A Guide in Synthetic Biology: Designing Genetic Circuits and Their Applications in Stem Cells
Abstract
1. Introduction
2. Synthetic Biology Concepts and Toolkit
2.1. Synthetic DNA
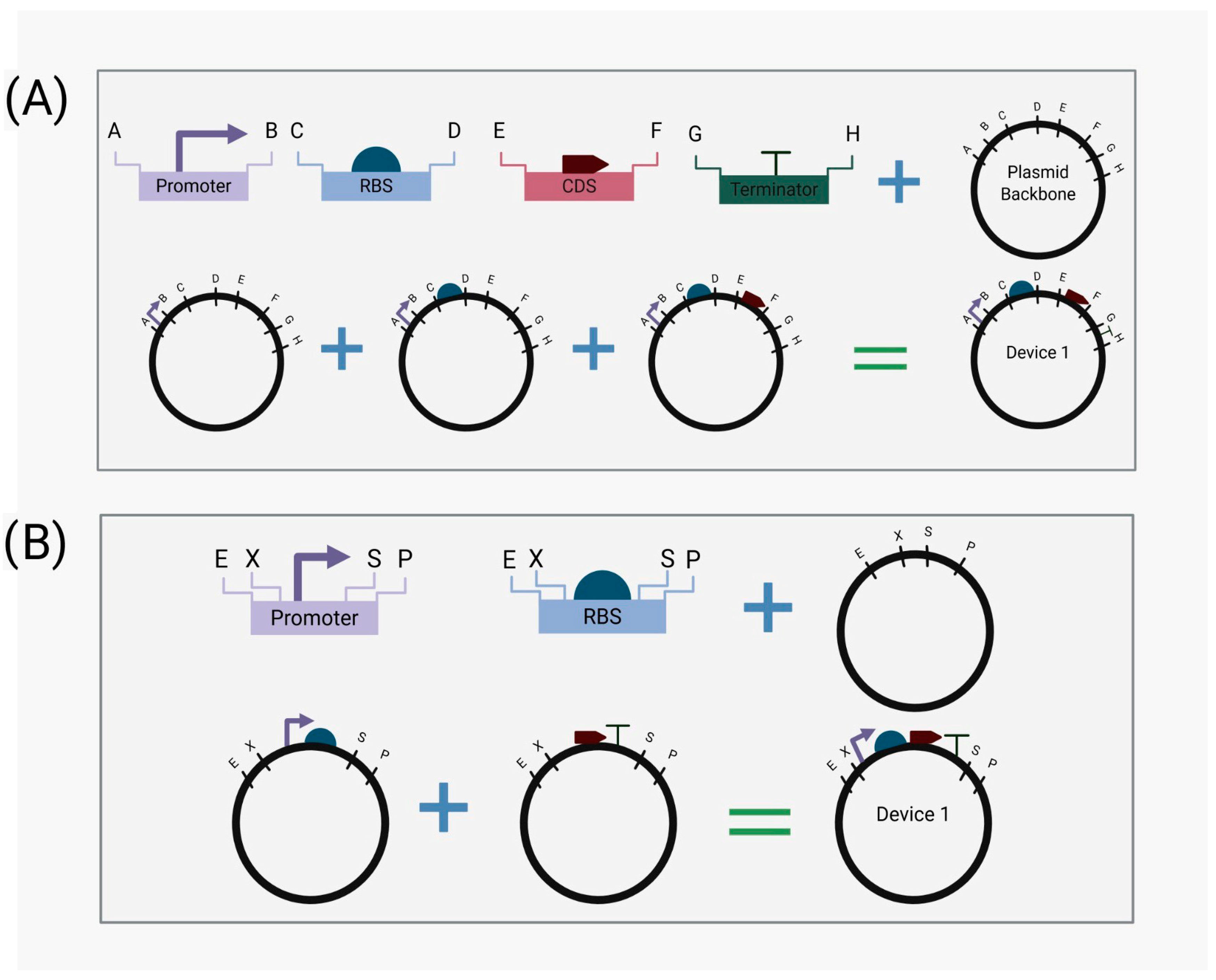
2.2. Standardization
2.3. Abstraction Hierarchy
3. Designing Genetic Circuits
3.1. Fundamental Biological Parts
3.1.1. Gene Expression Regulatory Parts
Promoters/Synthetic Promoters
Riboswitches
Toehold Switches
Synthetic Transcription Factors
3.1.2. Other Parts: Ribosomal Binding Sites, Coding Sequences, and Terminators
3.2. Circuit Architectures
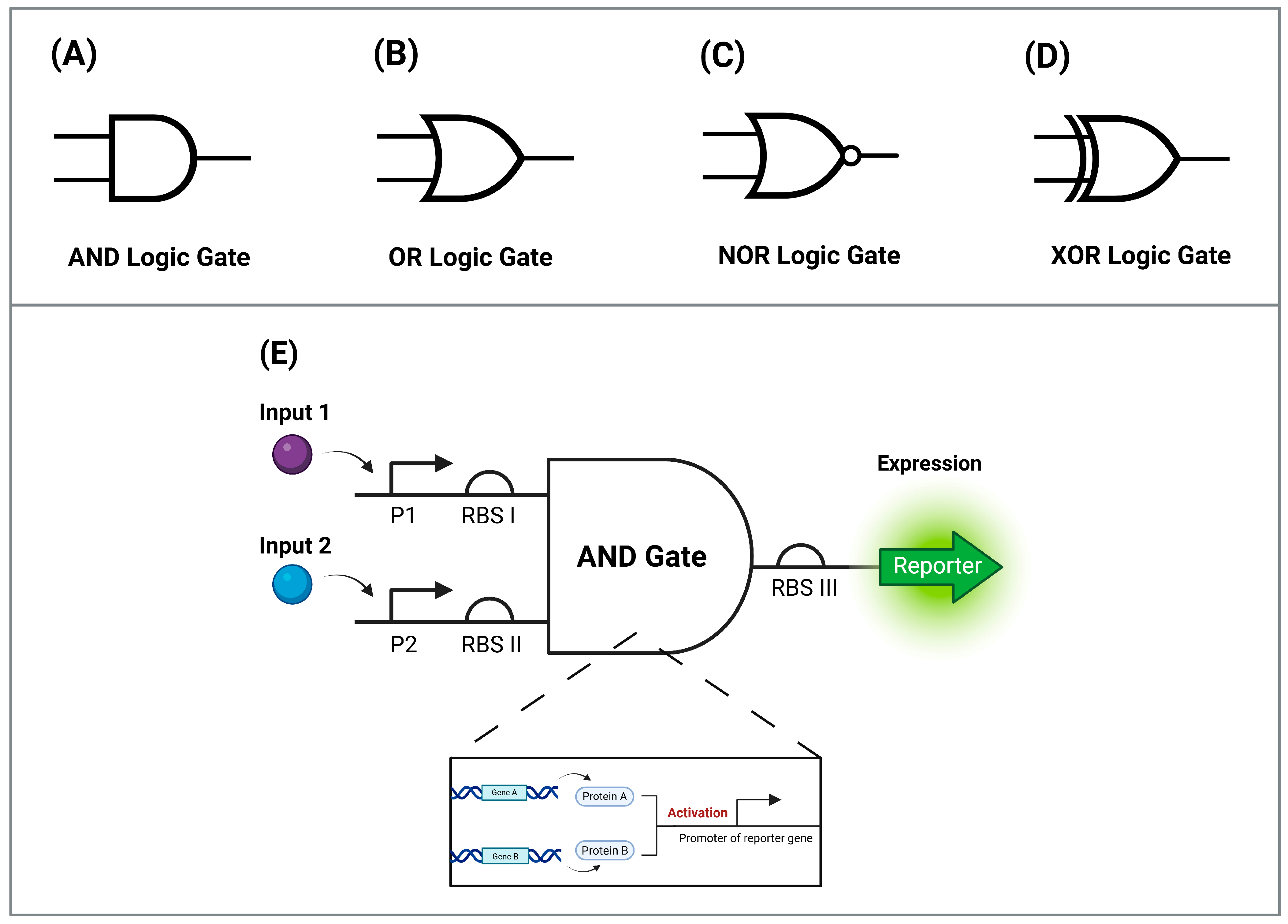
3.2.1. Logic Gates
3.2.2. Toggle Switches
3.2.3. Oscillators
3.3. Implementing Genetic Circuits
3.3.1. Computational Tools
COPASI
Cello
iBioSim
3.3.2. Wet Lab Assembly Approaches
BioBrick Standard Assembly
Golden Gate Assembly
Gibson Assembly
Sequence and Ligation-Independent Cloning (SLIC)
CRISPR-Based In Situ Integration
4. Integrating Genetic Circuits with Stem Cells
4.1. Cell Differentiation
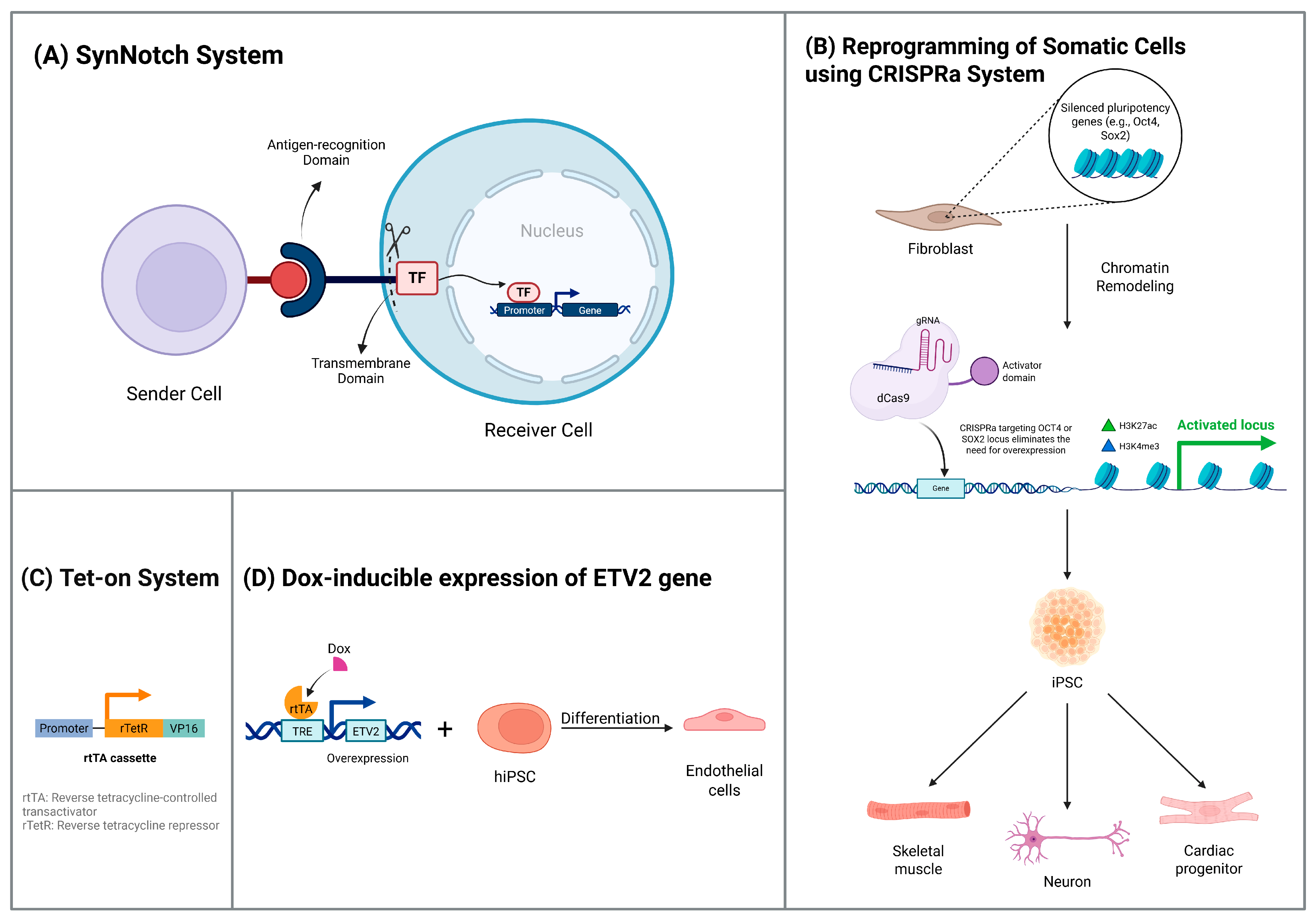
4.2. Cell Reprogramming
4.3. Cell Therapies
4.4. Tissue Engineering
5. Challenges and Limitations of Integrating Synthetic Biology with Stem Cells
5.1. Major Types of Stem Cells and Their Characteristics
5.2. SynBio Challenges
5.3. Stem Cell Challenges
6. Future Aspects and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Aly, R.M. Current state of stem cell-based therapies: An overview. Stem Cell Investig. 2020, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P.; Vanneaux, V.; Hagège, A.; Bel, A.; Cholley, B.; Cacciapuoti, I.; Parouchev, A.; Benhamouda, N.; Tachdjian, G.; Tosca, L.; et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report: Figure 1. Eur. Heart J. 2015, 36, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.-P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Ogilvie, C. Concise Review: Human Embryonic Stem Cells—What Have We Done? What Are We Doing? Where Are We Going? Stem Cells 2016, 35, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Lezmi, E.; Jung, J.; Benvenisty, N. High prevalence of acquired cancer-related mutations in 146 human pluripotent stem cell lines and their differentiated derivatives. Nat. Biotechnol. 2024, 42, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.P.; Deans, T.L. Genetic circuits to engineer tissues with alternative functions. J. Biol. Eng. 2019, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Ohneda, K.; Yamamoto, M.; Philipsen, S. GATA1 Function, a Paradigm for Transcription Factors in Hematopoiesis. Mol. Cell. Biol. 2005, 25, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Wobus, A.M.; Boheler, K.R. Embryonic Stem Cells: Prospects for Developmental Biology and Cell Therapy. Physiol. Rev. 2005, 85, 635–678. [Google Scholar] [CrossRef] [PubMed]
- Itakura, G.; Kawabata, S.; Ando, M.; Nishiyama, Y.; Sugai, K.; Ozaki, M.; Iida, T.; Ookubo, T.; Kojima, K.; Kashiwagi, R.; et al. Fail-Safe System against Potential Tumorigenicity after Transplantation of iPSC Derivatives. Stem Cell Rep. 2017, 8, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Miyoshi, H.; Nagoshi, N.; Kohyama, J.; Itakura, G.; Kawabata, S.; Ozaki, M.; Iida, T.; Sugai, K.; Ito, S.; et al. Selective Ablation of Tumorigenic Cells Following Human Induced Pluripotent Stem Cell-Derived Neural Stem/Progenitor Cell Transplantation in Spinal Cord Injury. Stem Cells Transl. Med. 2018, 8, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Kurtoğlu, A.; Yıldız, A.; Arda, B. The view of synthetic biology in the field of ethics: A thematic systematic review. Front. Bioeng. Biotechnol. 2024, 12, 1397796. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.S.; Collins, J.J. Synthetic biology: Applications come of age. Nat. Rev. Genet. 2010, 11, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Glass, J.I.; Lartigue, C.; Noskov, V.N.; Chuang, R.-Y.; Algire, M.A.; Benders, G.A.; Montague, M.G.; Ma, L.; Moodie, M.M.; et al. Creation of a Bacterial Cell Controlled by a Chemically Synthesized Genome. Science 2010, 329, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.A. Synthetic DNA; Humana: New York, NY, USA, 2017; Volume 1472. [Google Scholar] [CrossRef]
