Stoichiometric Multiprotein Assembly Scaffolded by a Heterotrimeric DNA Clamp for Enzyme Colocalization and DNA Functionalization
Abstract
1. Introduction
2. Results and Discussion
2.1. Binding of PCNA on Different DNA Scaffolds
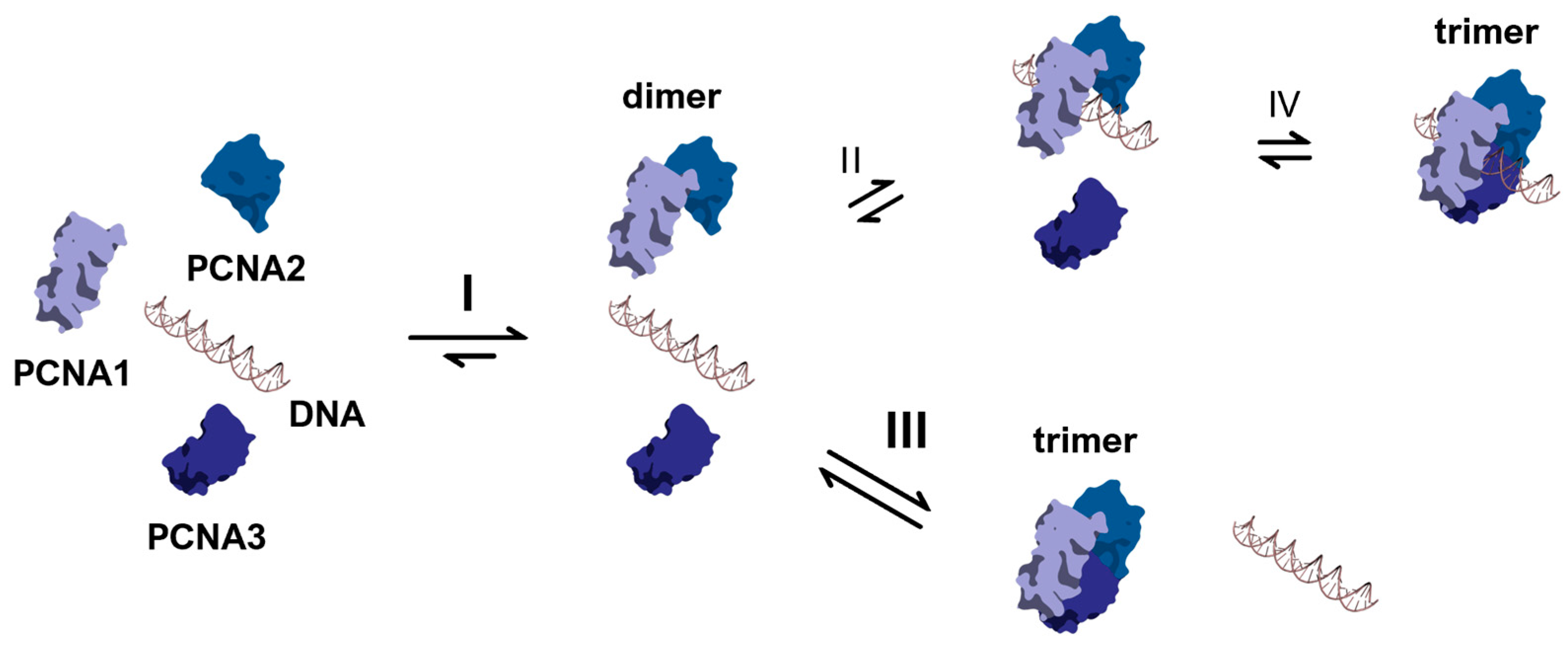
2.2. DNA Binding at Different PCNA Multimerization Levels

2.3. Clamp Loader-Independent DNA Binding Mechanism of PCNA
2.4. DNA Binding of Cys-Mutated PCNA

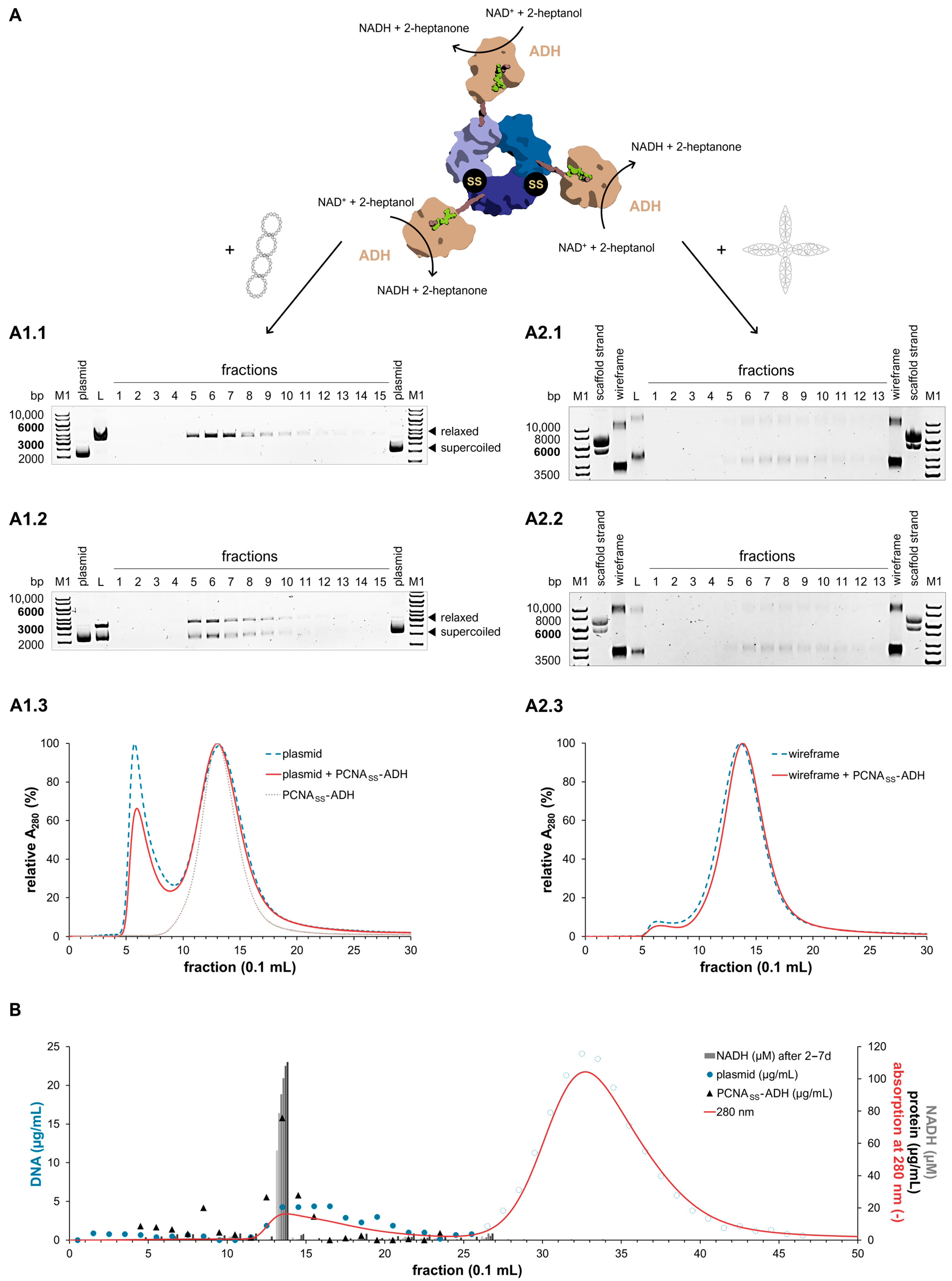
2.5. Activity of PCNASS-ADH Fusion Proteins on DNA
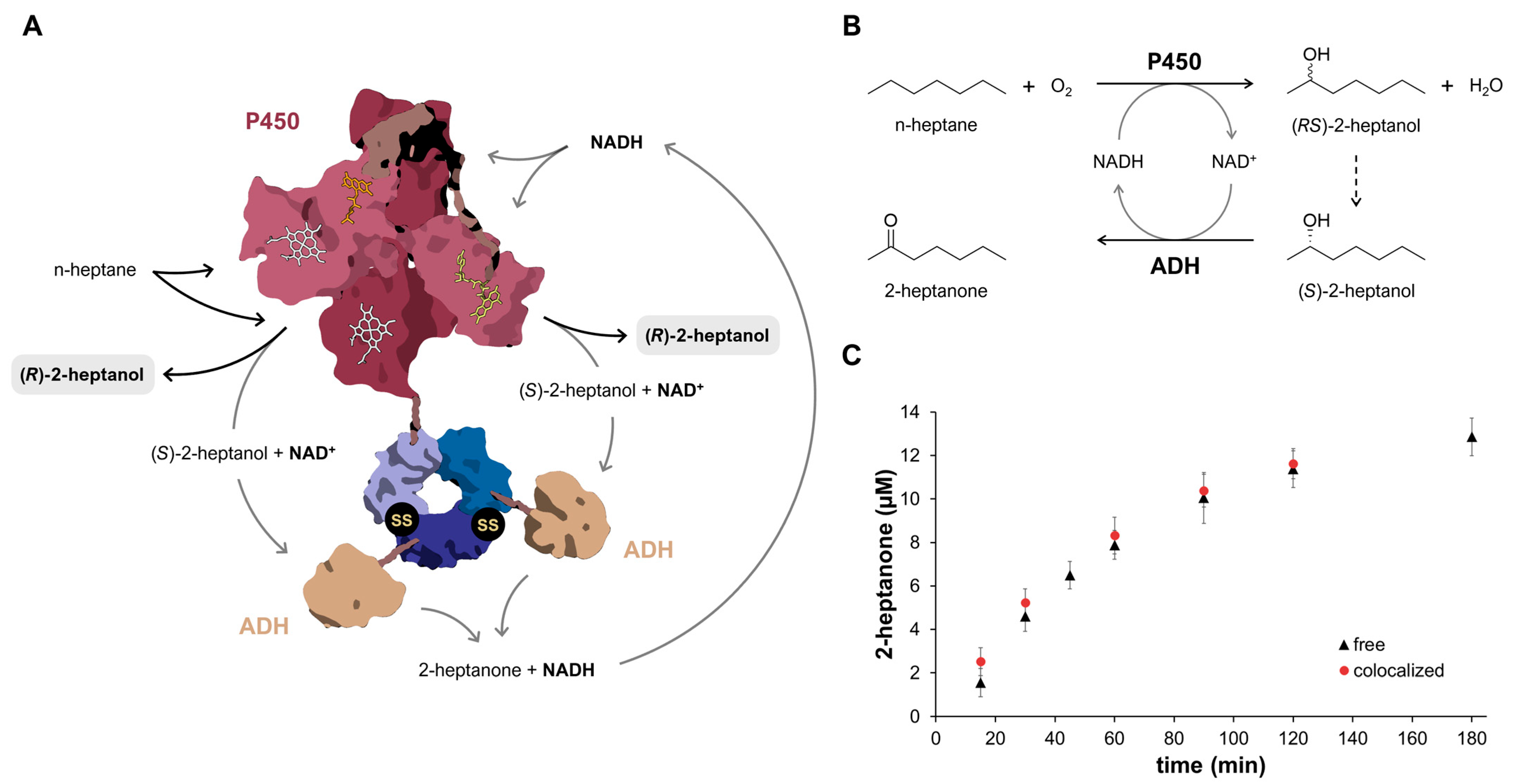
2.6. Colocalization of a Two-Enzyme System
3. Materials and Methods
3.1. Cloning and Site-Directed Mutagenesis
3.2. Protein Expression and Purification
3.3. SDS-PAGE
3.4. Preparation of the DNA Scaffolds
3.5. DNA Binding Assay
3.6. Electrophoretic Mobility Shift Assay (EMSA)
3.7. Preparation of the PCNASS-ADH-DNA Complex
3.8. ADH Activity Assay
3.9. Preparation of the Colocalized Enzyme System
3.10. Analysis of the Two-Enzyme System by Gas Chromatography
4. Conclusions and Prospect
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADH | Alcohol dehydrogenase |
| AFM | Atomic force microscopy |
| E. coli | Escherichia coli |
| GC-FID | Gas chromatography—flame ionization detector |
| Kd | Dissociation constant/binding constant |
| Km | Michaelis constant |
| MTBE | Methyl tert-butyl ether |
| NAD+/NADH | Nicotinamide adenine dinucleotide |
| P450 | Cytochrome P450 monooxygenase |
| PCNA | Proliferating cell nuclear antigen |
| SEC | Size exclusion chromatography |
References
- Hwang, E.T.; Lee, S. Multienzymatic cascade reactions via enzyme complex by immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Rabe, K.S.; Müller, J.; Skoupi, M.; Niemeyer, C.M. Cascades in compartments: En route to machine-assisted biotechnology. Angew. Chem. Int. Ed. 2017, 56, 13574–13589. [Google Scholar] [CrossRef]
- Conrado, R.J.; Varner, J.D.; DeLisa, M.P. Engineering the spatial organization of metabolic enzymes: Mimicking nature’s synergy. Curr. Opin. Biotechnol. 2008, 19, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.A.; Klein, W.P.; Lasarte-Aragones, G.; Thakur, M.; Walper, S.A.; Medintz, I.L. Artificial multienzyme scaffolds: Pursuing in vitro substrate channeling with an overview of current progress. ACS Catal. 2019, 9, 10812–10869. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Yuan, H.; Huang, D.; Wang, R.; Liu, H.; Wang, T. Research progress and the biotechnological applications of multienzyme complex. Appl. Microbiol. Biotechnol. 2021, 105, 1759–1777. [Google Scholar] [CrossRef] [PubMed]
