J. 1995, 4, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.M.K.; Manek, N.J.; Sambrook, P.N.; Spector, T.D.; Macgregor, A.J. Schmorl’s nodes: Common, highly heritable, and related to lumbar disc disease. Arthritis Rheum. 2007, 57, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, D.; Mok, F.; Karppinen, J.; Fong, D.; Luk, K.; Cheung, K. Classification of Schmorl’s nodes of the lumbar spine and association with disc degeneration: A large-scale population-based MRI study. Osteoarthr. Cartil. 2016, 24, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.L.; Hensinger, R.N.; Hunter, L.Y. Back pain and vertebral change simulating Scheuermann’s disease. J. Pediatric. Orthop. 1985, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Swischuk, L.E.; John, S.D.; Allbery, S. Disk degenerative disease in childhood: Scheuermann’s disease Schmorl’s nodes, and the limbus vertebra. MRI findings in 12 patients. Pediatr. Radiol. 1998, 28, 334–338. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, H.S.; Kim, S.W. Severe Vertebral Erosion by Huge Symptomatic Pulsating Aortic Aneurysm. J. Korean Neurosurg. Soc. 2008, 43, 117–118. [Google Scholar] [CrossRef][Green Version]
- Pinheiro, J.F.; Blanco, J.F.; Hernández, D.P.; García, F.F. Vertebral destruction due to abdominal aortic aneurysm. Int. J. Surg. Case Rep. 2015, 6, 296–299. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Cohn, L.; Aviza, A.; Yoon, B.H. Aortic aneurysm producing back pain, bone destruction, and paraplegia. Clin. Orthop. Rel. Res. 1982, 164, 123–125. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Surmik, D.; Bertozzo, F. Modern Paleopathology, the Study of Diagnostic Approach to Ancient Diseases, their Pathology and Epidemiology: Let there Be Light, the Light of Science and Critical Thinking; Springer: New York, NY, USA, 2023. [Google Scholar]
- Smith, B.M.T.; Hurwitz, E.L.; Solsberg, D.; Rubinstein, D.; Corenman, D.S.; Dwyer, A.P.; Kleiner, J. Interobserver Reliability of Detecting Lumbar Intervertebral Disc High-Intensity Zone on Magnetic Resonance Imaging and Association of High-Intensity Zone With Pain and Anular Disruption. Spine 1998, 23, 2074–2080. [Google Scholar] [CrossRef]
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Twomey, L.; Taylor, J. Age changes in lumbar intervertebral discs. Acta Orthop. Scand. 1985, 56, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Dolan, P. Intervertebral disc degeneration: Evidence for two distinct phenotypes. J. Anat. 2012, 221, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, B.M. Diffuse Idiopathic Skeletal Hyperostosis (DISH). Medscape Drugs & Diseases. 2020. Available online: https://authoring.medscape.com/content/1258514 (accessed on 5 July 2023).
- Seawright, A.; English, P.; Gartner, R. Hypervitaminosis A and deforming cervical spondylosis of the cat. J. Comp. Pathol. 1967, 77, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, B.M. Universal nature of spondyloarthropathy as a reactive disease, reflecting differential sensitivities. Curr. Rheumatol. Rev. 2013, 9, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, B.M.; Woods, R.J. Spondyloarthropathy: Erosive arthritis in representative defleshed bones. Am. J. Phys. Anthropol. 1991, 85, 125–134. [Google Scholar] [CrossRef]
- Forrester, D.M. Infectious spondylitis. Semin. Ultrasound CT MRI 2004, 25, 461–473. [Google Scholar] [CrossRef]
- Amini, M.H.; Salzman, G.A. Infectious spondylodiscitis: Diagnosis and treatment. Mo. Med. 2013, 110, 80–84. [Google Scholar]
- Perronne, C.; Saba, J.; Behloul, Z.; Salmon-Ceron, D.; Leport, C.; Vilde, J.L.; Kahn, M.F. Pyogenic and Tuberculous Spondylodiskitis (Vertebral Osteomyelitis) in 80 Adult Patients. Clin. Infect. Dis. 1994, 19, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, B.M. Lumbar Spondylosis. 2020. Available online: https://authoring.medscape.com/content/249036/view/print-view (accessed on 5 July 2023).