- Hughes, R.A.; Ellington, A.D. Synthetic DNA Synthesis and Assembly: Putting the Synthetic in Synthetic Biology. Cold Spring Harb. Perspect. Biol. 2017, 9, a023812. [Google Scholar] [CrossRef] [PubMed]
- Demissie, E.A.; Park, S.-Y.; Moon, J.H.; Lee, D.-Y. Comparative Analysis of Codon Optimization Tools: Advancing toward a Multi-Criteria Framework for Synthetic Gene Design. J. Microbiol. Biotechnol. 2025, 35, e2411066. [Google Scholar] [CrossRef] [PubMed]
- Canton, B.; Labno, A.; Endy, D. Refinement and standardization of synthetic biological parts and devices. Nat. Biotechnol. 2008, 26, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Kuldell, N.; Bernstein, R.; Ingram, K.; Hart, K. BioBuilder: Synthetic Biology in the Lab; O’Reilly Media: Sebastopol, CA, USA, 2015; Volume 237. [Google Scholar]
- Arkin, A. Setting the standard in synthetic biology. Nat. Biotechnol. 2008, 26, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.M.; Arndt, K.M. Standardization in synthetic biology. Methods Mol. Biol. 2012, 813, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Galdzicki, M.; Clancy, K.P.; Oberortner, E.; Pocock, M.; Quinn, J.Y.; Rodriguez, C.A.; Roehner, N.; Wilson, M.L.; Adam, L.; Anderson, J.C.; et al. The Synthetic Biology Open Language (SBOL) provides a community standard for communicating designs in synthetic biology. Nat. Biotechnol. 2014, 32, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Spitz, F.; Furlong, E.E.M. Transcription factors: From enhancer binding to developmental control. Nat. Rev. Genet. 2012, 13, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.A.; Voigt, C.A. Multi-input CRISPR/Cas genetic circuits that interface host regulatory networks. Mol. Syst. Biol. 2014, 10, 763. [Google Scholar] [CrossRef] [PubMed]
- Lenhard, B.; Sandelin, A.; Carninci, P. Metazoan promoters: Emerging characteristics and insights into transcriptional regulation. Nat. Rev. Genet. 2012, 13, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Kim, W.C. A Fruitful Decade Using Synthetic Promoters in the Improvement of Transgenic Plants. Front. Plant Sci. 2019, 10, 493712. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Voigt, C.A.; Arkin, A.P. Environmental signal integration by a modular AND gate. Mol. Syst. Biol. 2007, 3, 133. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Stewart, C.N., Jr. Plant synthetic promoters and transcription factors. Curr. Opin. Biotechnol. 2016, 37, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Rai, K.M.; Srivastava, M.; Kumar, V.; Pandey, B.; Singh, S.P.; Bag, S.K.; Singh, B.D.; Tuli, R.; Sawant, S.V. Distinct Role of Core Promoter Architecture in Regulation of Light-Mediated Responses in Plant Genes. Mol. Plant 2014, 7, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Artemyev, V.; Gubaeva, A.; Paremskaia, A.I.; Dzhioeva, A.A.; Deviatkin, A.; Feoktistova, S.G.; Mityaeva, O.; Volchkov, P.Y. Synthetic Promoters in Gene Therapy: Design Approaches, Features and Applications. Cells 2024, 13, 1963. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, E.; Wang, J.; Riaz, M.; Zhang, L.; Zuo, K. Designing artificial synthetic promoters for accurate, smart, and versatile gene expression in plants. Plant Commun. 2023, 4, 100558. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, I.Z.; Abubakar, F.S.; Katsayal, B.S.; Ibrahim, B.; Adamu, A.; Usman, M.A.; Aliyu, M.; Suleiman, M.A.; Muhammad, A. Stem Cells in Regenerative Medicine: Unlocking Therapeutic Potential Through Stem Cell Therapy, 3D Bioprinting, Gene Editing, and Drug Discovery. Biomed. Eng. Adv. 2025, 9, 100172. [Google Scholar] [CrossRef]
- Vogel, A.M.; Persson, K.M.; Seamons, T.R.; Deans, T.L.; Bayley, H. Synthetic biology for improving cell fate decisions and tissue engineering outcomes. Emerg. Top. Life Sci. 2019, 3, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Findeiß, S.; Etzel, M.; Will, S.; Mörl, M.; Stadler, P.F. Design of Artificial Riboswitches as Biosensors. Sensors 2017, 17, 1990. [Google Scholar] [CrossRef] [PubMed]