- Fessner, W.-D.; Badia, J.; Eyrisch, O.; Schneider, A.; Sinerius, G. Enzymatic syntheses of rare ketose 1-phosphates. Tetrahedron Lett. 1992, 33, 5231–5234. [Google Scholar] [CrossRef]
- Wang, Z.; Sekar, B.S.; Li, Z. Recent advances in artificial enzyme cascades for the production of value-added chemicals. Bioresour. Technol. 2021, 323, 124551. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Enzyme immobilization and co-immobilization: Main framework, advances and some applications. Processes 2022, 10, 494. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Noreen, S.; Shah, S.Z.H.; Bharagava, R.N.; Iqbal, H.M.N. Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal. Biotransformation 2019, 37, 159–182. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Hoarau, M.; Badieyan, S.; Marsh, E.N.G. Immobilized enzymes: Understanding enzyme–surface interactions at the molecular level. Org. Biomol. Chem. 2017, 15, 9539–9551. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P. Substrate channeling and enzyme complexes for biotechnological applications. Biotechnol. Adv. 2011, 29, 715–725. [Google Scholar] [CrossRef]
- Dubey, N.C.; Tripathi, B.P. Nature inspired multienzyme immobilization: Strategies and concepts. ACS Appl. Bio Mater. 2021, 4, 1077–1114. [Google Scholar] [CrossRef] [PubMed]
- Geck, M.K.; Kirsch, J.F. A novel, definitive test for substrate channeling illustrated with the aspartate aminotransferase/malate dehydrogenase system. Biochemistry 1999, 38, 8032–8037. [Google Scholar] [CrossRef] [PubMed]
- Dueber, J.E.; Wu, G.C.; Malmirchegini, G.R.; Moon, T.S.; Petzold, C.J.; Ullal, A.V.; Prather, K.L.J.; Keasling, J.D. Synthetic protein scaffolds provide modular control over metabolic flux. Nat. Biotechnol. 2009, 27, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Svedružić, Ž.M.; Odorčić, I.; Chang, C.H.; Svedružić, D. Substrate channeling via a transient protein-protein complex: The case of D-glyceraldehyde-3-phosphate dehydrogenase and L-lactate dehydrogenase. Sci. Rep. 2020, 10, 10404. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Liu, H.; Yuan, H.; Huang, D.; Wang, T. Research progress of multi-enzyme complexes based on the design of scaffold protein. Bioresour. Bioprocess. 2023, 10, 72. [Google Scholar] [CrossRef]
- Zdarta, J.; Kołodziejczak-Radzimska, A.; Bachosz, K.; Rybarczyk, A.; Bilal, M.; Iqbal, H.M.N.; Buszewski, B.; Jesionowski, T. Nanostructured supports for multienzyme co-immobilization for biotechnological applications: Achievements, challenges and prospects. Adv. Colloid Interface Sci. 2023, 315, 102889. [Google Scholar] [CrossRef]
- Xu, K.; Chen, X.; Zheng, R.; Zheng, Y. Immobilization of multi-enzymes on support materials for efficient biocatalysis. Front. Bioeng. Biotechnol. 2020, 8, 660. [Google Scholar] [CrossRef]
- Meena, J.; Gupta, A.; Ahuja, R.; Singh, M.; Panda, A.K. Recent advances in nano-engineered approaches used for enzyme immobilization with enhanced activity. J. Mol. Liq. 2021, 338, 116602. [Google Scholar] [CrossRef]
- Gad, S.; Ayakar, S. Protein scaffolds: A tool for multi-enzyme assembly. Biotechnol. Rep. 2021, 32, e00670. [Google Scholar] [CrossRef]
- Kazenwadel, F.; Franzreb, M.; Rapp, B.E. Synthetic enzyme supercomplexes: Co-immobilization of enzyme cascades. Anal. Methods 2015, 7, 4030–4037. [Google Scholar] [CrossRef]
- Vanderstraeten, J.; Briers, Y. Synthetic protein scaffolds for the colocalisation of co-acting enzymes. Biotechnol. Adv. 2020, 44, 107627. [Google Scholar] [CrossRef]
- Artzi, L.; Bayer, E.A.; Moraïs, S. Cellulosomes: Bacterial nanomachines for dismantling plant polysaccharides. Nat. Rev. Microbiol. 2017, 15, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.S.; Dueber, J.E.; Shiue, E.; Prather, K.L.J. Use of modular, synthetic scaffolds for improved production of glucaric acid in engineered E. coli. Metab. Eng. 2010, 12, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Nygren, P.-Å. Alternative binding proteins: Affibody binding proteins developed from a small three-helix bundle scaffold. FEBS J. 2008, 275, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.M.; Müller, K.M.; Arndt, K.M. Considerations in the Design and Optimization of Coiled Coil Structures. In Protein Engineering Protocols, 1st ed.; Arndt, K.M., Müller, K.M., Eds.; Humana Press: Totowa, NJ, USA, 2007; Volume 352, pp. 35–70. [Google Scholar]