- Jurmain, R.D.; Kilgore, L. Skeletal evidence of osteoarthritis: A palaeopathological perspective. Ann. Rheum. Dis. 1995, 54, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Luetmer, P.; Comstock, B.; Bresnahan, B.; Chen, L.; Deyo, R.; Halabi, S.; Turner, J.; Avins, A.; James, K.; et al. Systematic Literature Review of Imaging Features of Spinal Degeneration in Asymptomatic Populations. AJNR Am. J. Neuroradiol. 2015, 36, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Chen, Z.; Qi, Q.; Shi, Z. The relationship of symptomatic thoracolumbar disc herniation and Scheuermann’s disease. Eur. Spine J. 2014, 23, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Raja, S.N. Pathogenesis, Diagnosis, and Treatment of Lumbar Zygapophysial (Facet) Joint Pain. J. Am. Soc. Anesthesiol. 2007, 106, 591–614. [Google Scholar] [CrossRef]
- Moshrif, A.; Laredo, J.D.; Bassiouni, H.; Abdelkareem, M.; Richette, P.; Rigon, M.R.; Bardin, T. Spinal involvement with calcium pyrophosphate deposition disease in an academic rheumatology center: A series of 37 patients. Semin. Arthritis Rheum. 2018, 48, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, F.R. Computed tomography and fluoroscopy guided anesthesia and steroid injection in facet syndrome. Spine 1988, 13, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Wiesel, S.W.; Tsourmas, N.; Feffer, H.L.; Citrin, C.M.; Patronas, N. A study of computer-assisted tomography. I. The incidence of positive CAT scans in an asymptomatic group of patients. Spine 1984, 9, 549–551. [Google Scholar] [CrossRef]
- Perolat, R.; Kastler, A.; Nicot, B.; Pellat, J.-M.; Tahon, F.; Attye, A.; Heck, O.; Boubagra, K.; Grand, S.; Krainik, A. Facet joint syndrome: From diagnosis to interventional management. Insights Imaging 2018, 9, 773–789. [Google Scholar] [CrossRef]
- Said, N.; Amrhein, T.J.; Joshi, A.B.; Nacey, N.; Kranz, P.G. Facets of facet joint interventions. Skel. Radiol. 2022, 52, 1873–1886. [Google Scholar] [CrossRef]
- Won, H.-S.; Yang, M.; Kim, Y.-D. Facet joint injections for management of low back pain: A clinically focused review. Anesth. Pain Med. 2020, 15, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Hurley, R.W.; Christo, P.J.; Winkley, J.; Mohiuddin, M.M.; Stojanovic, M.P. Clinical Predictors of Success and Failure for Lumbar Facet Radiofrequency Denervation. Clin. J. Pain 2007, 23, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Feldtkeller, E.; Rothschild, B. Spondyloarthritis in antiquity and history. In Oxford Textbook of Axial Spondyloarthritis; Oxford University: Oxford, UK, 2016; pp. 3–12. [Google Scholar]
- Rothschild, B.M.; Breit, S.M. Recognition and Breed Specificity of Canine Spondyloarthropathy. J. Spine 2016, 43, 1251–1252. [Google Scholar] [CrossRef]
- Beal, M.C. The sacroiliac problem: Review of anatomy, mechanics, and diagnosis. J. Am. Osteopath. Assoc. 1982, 81, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, B.M.; Poteat, G.B.; Williams, E.; Crawford, W.L. Inflammatory sacroiliac joint pathology: Evaluation of radiologic assessment techniques. Clin. Exp. Rheumatol. 1994, 12, 267–274. [Google Scholar] [PubMed]
- Yazici, H.; Turunc, M.; Ozdogan, H.; Yurdakul, S.; Akinci, A.; Barnes, C.G. Observer variation in grading sacroiliac radiographs might be a cause of ‘sacroiliitis’ reported in certain disease states. Ann. Rheum. Dis. 1987, 46, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, C.; Griffith, J.F.; Lee, R.K.L.; Wong, P.C.H.; Tam, L.S. Imaging of sacroiliitis: Current status, limitations and pitfalls. Quant. Imaging Med. Surg. 2019, 9, 318–335. [Google Scholar] [CrossRef]
- Diekhoff, T.; Hermann, K.G.; Greese, J.; Schwenke, C.; Poddubnyy, D.; Hamm, B.; Sieper, J. Comparison of MRI with radiography for detecting structural lesions of the sacroiliac joint using CT as standard of reference: Results from the SIMACT study. Ann. Rheum. Dis. 2017, 76, 1502–1508. [Google Scholar] [CrossRef]
- Rezvani, A.; Ergin, O.; Karacan, I.; Oncu, M. Validity and Reliability of the Metric Measurements in the Assessment of Lumbar Spine Motion in Patients With Ankylosing Spondylitis. Spine 2012, 37, E1189–E1196. [Google Scholar] [CrossRef]
- Cidem, M.; Karacan, I.; Uludag, M. Normal range of spinal mobility for healthy young adult Turkish men. Rheumatol. Int. 2012, 32, 2265–2269. [Google Scholar] [CrossRef]
- Rothschild, B. Overcoming the challenge of back pain complaints. Rheumatol. Res. 2017, 2, 79–83. [Google Scholar] [CrossRef]
- Hughes, R.J.; Saifuddin, A. Numbering of lumbosacral transitional vertebrae on MRI: Role of the iliolumbar ligaments. Am. J. Roentgenol. 2006, 187, W59–W65. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, B. Mechanical/Enthesial Origin for Ankylosing Spondylitis Axial Involvement? Clues from a Therapeutic Viewpoint. J. Arthritis 2014, 3, 2. [Google Scholar] [CrossRef]
- Rothschild, B. Back to Basics: Clinical versus Radiologic Recognition of Spondyloarthropathy. J. Rheumatol. 2017, 44, 957. [Google Scholar] [CrossRef] [PubMed]
- Elgafy, H.; Liu, X.; Herron, J. Spinal gout: A review with case illustration. World J. Orthop. 2016, 7, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Tan, W.L.B.; Wei, W.; Vellayappan, B.A. An overview of the tumors affecting the spine—Inside to out. Neuro-Oncol. Pract. 2020, 7 (Suppl. S1), i10–i17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).