- Serganov, A.; Nudler, E. A Decade of Riboswitches. Cell 2013, 152, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Jang, S.; Yang, J.; Seo, S.W.; Jung, G.Y. RNA-based dynamic genetic controllers: Development strategies and applications. Curr. Opin. Biotechnol. 2017, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Machtel, P.; Bąkowska-Żywicka, K.; Żywicki, M. Emerging applications of riboswitches—From antibacterial targets to molecular tools. J. Appl. Genet. 2016, 57, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jensen, M.K.; Keasling, J.D. Development of biosensors and their application in metabolic engineering. Curr. Opin. Chem. Biol. 2015, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, Z.F.; Su, Y.; Kitto, R.Z.; Hammond, M.C. Engineering and In Vivo Applications of Riboswitches. Annu. Rev. Biochem. 2017, 86, 515–539. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.H.T.; Mai, D.H.A.; Pham, D.N.; Le, H.T.Q.; Lee, E.Y. Developments of Riboswitches and Toehold Switches for Molecular Detection—Biosensing and Molecular Diagnostics. Int. J. Mol. Sci. 2020, 21, 3192. [Google Scholar] [CrossRef] [PubMed]
- Winkler, W.C.; Breaker, R.R. Regulation of bacterial gene expression by riboswitches. Annu. Rev. Microbiol. 2005, 59, 487–517. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, F.; Leonardi, A.; Crescenzi, E. Glutamine Metabolism in Cancer Stem Cells: A Complex Liaison in the Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 2337. [Google Scholar] [CrossRef] [PubMed]
- Wieland, M.; Ausländer, D.; Fussenegger, M. Engineering of ribozyme-based riboswitches for mammalian cells. Methods 2012, 56, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Topp, S.; Gallivan, J.P. Emerging Applications of Riboswitches in Chemical Biology. ACS Chem. Biol. 2010, 5, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Yokobayashi, Y. Scalable control of stem cell fate by riboswitch-regulated RNA viral vector without genomic integration. Mol. Ther. 2025, 33, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Green, A.A.; Silver, P.A.; Collins, J.J.; Yin, P. Toehold Switches: De-Novo-Designed Regulators of Gene Expression. Cell 2014, 159, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Ahmed, E.I.; Elareer, N.R.; Junejo, K.; Steinhoff, M.; Uddin, S. Role of miRNA-Regulated Cancer Stem Cells in the Pathogenesis of Human Malignancies. Cells 2019, 8, 840. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ferrai, C.; Schulte, C. Mechanotransduction in stem cells. Eur. J. Cell Biol. 2024, 103, 151417. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.T.; Handorf, A.M.; Bae Jeon, W.; Li, W.-J. Stem Cell-based Tissue Engineering Approaches for Musculoskeletal Regeneration. Curr. Pharm. Des. 2013, 19, 3429–3445. [Google Scholar] [CrossRef] [PubMed]
- Heiderscheit, E.A.; Eguchi, A.; Spurgat, M.C.; Ansari, A.Z. Reprogramming cell fate with artificial transcription factors. FEBS Lett. 2018, 592, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Hersey, A.N.; Kay, V.E.; Lee, S.; Realff, M.J.; Wilson, C.J. Engineering allosteric transcription factors guided by the LacI topology. Cell Syst. 2023, 14, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Lee, G.O.; Wan, F.; Erwin, G.S.; Ansari, A.Z. Controlling gene networks and cell fate with precision-targeted DNA-binding proteins and small-molecule-based genome readers. Biochem. J. 2014, 462, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Slusarczyk, A.L.; Lin, A.; Weiss, R. Foundations for the design and implementation of synthetic genetic circuits. Nat. Rev. Genet. 2012, 13, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Lohmueller, J.J.; Armel, T.Z.; Silver, P.A. A tunable zinc finger-based framework for Boolean logic computation in mammalian cells. Nucleic Acids Res. 2012, 40, 5180–5187. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.; Dalgarno, L. The 3′-Terminal Sequence of Escherichia coli 16S Ribosomal RNA: Complementarity to Nonsense Triplets and Ribosome Binding Sites. Proc. Natl. Acad. Sci. USA 1974, 71, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK21054/ (accessed on 10 June 2025).