- Moldovan, G.-L.; Pfander, B.; Jentsch, S. PCNA, the maestro of the replication fork. Cell 2007, 129, 665–679. [Google Scholar] [CrossRef]
- Kelman, Z. PCNA: Structure, functions and interactions. Oncogene 1997, 14, 629–640. [Google Scholar] [CrossRef]
- Pan, M.; Kelman, L.M.; Kelman, Z. The archaeal PCNA proteins. Biochem. Soc. Trans. 2011, 39, 20–24. [Google Scholar] [CrossRef]
- Williams, G.J.; Johnson, K.; Rudolf, J.; McMahon, S.A.; Carter, L.; Oke, M.; Liu, H.; Taylor, G.L.; White, M.F.; Naismith, J.H. Structure of the heterotrimeric PCNA from Sulfolobus solfataricus. Acta Crystallogr. F Struct. Biol. Commun. 2006, 62, 944–948. [Google Scholar] [CrossRef]
- Hirakawa, H.; Nagamune, T. Molecular assembly of P450 with ferredoxin and ferredoxin reductase by fusion to PCNA. ChemBioChem 2010, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- De Biasio, A.; de Opakua, A.I.; Mortuza, G.B.; Molina, R.; Cordeiro, T.N.; Castillo, F.; Villate, M.; Merino, N.; Delgado, S.; Gil-Cartón, D.; et al. Structure of p15(PAF)-PCNA complex and implications for clamp sliding during DNA replication and repair. Nat. Commun. 2015, 6, 6439. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Liu, Y.; Yan, H. DNA Nanostructures as Programmable Biomolecular Scaffolds. Bioconjug. Chem. 2015, 26, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Meyer, T.A.; Pan, V.; Dutta, P.K.; Ke, Y. The Beauty and Utility of DNA Origami. Chem 2017, 2, 359–382. [Google Scholar] [CrossRef]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef]
- Nummelin, S.; Kommeri, J.; Kostiainen, M.A.; Linko, V. Evolution of Structural DNA Nanotechnology. Adv. Mater. 2018, 30, e1703721. [Google Scholar] [CrossRef]
- Saccà, B.; Niemeyer, C.M. DNA origami: The art of folding DNA. Angew. Chem. Int. Ed. Engl. 2012, 51, 58–66. [Google Scholar] [CrossRef]
- Benson, E.; Mohammed, A.; Gardell, J.; Masich, S.; Czeizler, E.; Orponen, P.; Högberg, B. DNA rendering of polyhedral meshes at the nanoscale. Nature 2015, 523, 441–444. [Google Scholar] [CrossRef]