- Laursen, B.S.; Sørensen, H.P.; Mortensen, K.K.; Sperling-Petersen, H.U. Initiation of Protein Synthesis in Bacteria. Microbiol. Mol. Biol. Rev. 2005, 69, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pomeroy-Cloney, L.; Bjerknes, M.; Tam, J.; Jay, E. The Influence of Adenine-rich Motifs in the 3′ Portion of the Ribosome Binding Site on Human IFN-γ Gene Expression in Escherichia coli. J. Mol. Biol. 1994, 240, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, V.K.; Guimaraes, J.C.; Cambray, G.; Lam, C.; Christoffersen, M.J.; Mai, Q.-A.; Tran, A.B.; Paull, M.; Keasling, J.D.; Arkin, A.P.; et al. Precise and reliable gene expression via standard transcription and translation initiation elements. Nat. Methods 2013, 10, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Elowitz, M.B.; Leibler, S. A synthetic oscillatory network of transcriptional regulators. Nature 2000, 403, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.E.; Bashor, C.J.; Collins, J.J. A brief history of synthetic biology. Nat. Rev. Microbiol. 2014, 12, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Liu, P.; Nielsen, A.A.K.; Brophy, J.A.N.; Clancy, K.; Peterson, T.; Voigt, C.A. Characterization of 582 natural and synthetic terminators and quantification of their design constraints. Nat. Methods 2013, 10, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Brophy, J.A.N.; Voigt, C.A. Principles of genetic circuit design. Nat. Methods 2014, 11, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.A.K.; Der, B.S.; Shin, J.; Vaidyanathan, P.; Paralanov, V.; Strychalski, E.A.; Ross, D.; Densmore, D.; Voigt, C.A. Genetic circuit design automation. Science 2016, 352, aac7341. [Google Scholar] [CrossRef] [PubMed]
- Bashor, C.J.; Helman, N.C.; Yan, S.; Lim, W.A. Using Engineered Scaffold Interactions to Reshape MAP Kinase Pathway Signaling Dynamics. Science 2008, 319, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.S.; Cantor, C.R.; Collins, J.J. Construction of a genetic toggle switch in Escherichia coli. Nature 2000, 403, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Garner, K.L. Principles of synthetic biology. Essays Biochem. 2021, 65, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, J.C. Molecular Bases for Circadian Clocks. Cell 1999, 96, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Bell-Pedersen, D.; Cassone, V.M.; Earnest, D.J.; Golden, S.S.; Hardin, P.E.; Thomas, T.L.; Zoran, M.J. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat. Rev. Genet. 2005, 6, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Virshup, D.M. Post-translational modifications regulate the ticking of the circadian clock. Nat. Rev. Mol. Cell Biol. 2007, 8, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Purcell, O.; Savery, N.J.; Grierson, C.S.; di Bernardo, M. A comparative analysis of synthetic genetic oscillators. J. R. Soc. Interface 2010, 7, 1503–1524. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.T.; Barnes, C.; Kitney, R.I.; Freemont, P.S.; Stan, G.-B.V. Computational design approaches and tools for synthetic biology. Integr. Biol. 2011, 3, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, A.; Ness, J.E.; Gustafsson, C.; Minshull, J.; Govindarajan, S. Gene Designer: A synthetic biology tool for constructing artificial DNA segments. BMC Bioinform. 2006, 7, 285. [Google Scholar] [CrossRef] [PubMed]
- Radha KesavanNair, L. Computational design of guide RNAs and vector to knockout LasR gene of Pseudomonas aeruginosa. Gene Genome Ed. 2023, 6, 100028. [Google Scholar] [CrossRef]
- Boeing, P.; Ozdemir, T.; Barnes, C.P. Design Tools for Synthetic Biology. Synthetic Biology Handbook; CRC Press: Boca Raton, FL, USA, 2016; pp. 259–279. [Google Scholar] [CrossRef]
- Liu, B.; Åberg, C.; van Eerden, F.J.; Marrink, S.J.; Poolman, B.; Boersma, A.J. Design and Properties of Genetically Encoded Probes for Sensing Macromolecular Crowding. Biophys. J. 2017, 112, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Zulkower, V.; Rosser, S.; Ponty, Y. DNA Chisel, a versatile sequence optimizer. Bioinformatics 2020, 36, 4508–4509. [Google Scholar] [CrossRef] [PubMed]
- Diez, M.; Medina-Muñoz, S.G.; Castellano, L.A.; Pescador, G.d.S.; Wu, Q.; Bazzini, A.A. iCodon customizes gene expression based on the codon composition. Sci. Rep. 2022, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Grote, A.; Hiller, K.; Scheer, M.; Münch, R.; Nörtemann, B.; Hempel, D.C.; Jahn, D. JCat: A novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 2005, 33, W526–W531. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.A.; Beal, J.; Mısırlı, G.; Grünberg, R.; Bartley, B.A.; Scott-Brown, J.; Vaidyanathan, P.; Fontanarrosa, P.; Oberortner, E.; Wipat, A.; et al. The Synthetic Biology Open Language (SBOL) Version 3: Simplified Data Exchange for Bioengineering. Front. Bioeng. Biotechnol. 2020, 8, 567377. [Google Scholar] [CrossRef] [PubMed]
- Golebiewski, M.; Bader, G.; Gleeson, P.; Gorochowski, T.E.; Keating, S.M.; König, M.; Myers, C.J.; Nickerson, D.P.; Sommer, B.; Waltemath, D.; et al. Specifications of standards in systems and synthetic biology: Status, developments, and tools in 2024. J. Integr. Bioinform. 2024, 21, 20240015. [Google Scholar] [CrossRef]
- Shetty, R.P.; Endy, D.; Knight, T.F., Jr. Engineering BioBrick vectors from BioBrick parts. J. Biol. Eng. 2008, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.A.; Myers, C.J.; Zundel, Z.; Mısırlı, G.; Zhang, M.; Ofiteru, I.D.; Goñi-Moreno, A.; Wipat, A. SynBioHub: A Standards-Enabled Design Repository for Synthetic Biology. ACS Synth. Biol. 2018, 7, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Ham, T.S.; Dmytriv, Z.; Plahar, H.; Chen, J.; Hillson, N.J.; Keasling, J.D. Design, implementation and practice of JBEI-ICE: An open source biological part registry platform and tools. Nucleic Acids Res. 2012, 40, e141. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, P.; Zhuang, X.; Ling, Y.; Liu, H.; Wang, S.; Yu, H.; Ma, L.; Jiang, Y.; Zhao, G.; et al. RDBSB: A database for catalytic bioparts with experimental evidence. Nucleic Acids Res. 2024, 53, D709–D716. [Google Scholar] [CrossRef] [PubMed]
- Seiler, C.Y.; Park, J.G.; Sharma, A.; Hunter, P.; Surapaneni, P.; Sedillo, C.; Field, J.; Algar, R.; Price, A.; Steel, J.; et al. DNASU plasmid and PSI:Biology-Materials repositories: Resources to accelerate biological research. Nucleic Acids Res. 2013, 42, D1253–D1260. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.S.; Nishikata, K.; Shimoyama, S.; Yoshida, Y.; Matsui, M.; Makita, Y.; Toyoda, T. PromoterCAD: Data-driven design of plant regulatory DNA. Nucleic Acids Res. 2013, 41, W569–W574. [Google Scholar] [CrossRef] [PubMed]
- Dudek, C.-A.; Jahn, D. PRODORIC: State-of-the-art database of prokaryotic gene regulation. Nucleic Acids Res. 2021, 50, D295–D302. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, Z.; Dominguez, A.; Li, Y.; Wang, X.; Qi, L.S. CRISPR-ERA: A comprehensive design tool for CRISPR-mediated gene editing, repression and activation: Fig. 1. Bioinformatics 2015, 31, 3676–3678. [Google Scholar] [CrossRef] [PubMed]
- Fornace, M.E.; Huang, J.; Newman, C.T.; Porubsky, N.J.; Pierce, M.B.; Pierce, N.A. NUPACK: Analysis and Design of Nucleic Acid Structures, Devices, and Systems. ChemRxiv 2022. [Google Scholar] [CrossRef]
- Varenyk, Y.; Spicher, T.; Hofacker, I.L.; Lorenz, R.; Cowen, L. Modified RNAs and predictions with the ViennaRNA Package. Bioinformatics 2023, 39, btad696. [Google Scholar] [CrossRef] [PubMed]
- Borujeni, A.E.; Mishler, D.M.; Wang, J.; Huso, W.; Salis, H.M. Automated physics-based design of synthetic riboswitches from diverse RNA aptamers. Nucleic Acids Res. 2015, 44, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.J.; Andreasson, J.O.L.; Kladwang, W.; Greenleaf, W.; Das, R. Automated Design of Diverse Stand-Alone Riboswitches. ACS Synth. Biol. 2019, 8, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kladwang, W.; Lee, M.; Cantu, D.; Azizyan, M.; Kim, H.; Limpaecher, A.; Gaikwad, S.; Yoon, S.; Treuille, A.; et al. RNA design rules from a massive open laboratory. Proc. Natl. Acad. Sci. USA 2014, 111, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- To, A.C.-Y.; Chu, D.H.-T.; Wang, A.R.; Li, F.C.-Y.; Chiu, A.W.-O.; Gao, D.Y.; Choi, C.H.J.; Kong, S.-K.; Chan, T.-F.; Chan, K.-M.; et al. A comprehensive web tool for toehold switch design. Bioinformatics 2018, 34, 2862–2864. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, A.F.; Rouleau, F.D.; Bautista, C.; Lemieux, P.; Dumont-Leblond, N. Toeholder: A software for automated design and in silico validation of toehold riboswitches. PeerJ Phys. Chem. 2023, 5, e28. [Google Scholar] [CrossRef]
- Magana Gomez, P.G.; Kovalevskiy, O. AlphaFold A Practical Guide Online Tutorial–EBI-EMBL. Available online: https://doi.org/10.6019/TOL.AlphaFold-w.2024.00001.1 (accessed on 10 June 2025).
- Lyskov, S.; Chou, F.-C.; Conchúir, S.Ó.; Der, B.S.; Drew, K.; Kuroda, D.; Xu, J.; Weitzner, B.D.; Renfrew, P.D.; Sripakdeevong, P.; et al. Serverification of Molecular Modeling Applications: The Rosetta Online Server That Includes Everyone (ROSIE). PLoS ONE 2013, 8, e63906. [Google Scholar] [CrossRef] [PubMed]
- Mandell, J.G.; Barbas, C.F. Zinc Finger Tools: Custom DNA-binding domains for transcription factors and nucleases. Nucleic Acids Res. 2006, 34, W516–W523. [Google Scholar] [CrossRef] [PubMed]
- Heigwer, F.; Kerr, G.; Walther, N.; Glaeser, K.; Pelz, O.; Breinig, M.; Boutros, M. E-TALEN: A web tool to design TALENs for genome engineering. Nucleic Acids Res. 2013, 41, e190. [Google Scholar] [CrossRef] [PubMed]
- Minniti, J.; Checler, F.; Duplan, E.; Alves da Costa, C. TFinder: A Python Web Tool for Predicting Transcription Factor Binding Sites. J. Mol. Biol. 2025, 437, 168921. [Google Scholar] [CrossRef] [PubMed]
- Wingender, E.; Kel, A.; Krull, M. Transcription factor databases. Encycl. Bioinform. Comput. Biol. ABC Bioinform. 2018, 2, 134–141. [Google Scholar] [CrossRef]
- Bryne, J.C.; Valen, E.; Tang, M.-H.E.; Marstrand, T.; Winther, O.; da Piedade, I.; Krogh, A.; Lenhard, B.; Sandelin, A. JASPAR, the open access database of transcription factor-binding profiles: New content and tools in the 2008 update. Nucleic Acids Res. 2007, 36, D102–D106. [Google Scholar] [CrossRef] [PubMed]
- Salis, H.M. The Ribosome Binding Site Calculator. Methods Enzymol. 2011, 498, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Na, D.; Lee, D. RBSDesigner: Software for designing synthetic ribosome binding sites that yields a desired level of protein expression. Bioinformatics 2010, 26, 2633–2634. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.W.; Yang, J.-S.; Kim, I.; Yang, J.; Min, B.E.; Kim, S.; Jung, G.Y. Predictive design of mRNA translation initiation region to control prokaryotic translation efficiency. Metab. Eng. 2013, 15, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bonde, M.T.; Pedersen, M.; Klausen, M.S.; Jensen, S.I.; Wulff, T.; Harrison, S.; Nielsen, A.T.; Herrgård, M.J.; Sommer, M.O. Predictable tuning of protein expression in bacteria. Nat. Methods 2016, 13, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Farasat, I.; Kushwaha, M.; Collens, J.; Easterbrook, M.; Guido, M.; Salis, H.M. Efficient search, mapping, and optimization of multi-protein genetic systems in diverse bacteria. Mol. Syst. Biol. 2014, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.S.; Oliveira, S.M.D.; Myers, C.J.; Voigt, C.A.; Densmore, D. Genetic circuit design automation with Cello 2.0. Nat. Protoc. 2022, 17, 1097–1113. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, L.; Nguyen, T.; Zhang, M.; Zundel, Z.; Zhang, Z.; Madsen, C.; Roehner, N.; Myers, C. iBioSim 3: A Tool for Model-Based Genetic Circuit Design. ACS Synth. Biol. 2018, 8, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Kaznessis, Y.N. SynBioSS-Aided Design of Synthetic Biological Constructs. Methods Enzymol. 2011, 498, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Chandran, D.; Bergmann, F.T.; Sauro, H.M. Computer-aided design of biological circuits using tinkercell. Bioeng. Bugs 2010, 1, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Czar, M.J.; Cai, Y.; Peccoud, J. Writing DNA with GenoCADTM. Nucleic Acids Res. 2009, 37, W40–W47. [Google Scholar] [CrossRef] [PubMed]
- Jelemenská, K.; Siebert, M.; Macko, D.; Čičák, P. Logic circuit design verification support tool-Fit Board. Procedia-Soc. Behav. Sci. 2011, 28, 305–310. [Google Scholar] [CrossRef]
- Beal, J.; Weiss, R.; Densmore, D.; Adler, A.; Babb, J.; Bhatia, S.; Davidsohn, N.; Haddock, T.; Yaman, F.; Schantz, R.; et al. TASBE: A Tool-Chain to Accelerate Synthetic Biological Engineering. In Proceedings of the 3rd International Workshop on Bio-Design Automation, San Diego, CA, USA, 6–7 January 2011. [Google Scholar]
- Marchisio, M.A.; Stelling, J.; Papin, J.A. Automatic Design of Digital Synthetic Gene Circuits. PLoS Comput. Biol. 2011, 7, e1001083. [Google Scholar] [CrossRef] [PubMed]
- Hoops, S.; Sahle, S.; Gauges, R.; Lee, C.; Pahle, J.; Simus, N.; Singhal, M.; Xu, L.; Mendes, P.; Kummer, U. COPASI—A COmplex PAthway SImulator. Bioinformatics 2006, 22, 3067–3074. [Google Scholar] [CrossRef] [PubMed]
- Matzko, R.; Konur, S. Technologies for design-build-test-learn automation and computational modelling across the synthetic biology workflow: A review. Netw. Model. Anal. Health Inform. Bioinform. 2024, 13, 1–23. [Google Scholar] [CrossRef]
- Tiwari, K.; Kananathan, S.; Roberts, M.G.; Meyer, J.P.; Shohan, M.U.S.; Xavier, A.; Maire, M.; Zyoud, A.; Men, J.; Ng, S.; et al. Reproducibility in systems biology modelling. Mol. Syst. Biol. 2021, 17, e9982. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Antunes, F.; Salvador, A. Tools for kinetic modeling of biochemical networks. Nat. Biotechnol. 2006, 24, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Buecherl, L.; Myers, C.J. Engineering genetic circuits: Advancements in genetic design automation tools and standards for synthetic biology. Curr. Opin. Microbiol. 2022, 68, 102155. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, D.; Ninfa, A.J.; Sontag, E.D. Modular cell biology: Retroactivity and insulation. Mol. Syst. Biol. 2008, 4, 161. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.J.; Barker, N.; Jones, K.; Kuwahara, H.; Madsen, C.; Nguyen, N.-P.D. iBioSim: A tool for the analysis and design of genetic circuits. Bioinformatics 2009, 25, 2848–2849. [Google Scholar] [CrossRef] [PubMed]
- Hucka, M.; Finney, A.; Sauro, H.M.; Bolouri, H.; Doyle, J.C.; Kitano, H.; Arkin, A.P.; Bornstein, B.J.; Bray, D.; Cornish-Bowden, A. The systems biology markup language (SBML): A medium for representation and exchange of biochemical network models. Bioinformatics 2003, 19, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Roehner, N.; Beal, J.; Clancy, K.; Bartley, B.; Misirli, G.; Grünberg, R.; Oberortner, E.; Pocock, M.; Bissell, M.; Madsen, C.; et al. Sharing Structure and Function in Biological Design with SBOL 2.0. ACS Synth. Biol. 2016, 5, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Young, L.; Chuang, R.-Y.; Venter, J.C.; Hutchison, C.A., III; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Knight, T. Idempotent Vector Design for Standard Assembly of Biobricks; MIT Artificial Intelligence Laboratory: Cambridge, MA, USA, 2003. [Google Scholar]
- Shetty, R.; Lizarazo, M.; Rettberg, R.; Knight, T.F. Assembly of BioBrick Standard Biological Parts Using Three Antibiotic Assembly. Methods Enzymol. 2011, 498, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Storch, M.; Baldwin, G.S.; Ellis, T. Bricks and blueprints: Methods and standards for DNA assembly. Nat. Rev. Mol. Cell Biol. 2015, 16, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, A.P.; Tabatabaei, S.K.; Lee, Y.; Lund, S.; Lohman, G.J.S. High-Complexity One-Pot Golden Gate Assembly. Curr. Protoc. 2023, 3, e882. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Engler, C.; Gruetzner, R.; Werner, S.; Marillonnet, S.; Peccoud, J. A Modular Cloning System for Standardized Assembly of Multigene Constructs. PLoS ONE 2011, 6, e16765. [Google Scholar] [CrossRef] [PubMed]
- Avilan, L. Assembling Multiple Fragments: The Gibson Assembly. In DNA Manipulation and Analysis; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2023; Volume 2633, pp. 45–53. [Google Scholar] [CrossRef]
- Li, M.Z.; Elledge, S.J. SLIC: A Method for Sequence-and Ligation-Independent Cloning. In Gene Synthesis; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2012; Volume 852. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Yim, H.-S.; Ryu, J.-Y.; Lee, H.S.; Lee, J.-H.; Seen, D.-S.; Kang, S.G. One-Step Sequence- and Ligation-Independent Cloning as a Rapid and Versatile Cloning Method for Functional Genomics Studies. Appl. Environ. Microbiol. 2012, 78, 5440–5443. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.M.; Fu, X.; Zhu, J.; Katrekar, D.; Shih, Y.-R.V.; Marlett, J.; Cabotaje, J.; Tat, J.; Naughton, J.; Lisowski, L.; et al. In Situ Gene Therapy via AAV-CRISPR-Cas9-Mediated Targeted Gene Regulation. Mol. Ther. 2018, 26, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Damage, G.; Centre, S.; O’DRiscoll, M.; Jeggo, P.A. The role of double-strand break repair—Insights from human genetics. Nat. Rev. Genet. 2006, 7, 45–54. [Google Scholar] [CrossRef]
- Lienert, F.; Lohmueller, J.J.; Garg, A.; Silver, P.A. Synthetic biology in mammalian cells: Next generation research tools and therapeutics. Nat. Rev. Mol. Cell Biol. 2014, 15, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Ruder, W.C.; Lu, T.; Collins, J.J. Synthetic Biology Moving into the Clinic. Science 2011, 333, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, C.; Bates, D. Feedback Control in Systems Biology; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–278. [Google Scholar] [CrossRef]