- Muscat, R.A.; Bath, J.; Turberfield, A.J. A programmable molecular robot. Nano Lett. 2011, 11, 982–987. [Google Scholar] [CrossRef]
- Lund, K.; Manzo, A.J.; Dabby, N.; Michelotti, N.; Johnson-Buck, A.; Nangreave, J.; Taylor, S.; Pei, R.; Stojanovic, M.N.; Walter, N.G.; et al. Molecular robots guided by prescriptive landscapes. Nature 2010, 465, 206–210. [Google Scholar] [CrossRef]
- Bath, J.; Turberfield, A.J. DNA nanomachines. Nat. Nanotech 2007, 2, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Fernie, A.R. The role of dynamic enzyme assemblies and substrate channelling in metabolic regulation. Nat. Commun. 2018, 9, 2136. [Google Scholar] [CrossRef] [PubMed]
- Wilchek, M.; Bayer, E.A. The avidin-biotin complex in bioanalytical applications. Anal. Biochem. 1988, 171, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Bock, L.C.; Griffin, L.C.; Latham, J.A.; Vermaas, E.H.; Toole, J.J. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 1992, 355, 564–566. [Google Scholar] [CrossRef]
- Ngo, T.A.; Dinh, H.; Nguyen, T.M.; Liew, F.F.; Nakata, E.; Morii, T. Protein adaptors assemble functional proteins on DNA scaffolds. Chem. Commun. 2019, 55, 12428–12446. [Google Scholar] [CrossRef]
- Dionne, I.; Brown, N.J.; Woodgate, R.; Bell, S.D. On the mechanism of loading the PCNA sliding clamp by RFC. Mol. Microbiol. 2008, 68, 216–222. [Google Scholar] [CrossRef]
- Kelman, Z.; Hurwitz, J. Biochemical characterization of a clamp-loader complex homologous to eukaryotic replication factor C from the hyperthermophilic archaeon Sulfolobus solfataricus. J. Mol. Biol. 2000, 298, 779–789. [Google Scholar]
- Roberts, J.A.; White, M.F. An archaeal endonuclease displays key properties of both eukaryal XPF-ERCC1 and Mus81. J. Biol. Chem. 2005, 280, 5924–5928. [Google Scholar] [CrossRef]
- De March, M.; Merino, N.; Barrera-Vilarmau, S.; Crehuet, R.; Onesti, S.; Blanco, F.J.; de Biasio, A. Structural basis of human PCNA sliding on DNA. Nat. Commun. 2017, 8, 13935. [Google Scholar] [CrossRef]
- Georgescu, R.E.; Kim, S.-S.; Yurieva, O.; Kuriyan, J.; Kong, X.-P.; O’Donnell, M. Structure of a sliding clamp on DNA. Cell 2008, 132, 43–54. [Google Scholar] [CrossRef]