- Tewary, M.; Shakiba, N.; Zandstra, P.W. Stem cell bioengineering: Building from stem cell biology. Nat. Rev. Genet. 2018, 19, 595–614. [Google Scholar] [CrossRef] [PubMed]
- Becskei, A.; Serrano, L. Engineering stability in gene networks by autoregulation. Nature 2000, 405, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Balázsi, G.; van Oudenaarden, A.; Collins, J.J. Cellular Decision Making and Biological Noise: From Microbes to Mammals. Cell 2011, 144, 910–925. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.; Gibbs, C.; Shimpi, A.A.; Deans, T.L. Adoption of the Q Transcriptional System for Regulating Gene Expression in Stem Cells. ACS Synth. Biol. 2017, 6, 2014–2020. [Google Scholar] [CrossRef] [PubMed]
- Kemmer, C.; Gitzinger, M.; Baba, M.D.-E.; Djonov, V.; Stelling, J.; Fussenegger, M. Self-sufficient control of urate homeostasis in mice by a synthetic circuit. Nat. Biotechnol. 2010, 28, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Busskamp, V.; Lewis, N.E.; Guye, P.; Ng, A.H.; Shipman, S.L.; Byrne, S.M.; E Sanjana, N.; Murn, J.; Li, Y.; Li, S.; et al. Rapid neurogenesis through transcriptional activation in human stem cells. Mol. Syst. Biol. 2014, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Guye, P.; Ebrahimkhani, M.R.; Kipniss, N.; Velazquez, J.J.; Schoenfeld, E.; Kiani, S.; Griffith, L.G.; Weiss, R. Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6. Nat. Commun. 2016, 7, 10243. [Google Scholar] [CrossRef] [PubMed]
- Ran, D.; Shia, W.-J.; Lo, M.-C.; Fan, J.-B.; Knorr, D.A.; Ferrell, P.I.; Ye, Z.; Yan, M.; Cheng, L.; Kaufman, D.S.; et al. RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood 2013, 121, 2882–2890. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, M.; Ortmann, D.; Bertero, A.; Tavares, J.M.; Pedersen, R.A.; Vallier, L.; Kotter, M.R. Inducible and Deterministic Forward Programming of Human Pluripotent Stem Cells into Neurons, Skeletal Myocytes, and Oligodendrocytes. Stem Cell Rep. 2017, 8, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Trentesaux, C.; Yamada, T.; Klein, O.D.; Lim, W.A. Harnessing synthetic biology to engineer organoids and tissues. Cell Stem Cell 2023, 30, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, M.; Migueles, R.P.; Annoh, J.; Sadurska, D.; Blin, G.; Lowell, S. SyNPL: Synthetic Notch pluripotent cell lines to monitor and manipulate cell interactions in vitro and in vivo. Development 2022, 149, dev200226. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.G.; Lee, H.J.; Asatsuma, T.; Vento-Tormo, R.; Haque, A. An introduction to spatial transcriptomics for biomedical research. Genome Med. 2022, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Graf, T.; Enver, T. Forcing cells to change lineages. Nature 2009, 462, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Südhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.B.; Beitz, A.M.; Galloway, K. Engineering cell fate: Applying synthetic biology to cellular reprogramming. Curr. Opin. Syst. Biol. 2020, 24, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Balboa, D.; Weltner, J.; Eurola, S.; Trokovic, R.; Wartiovaara, K.; Otonkoski, T. Conditionally Stabilized dCas9 Activator for Controlling Gene Expression in Human Cell Reprogramming and Differentiation. Stem Cell Rep. 2015, 5, 448–459. [Google Scholar] [CrossRef]
- Liu, P.; Chen, M.; Liu, Y.; Qi, L.S.; Ding, S. CRISPR-Based Chromatin Remodeling of the Endogenous Oct4 or Sox2 Locus Enables Reprogramming to Pluripotency. Cell Stem Cell 2018, 22, 252–261.e4. [Google Scholar] [CrossRef] [PubMed]
- Chavez, A.; Tuttle, M.; Pruitt, B.W.; Ewen-Campen, B.; Chari, R.; Ter-Ovanesyan, D.; Haque, S.J.; Cecchi, R.J.; Kowal, E.J.K.; Buchthal, J.; et al. Comparison of Cas9 activators in multiple species. Nat. Methods 2016, 13, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ji, H.; Kabadi, A.M.; Gersbach, C.A.; Christoforou, N.; Leong, K.W. A CRISPR/Cas9-Based System for Reprogramming Cell Lineage Specification. Stem Cell Rep. 2014, 3, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, X.; Zhao, L.; Zuo, S.; Chen, X.; Zhang, L.; Lin, Z.; Zhao, X.; Qin, Y.; Zhou, X.; et al. Lineage reprogramming of fibroblasts into induced cardiac progenitor cells by CRISPR/Cas9-based transcriptional activators. Acta Pharm. Sin. B 2020, 10, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Babos, K.N.; Galloway, K.E.; Kisler, K.; Zitting, M.; Li, Y.; Shi, Y.; Quintino, B.; Chow, R.H.; Zlokovic, B.V.; Ichida, J.K. Mitigating Antagonism between Transcription and Proliferation Allows Near-Deterministic Cellular Reprogramming. Cell Stem Cell 2019, 25, 486–500.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Londono, P.; Cao, Y.; Sharpe, E.J.; Proenza, C.; O’rOurke, R.; Jones, K.L.; Jeong, M.Y.; Walker, L.A.; Buttrick, P.M.; et al. High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling. Nat. Commun. 2015, 6, 8243. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chang, Y.; Sun, P.; Li, H.; Guo, Z. Inhibition of profibrotic signalling enhances the 5-azacytidine-induced reprogramming of fibroblasts into cardiomyocytes. Int. J. Biochem. Cell Biol. 2020, 122, 105733. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.E.; Amlani, B.; Chen, T.; Tsirigos, A.; Stadtfeld, M. Combinatorial Modulation of Signaling Pathways Reveals Cell-Type-Specific Requirements for Highly Efficient and Synchronous iPSC Reprogramming. Stem Cell Rep. 2014, 3, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Suzuki, H.I. TGF-β Signaling in Cellular Senescence and Aging-Related Pathology. Int. J. Mol. Sci. 2019, 20, 5002. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, N.; Nara, K.; Tamura, F.; Kojima, H.; Yamakawa, H.; Sadahiro, T.; Miyamoto, K.; Isomi, M.; Haginiwa, S.; Tani, H.; et al. Role of cyclooxygenase-2-mediated prostaglandin E2-prostaglandin E receptor 4 signaling in cardiac reprogramming. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Weltner, J.; Balboa, D.; Katayama, S.; Bespalov, M.; Krjutškov, K.; Jouhilahti, E.-M.; Trokovic, R.; Kere, J.; Otonkoski, T. Human pluripotent reprogramming with CRISPR activators. Nat. Commun. 2018, 9, 2643. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, F.W.; Millman, J.R.; Gürtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of Functional Human Pancreatic β Cells In Vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Brown, J.; Kanarek, A.; Rajagopal, J.; Melton, D.A. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 2008, 455, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Ariyachet, C.; Tovaglieri, A.; Xiang, G.; Lu, J.; Shah, M.S.; Richmond, C.A.; Verbeke, C.; Melton, D.A.; Stanger, B.Z.; Mooney, D.; et al. Reprogrammed Stomach Tissue as a Renewable Source of Functional β Cells for Blood Glucose Regulation. Cell Stem Cell 2016, 18, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Heng, B.C.; Bai, P.; Folcher, M.; Zulewski, H.; Fussenegger, M. A programmable synthetic lineage-control network that differentiates human IPSCs into glucose-sensitive insulin-secreting beta-like cells. Nat. Commun. 2016, 7, 11247. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.J.C.; Katayama, S.; Miki, K.; Kuang, Y.; Yoshida, Y.; Morizane, A.; Takahashi, J.; Yamanaka, S.; Saito, H. MicroRNA-302 switch to identify and eliminate undifferentiated human pluripotent stem cells. Sci. Rep. 2016, 6, 32532. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Gersbach, C.A.; Le Doux, J.M.; Guldberg, R.E.; García, A.J. Inducible regulation of Runx2-stimulated osteogenesis. Gene Ther. 2006, 13, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Baron, U.; Bujard, H. Tet repressor-based system for regulated gene expression in eukaryotic cells: Principles and advances. Methods Enzymol. 2000, 327, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Denduluri, S.; Zhang, B.; Wang, Z.; Yin, L.; Yan, Z.; Kang, R.; Shi, L.L.; Mok, J.; Lee, M.J.; et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2014, 1, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Glass, K.A.; Link, J.M.; Brunger, J.M.; Moutos, F.T.; Gersbach, C.A.; Guilak, F. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 2014, 35, 5921–5931. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Morsut, L. Novel synthetic biology approaches for developmental systems. Stem Cell Rep. 2021, 16, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Elcheva, I.; Brok-Volchanskaya, V.; Kumar, A.; Liu, P.; Lee, J.-H.; Tong, L.; Vodyanik, M.; Swanson, S.; Stewart, R.; Kyba, M.; et al. Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lange, L.; Hoffmann, D.; Schwarzer, A.; Ha, T.-C.; Philipp, F.; Lenz, D.; Morgan, M.; Schambach, A. Inducible Forward Programming of Human Pluripotent Stem Cells to Hemato-endothelial Progenitor Cells with Hematopoietic Progenitor Potential. Stem Cell Rep. 2020, 14, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, D.; Yu, Y.Y.L.; Kang, I.; Cha, M.-J.; Kim, J.Y.; Park, C.; Watson, D.K.; Wang, T.; Choi, K. Induction of hematopoietic and endothelial cell program orchestrated by ETS transcription factor ER 71/ETV. EMBO Rep. 2015, 16, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Veldman, M.B.; Zhao, C.; Gomez, G.A.; Lindgren, A.G.; Huang, H.; Yang, H.; Yao, S.; Martin, B.L.; Kimelman, D.; Lin, S.; et al. Transdifferentiation of Fast Skeletal Muscle Into Functional Endothelium in Vivo by Transcription Factor Etv2. PLoS Biol. 2013, 11, e1001590. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.-J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.-S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Bacchus, W.; Lang, M.; El-Baba, M.D.; Weber, W.; Stelling, J.; Fussenegger, M. Synthetic two-way communication between mammalian cells. Nat. Biotechnol. 2012, 30, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Lian, X.; Hacker, T.A.; Schmuck, E.G.; Qian, T.; Bhute, V.J.; Han, T.; Shi, M.; Drowley, L.; Plowright, A.T.; et al. Long-term self-renewing human epicardial cells generated from pluripotent stem cells under defined xeno-free conditions. Nat. Biomed. Eng. 2016, 1, 0003. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Weissman, I.L. Stem Cells. Cell 2000, 100, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. Mammalian Neural Stem Cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Mobini, G.-R.; Abiri, A.; Shojaeian, A. Synthetic biology in various cellular and molecular fields: Applications, limitations, and perspective. Mol. Biol. Rep. 2020, 47, 6207–6216. [Google Scholar] [CrossRef] [PubMed]
- Trump, B.D.; Cegan, J.C.; Wells, E.; Keisler, J.; Linkov, I. A critical juncture for synthetic biology: Lessons from nanotechnology could inform public discourse and further development of synthetic biology. EMBO Rep. 2018, 19, e46153. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, B.; Carr, P.A. The limits of synthetic biology. Trends Biotechnol. 2015, 33, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yan, Q.; Jones, J.A.; Tang, Y.J.; Fong, S.S.; Koffas, M.A. Metabolic Burden: Cornerstones in Synthetic Biology and Metabolic Engineering Applications. Trends Biotechnol. 2016, 34, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M. Evolution of the mutation rate. Trends Genet. 2010, 26, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F., III. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2014, 31, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Serrano, L. Synthetic biology: Promises and challenges. Mol. Syst. Biol. 2007, 3, 158. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, W. Synthetic biology: Recent progress, biosafety and biosecurity concerns, and possible solutions. J. Biosaf. Biosecurity 2019, 1, 22–30. [Google Scholar] [CrossRef]
- Ehni, H.-J. Dual use and the ethical responsibility of scientists. Arch. Immunol. Ther. Exp. 2008, 56, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Kuhlau, F.; Eriksson, S.; Evers, K.; HÖglund, A.T. Taking due care: Moral obligations in dual use research. Bioethics 2008, 22, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, B.D. Issues in biosecurity and biosafety. Int. J. Antimicrob. Agents 2010, 36, S66–S69. [Google Scholar] [CrossRef] [PubMed]
- Gyngell, C.; Douglas, T.; Savulescu, J. The Ethics of Germline Gene Editing. J. Appl. Philos. 2016, 34, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Ronen, D.; Benvenisty, N. Genomic stability in reprogramming. Curr. Opin. Genet. Dev. 2012, 22, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Keller, G. Differentiation of Embryonic Stem Cells to Clinically Relevant Populations: Lessons from Embryonic Development. Cell 2008, 132, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Liras, A. Future research and therapeutic applications of human stem cells: General, regulatory, and bioethical aspects. J. Transl. Med. 2010, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Brons, I.G.M.; Smithers, L.E.; Trotter, M.W.B.; Rugg-Gunn, P.; Sun, B.; de Sousa Lopes, S.M.C.; Howlett, S.K.; Clarkson, A.; Ahrlund-Richter, L.; Pedersen, R.A.; et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nat. Cell Biol. 2007, 448, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Charron, D.; Suberbielle-Boissel, C.; Al-Daccak, R. Immunogenicity and Allogenicity: A Challenge of Stem Cell Therapy. J. Cardiovasc. Transl. Res. 2008, 2, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Gloor, J.; Cosio, F.; Lager, D.J.; Stegall, M.D.; Stegall, M.D. The Spectrum of Antibody-Mediated Renal Allograft Injury: Implications for Treatment. Am. J. Transplant. 2008, 8, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Pearl, J.I.; Lee, A.S.; Leveson-Gower, D.B.; Sun, N.; Ghosh, Z.; Lan, F.; Ransohoff, J.; Negrin, R.S.; Davis, M.M.; Wu, J.C. Short-Term Immunosuppression Promotes Engraftment of Embryonic and Induced Pluripotent Stem Cells. Cell Stem Cell 2011, 8, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Dunnill, P. A Brief Definition of Regenerative Medicine. Regen. Med. 2007, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Goshisht, M.K. Machine Learning and Deep Learning in Synthetic Biology: Key Architectures, Applications, and Challenges. ACS Omega 2024, 9, 9921–9945. [Google Scholar] [CrossRef] [PubMed]
- Beardall, W.A.; Stan, G.-B.; Dunlop, M.J. Deep Learning Concepts and Applications for Synthetic Biology. GEN Biotechnol. 2022, 1, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Ellis, T. The second decade of synthetic biology: 2010–2020. Nat. Commun. 2020, 11, 5174. [Google Scholar] [CrossRef] [PubMed]
- Angermueller, C.; Pärnamaa, T.; Parts, L.; Stegle, O. Deep learning for computational biology. Mol. Syst. Biol. 2016, 12, 878. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef] [PubMed]
- King, R.D.; Rowland, J.; Oliver, S.G.; Young, M.; Aubrey, W.; Byrne, E.; Liakata, M.; Markham, M.; Pir, P.; Soldatova, L.N.; et al. The Automation of Science. Science 2009, 324, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, P.; Radivojevic, T.; García Martín, H. Opportunities at the Intersection of Synthetic Biology, Machine Learning, and Automation. ACS Synth. Biol. 2019, 8, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.A.; June, C.H. The Principles of Engineering Immune Cells to Treat Cancer. Cell 2017, 168, 724–740. [Google Scholar] [CrossRef] [PubMed]

| A. Nucleic Acid Design and Assembly Tools | |||
| Tool | Description | Tool Link | Reference |
| Gene Designer | A tool to design synthetic DNA sequences. | https://www.atum.bio/gene-designer/, accessed on 10 June 2025 | [76] |
| Benchling | Design and analyze DNA, RNA, and amino acid sequences using smart software. | https://www.benchling.com/molecular-biology, accessed on 10 June 2025 | [77] |
| SnapGene | Software for DNA editing, cloning simulation, sequence alignment, and plasmid visualization. | https://www.snapgene.com/, accessed on 10 June 2025 | [78] |
| GeneArt | It is a reliable and cost-effective method for obtaining custom DNA constructs with 100% sequence accuracy. | https://www.thermofisher.com/eg/en/home/life-science/cloning/gene-synthesis/geneart-gene-synthesis.html, accessed on 10 June 2025 | [79] |
| DNAChisel | A Python library that optimizes DNA sequences based on a set of restrictions and objectives. Version 3.2.6. | https://pypi.org/project/dnachisel/, accessed on 10 June 2025 | [80] |
| IDT Codon Optimization Tool | Transfers the protein sequence, or DNA, from one organism to another for expression. Through sequence screening and filtering to reduce complexity and minimize secondary structures, the IDT algorithm offers the optimal sequence alternative. | https://eu.idtdna.com/pages/tools/codon-optimization-tool, accessed on 10 June 2025 | [81] |
| jCat | A method to modify a target gene’s codon use according to its possible expression host. | https://www.jcat.de/, accessed on 10 June 2025 | [82] |
| B. Standardization and Abstraction | |||
| Tool | Description | Tool Link | Reference |
| SBOL | Describes and shares details about synthetic biology parts, devices, and systems. Its companion, SBOL Visual, provides a clear set of symbols and guidelines for drawing genetic circuits, making complex designs easier to understand. | https://sbolstandard.org/, accessed on 10 June 2025 | [83] |
| SBML | An XML-based format was created to help computers interpret models of biological processes. | https://sbml.org/, accessed on 10 June 2025 | [84] |
| C. Biological Parts and Repositories | |||