- Essert, A.; Castiglione, K. Enhancing the activity of a monomeric alcohol dehydrogenase for site-specific applications by site-directed mutagenesis. Protein Eng. Des. Sel. 2023, 36, gzad006. [Google Scholar] [CrossRef] [PubMed]
- Essert, A.; Castiglione, K. Dimer Stabilization by SpyTag/SpyCatcher Coupling of the Reductase Domains of a Chimeric P450 BM3 Monooxygenase from Bacillus spp. Improves its Stability, Activity, and Purification. ChemBioChem 2024, 25, e202300650. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.A.; Akkapurathu, B.; Winkler, T.; Staudt, S.; Hummel, W.; Gröger, H.; Schwaneberg, U. In Vitro Double Oxidation of n-Heptane with Direct Cofactor Regeneration. Adv. Synth. Catal. 2013, 355, 1787–1798. [Google Scholar] [CrossRef]
- Dionne, I.; Nookala, R.K.; Jackson, S.P.; Doherty, A.J.; Bell, S.D. A heterotrimeric PCNA in the hyperthermophilic archaeon Sulfolobus solfataricus. Mol. Cell 2003, 11, 275–282. [Google Scholar] [CrossRef]
- Hirakawa, H.; Kakitani, A.; Nagamune, T. Introduction of selective intersubunit disulfide bonds into self-assembly protein scaffold to enhance an artificial multienzyme complex’s activity. Biotechnol. Bioeng. 2013, 110, 1858–1864. [Google Scholar] [CrossRef]
- Götzfried, M.A. Dynamic DNA Nanostructures Actuated by Hydrophobic and Magnetic Stimuli. Ph.D. Thesis, Technical University of Munich, Garching, Germany, 2021. [Google Scholar]
- Witz, G.; Stasiak, A. DNA supercoiling and its role in DNA decatenation and unknotting. Nucleic Acids Res. 2010, 38, 2119–2133. [Google Scholar] [CrossRef]
- Li, H.; Zheng, F.; O’Donnell, M. Water skating: How polymerase sliding clamps move on DNA. FEBS J. 2021, 288, 7256–7262. [Google Scholar] [CrossRef]
- Doré, A.S.; Kilkenny, M.L.; Jones, S.A.; Oliver, A.W.; Roe, S.M.; Bell, S.D.; Pearl, L.H. Structure of an archaeal PCNA1-PCNA2-FEN1 complex: Elucidating PCNA subunit and client enzyme specificity. Nucleic Acids Res. 2006, 34, 4515–4526. [Google Scholar] [CrossRef]
- Pascal, J.M.; Tsodikov, O.V.; Hura, G.L.; Song, W.; Cotner, E.A.; Classen, S.; Tomkinson, A.E.; Tainer, J.A.; Ellenberger, T. A flexible interface between DNA ligase and PCNA supports conformational switching and efficient ligation of DNA. Mol. Cell 2006, 24, 279–291. [Google Scholar] [CrossRef]