| Tool | Description | Tool Link | Reference |
| Registry of Standard Biological Parts | An online catalog of devices, systems, and DNA parts, each of which is configured as a BioBrick, allowing for standardized assembly. | https://parts.igem.org/, accessed on 10 June 2025 | [85] |
| SynBioHub | A design repository provides computational access for software and data integration and a graphical user interface that enables users to browse, upload, and share synthetic biology designs. | https://synbiohub.org/, accessed on 10 June 2025 | [86] |
| JBEI-ICEs | An open-source platform for managing biological part data, including plasmids and DNA parts in various assembly standards. | https://public-registry.jbei.org/, accessed on 10 June 2025 | [87] |
| RDBSB | A comprehensive resource containing experimentally validated catalytic bioparts. It offers detailed qualitative and quantitative catalytic information, including activities, substrates, optimal pH and temperature, and chassis specificity. | https://www.biosino.org/rdbsb/, accessed on 10 June 2025 | [88] |
| DNASU | A central repository for high-quality plasmid clones and online plasmid resources. | https://dnasu.org/, accessed on 10 June 2025 | [89] |
| D. Promoter and Regulatory Design | |||
| Tool | Description | Tool Link | Reference |
| PromoterCAD | An online tool to develop synthetic promoters with modified transcriptional regulation. | http://promotercad.org, accessed on 10 June 2025 | [90] |
| PRODORIC | One of the biggest databases of prokaryotic transcription factor binding sites from various bacterial sources, it offers tools for interpretation and visualization. | https://www.prodoric.de/, accessed on 10 June 2025 | [91] |
| CRISPR-ERA | A guide RNA design tool for wide-ranging CRISPR applications in gene repression, activation, and genome editing. | http://crispr-era.stanford.edu/, accessed on 10 June 2025 | [92] |
| E. Riboswitches and RNA Devices | |||
| Tool | Description | Tool Link | Reference |
| NUPACK | A software suite for designing and analyzing nucleic acid systems, devices, and structures. | https://www.nupack.org/, accessed on 10 June 2025 | [93] |
| ViennaRNA | A collection of stand-alone applications and libraries for secondary structure analysis and prediction of RNA nucleic acids. | http://rna.tbi.univie.ac.at/, accessed on 10 June 2025 | [94] |
| Riboswitch Calculator | A statistical thermodynamic model that predicts the role of riboswitches that regulate translation. | https://salislab.net/software/, accessed on 10 June 2025 | [95] |
| RiboLogic | An algorithm for creating RNA molecule sequences that adopt specific secondary structures. | https://github.com/wuami/RiboLogic, accessed on 10 June 2025 | [96] |
| EternaBot | An algorithm for determining an RNA sequence for a specific base pairing arrangement or secondary structure. | http://eternabot.org/, accessed on 10 June 2025 | [97] |
| F. Toehold Switches | |||
| Tool | Description | Tool Link | Reference |
| Toehold Switch Web Tool | A web tool for designing toehold switches while also predicting the efficacy of designed toehold switches. | https://yiplab.cse.cuhk.edu.hk/toehold/, accessed on 10 June 2025 | [98] |
| Toeholder | Open-source software for automated design and in silico comparison of toehold riboswitches. | https://github.com/igem-ulaval/toeholder, accessed on 10 June 2025 | [99] |
| G. Synthetic Transcription Factor Design | |||
| Tool | Description | Tool Link | Reference |
| Alphafold | An AI-based tool developed by DeepMind to predict 3D protein structures from amino acid sequences with near-experimental accuracy. | https://deepmind.google/science/alphafold/, accessed on 10 June 2025 | [100] |
| Rosetta | A versatile molecular modeling software for 3D structure prediction and high-resolution design of proteins, nucleic acids, and synthetic polymers. | https://rosettacommons.org/software/, accessed on 10 June 2025 | [101] |
| ZincFingerTools | Tools used for identifying target sites and designing custom zinc finger proteins (ZFPs). | http://www.zincfingers.org/, accessed on 10 June 2025 | [102] |
| E-TALEN | A web-based tool for designing TALENs targeting single or multiple genes across various experimental scales. | http://www.e-talen.org/, accessed on 10 June 2025 | [103] |
| TFinder | A Python-based web tool for identifying transcription factor binding sites and regulatory motifs. It extracts promoter or gene terminal regulatory regions using NCBI APIs and searches for Individual Motifs in different formats. It also provides binding scores and p-values. | https://tfinder-ipmc.streamlit.app/, accessed on 10 June 2025 | [104] |
| TRANSFAC | A database of transcription factors, their binding sites, and DNA binding specificity models like PWMs and DBDs. | https://genexplain.com/transfac-product/, accessed on 10 June 2025 | [105] |
| JASPAR | An open-access database of matrix models representing transcription factor and DNA binding motif preferences. | https://jaspar.elixir.no/, accessed on 10 June 2025 | [106] |
| H. Ribosome Binding Site (RBS) Design | |||
| Tool | Description | Tool Link | Reference |
| RBS Calculator | A design tool for predicting and managing bacterial translation initiation and protein expression. The tool might potentially optimize a synthetic RBS sequence to achieve the desired translation initiation. | https://salislab.net/software/predict_rbs_calculator, accessed on 10 June 2025 | [107] |
| RBSDesigner | Predicts translation efficiency and designs a synthetic RBS. | http://ssbio.cau.ac.kr/web/?page_id=195, accessed on 10 June 2025 | [108] |
| UTR Designer | A prediction tool for designing sequences around the TIR and predicting translation efficiency for the logical regulation of protein synthesis. | https://sbi.postech.ac.kr/utr_designer/, accessed on 10 June 2025 | [109] |
| EMOPEC | A tool used to modulate protein expression in E. coli species. | http://emopec.biosustain.dtu.dk, accessed on 10 June 2025 | [110] |
| SalisLab RBS Library Calculator | It finds the smallest degenerate RBS sequence with the highest search coverage for an input coding sequence by combining a genetic algorithm and a biophysical model of translation. | https://salislab.net/software/design_rbs_library_calculator, accessed on 10 June 2025 | [111] |
| I. Genetic Circuits Design | |||
| Tool | Description | Tool Link | Reference |
| Cello 2.0 | Automated design of logic genetic circuits using biological parts into DNA sequences. | https://www.cellocad.org/, accessed on 10 June 2025 | [112] |
| iBioSim | Enables circuit design, synthesis, and analysis through a model-based strategy. | https://async.ece.utah.edu/tools/ibiosim/, accessed on 10 June 2025 | [113] |
| SynBioSS | Modeling and simulation of gene regulatory networks and biological systems. | http://www.synbioss.org/, accessed on 10 June 2025 | [114] |
| TinkerCell | A visual modeling tool for constructing plasmids, artificial gene networks, and other synthetic genetic systems composed of standard genetic parts. | https://www.tinkercell.com/, accessed on 10 June 2025 | [115] |
| GenoCAD | Computer-aided design software for synthetic biology is used to design genetic parts and constructs. | http://www.genocad.org/, accessed on 10 June 2025 | [116] |
| LogicGate Designer | A software tool for designing, simulating, and testing digital logic circuits using logic gates. | https://logic.ly/, accessed on 10 June 2025 | [117] |
| TASBE | A toolchain to accelerate synthetic biological engineering, which allows researchers to incorporate their own design tools. | https://tasbe.github.io/, accessed on 10 June 2025 | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elnaggar, K.S.; Gamal, O.; Hesham, N.; Ayman, S.; Mohamed, N.; Moataz, A.; Elzayat, E.M.; Hassan, N. A Guide in Synthetic Biology: Designing Genetic Circuits and Their Applications in Stem Cells. SynBio 2025, 3, 11. https://doi.org/10.3390/synbio3030011
Elnaggar KS, Gamal O, Hesham N, Ayman S, Mohamed N, Moataz A, Elzayat EM, Hassan N. A Guide in Synthetic Biology: Designing Genetic Circuits and Their Applications in Stem Cells. SynBio. 2025; 3(3):11. https://doi.org/10.3390/synbio3030011
Chicago/Turabian StyleElnaggar, Karim S., Ola Gamal, Nouran Hesham, Sama Ayman, Nouran Mohamed, Ali Moataz, Emad M. Elzayat, and Nourhan Hassan. 2025. "A Guide in Synthetic Biology: Designing Genetic Circuits and Their Applications in Stem Cells" SynBio 3, no. 3: 11. https://doi.org/10.3390/synbio3030011
APA StyleElnaggar, K. S., Gamal, O., Hesham, N., Ayman, S., Mohamed, N., Moataz, A., Elzayat, E. M., & Hassan, N. (2025). A Guide in Synthetic Biology: Designing Genetic Circuits and Their Applications in Stem Cells. SynBio, 3(3), 11. https://doi.org/10.3390/synbio3030011