- Horiuchi, H.; Takagi, M.; Yano, K. Relaxation of supercoiled plasmid DNA by oxidative stresses in Escherichia coli. J. Bacteriol. 1984, 160, 1017–1021. [Google Scholar] [CrossRef]
- Land, H.; Humble, M.S. YASARA: A tool to obtain structural guidance in biocatalytic investigations. In Protein Engineering: Methods and Protocols, 1st ed.; Bornscheuer, U.T., Höhne, M., Eds.; Springer: New York, NY, USA, 2018; Volume 1685, pp. 43–67. [Google Scholar]
- Anslow, G.A.; Lyman, E.R. A Spectrophotometric Study of Glutathione. J. Opt. Soc. Am. 1941, 31, 114. [Google Scholar] [CrossRef]
- Kröll, S.; Niemeyer, C.M. Nucleic Acid-based Enzyme Cascades-Current Trends and Future Perspectives. Angew. Chem. Int. Ed. Engl. 2024, 63, e202314452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tsitkov, S.; Hess, H. Proximity does not contribute to activity enhancement in the glucose oxidase-horseradish peroxidase cascade. Nat. Commun. 2016, 7, 13982. [Google Scholar] [CrossRef] [PubMed]
- Studier, F.W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M. The Bicinchoninic Acid (BCA) Assay for Protein Quantitation. In Protein Protocols Handbook, 3rd ed.; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; Volume 1, pp. 11–14. [Google Scholar]
- Sambrook, J.; Russell, D.W. Bacterial Growth Media. In Molecular Cloning: A Laboratory Manual, 3rd ed.; Sambrook, J., Russell, D.W., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001; Volume 3, pp. 717–727. [Google Scholar]
- Stahl, E.; Martin, T.G.; Praetorius, F.; Dietz, H. Facile and Scalable Preparation of Pure and Dense DNA Origami Solutions. Angew. Chem. 2014, 126, 12949–12954. [Google Scholar] [CrossRef]
- Mandaji, M.; Rübensam, G.; Hoff, R.B.; Hillebrand, S.; Carrilho, E.; Kist, T.L. Sample stacking in CZE using dynamic thermal junctions I. Analytes with low dpKa/dT crossing a single thermally induced pH junction in a BGE with high dpH/dT. Electrophoresis 2009, 30, 1501–1509. [Google Scholar] [CrossRef]
- Barr, I.; Guo, F. Pyridine Hemochromagen Assay for Determining the Concentration of Heme in Purified Protein Solutions. Bio-protocol 2015, 5, e1594. [Google Scholar] [CrossRef]
- Müller, J.; Niemeyer, C.M. DNA-directed assembly of artificial multienzyme complexes. Biochem. Biophys. Res. Commun. 2008, 377, 62–67. [Google Scholar] [CrossRef]
- Kuzmak, A.; Carmali, S.; von Lieres, E.; Russell, A.J.; Kondrat, S. Can enzyme proximity accelerate cascade reactions? Sci. Rep. 2019, 9, 455. [Google Scholar] [CrossRef]
- Bauler, P.; Huber, G.; Leyh, T.; McCammon, J.A. Channeling by proximity: The catalytic advantages of active site colocalization using Brownian dynamics. J. Phys. Chem. Lett. 2010, 1, 1332–1335. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Electrophoretic Mobility and Stoke’s Law. In Biochemistry, 4th ed.; Voet, D., Voet, J.G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 1, pp. 165–172. [Google Scholar]
- Rill, R.L.; Beheshti, A.; Van Winkle, D.H. DNA electrophoresis in agarose gels: Effects of field and gel concentration on the exponential dependence of reciprocal mobility on DNA length. Electrophoresis 2002, 23, 2710–2719. [Google Scholar] [CrossRef]
- Kozlowski, L.P. Proteome-pI 2.0: Proteome isoelectric point database update. Nucleic Acids Res. 2022, 50, D1535–D1540. [Google Scholar] [CrossRef]
- Liu, H.; Naismith, J.H. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol. 2008, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.A.; Bunting, K.A. Rings in the extreme: PCNA interactions and adaptations in the archaea. Archaea 2012, 2012, 951010. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essert, A.; Castiglione, K. Stoichiometric Multiprotein Assembly Scaffolded by a Heterotrimeric DNA Clamp for Enzyme Colocalization and DNA Functionalization. SynBio 2025, 3, 16. https://doi.org/10.3390/synbio3040016
Essert A, Castiglione K. Stoichiometric Multiprotein Assembly Scaffolded by a Heterotrimeric DNA Clamp for Enzyme Colocalization and DNA Functionalization. SynBio. 2025; 3(4):16. https://doi.org/10.3390/synbio3040016
Chicago/Turabian StyleEssert, Arabella, and Kathrin Castiglione. 2025. "Stoichiometric Multiprotein Assembly Scaffolded by a Heterotrimeric DNA Clamp for Enzyme Colocalization and DNA Functionalization" SynBio 3, no. 4: 16. https://doi.org/10.3390/synbio3040016
APA StyleEssert, A., & Castiglione, K. (2025). Stoichiometric Multiprotein Assembly Scaffolded by a Heterotrimeric DNA Clamp for Enzyme Colocalization and DNA Functionalization. SynBio, 3(4), 16. https://doi.org/10.3390/synbio3040016





