Synthetic Proteins in Dental Applications
Abstract
:1. Introduction
2. Bacterial Biofilm Inhibition
3. Enamel Remineralization
4. Stimulation of the Dentin-Pulp Complex
5. Bone Regeneration
6. Challenges and Limitations
7. Ethical Considerations and Regulatory Aspects
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patil, S.; Albogami, S.; Hosmani, J.; Mujoo, S.; Kamil, M.A.; Mansour, M.A.; Abdul, H.N.; Bhandi, S.; Ahmed, S.S.S.J. Artificial Intelligence in the Diagnosis of Oral Diseases: Applications and Pitfalls. Diagnostics 2022, 12, 1029. [Google Scholar] [CrossRef] [PubMed]
- Singer, L.; Fouda, A.; Bourauel, C. Biomimetic approaches and materials in restorative and regenerative dentistry: Review article. BMC Oral. Health 2023, 23, 105. [Google Scholar] [CrossRef]
- Kumar, N.; Maher, N.; Amin, F.; Ghabbani, H.; Zafar, M.S.; Rodríguez-Lozano, F.J.; Oñate-Sánchez, R.E. Biomimetic Approaches in Clinical Endodontics. Biomimetics 2022, 7, 229. [Google Scholar] [CrossRef] [PubMed]
- Jafari, N.; Habashi, M.S.; Hashemi, A.; Shirazi, R.; Tanideh, N.; Tamadon, A. Application of bioactive glasses in various dental fields. Biomater. Res. 2022, 26, 31. [Google Scholar] [CrossRef]
- Hanczyc, M.M. Engineering Life: A Review of Synthetic Biology. Artif. Life 2020, 26, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Stein, V.; Alexandrov, K. Synthetic protein switches: Design principles and applications. Trends Biotechnol. 2015, 33, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Khoury, G.A.; Smadbeck, J.; Kieslich, C.A.; Floudas, C.A. Protein folding and de novo protein design for biotechnological applications. Trends Biotechnol. 2014, 32, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, Y.; Bi, J.; Huang, Y.; Cheng, Y.; Li, Y.; Wu, Y.; Cao, G.; Tian, Z. The use of supercytokines, immunocytokines, engager cytokines, and other synthetic cytokines in immunotherapy. Cell. Mol. Immunol. 2022, 19, 192–209. [Google Scholar] [CrossRef]
- Samiei, M.; Alipour, M.; Khezri, K.; Saadat, Y.R.; Forouhandeh, H.; Abdolahinia, E.D.; Vahed, S.Z.; Sharifi, S.; Dizaj, S.M. Application of collagen and mesenchymal stem cells in regenerative dentistry. Curr. Stem. Cell Res. Ther. 2022, 17, 606–620. [Google Scholar] [CrossRef]
- Tavelli, L.; Chen, C.Y.; Barootchi, S.; Kim, D.M. Efficacy of biologics for the treatment of periodontal infrabony defects: An American Academy of Periodontology best evidence systematic review and network meta-analysis. J. Periodontol. 2022, 93, 1803–1826. [Google Scholar] [CrossRef]
- Firipis, K.; Nisbet, D.R.; Franks, S.J.; Kapsa, R.M.I.; Pirogova, E.; Williams, R.J.; Quigley, A. Enhancing Peptide Biomaterials for Biofabrication. Polymers 2021, 13, 2590. [Google Scholar] [CrossRef]
- Wang, Y.; Katyal, P.; Montclare, J.K. Protein-engineered functional materials. Adv. Healthc. Mater. 2019, 8, 1801374. [Google Scholar] [CrossRef]
- Takahara, M.; Kamiya, N. Synthetic strategies for artificial lipidation of functional proteins. Chemistry 2020, 26, 4645–4655. [Google Scholar] [CrossRef]
- Ahn, W.; Lee, J.H.; Kim, S.R.; Lee, J.; Lee, E.J. Designed protein-and peptide-based hydrogels for biomedical sciences. J. Mater. Chem. B 2021, 9, 1919–1940. [Google Scholar] [CrossRef]
- Wise, S.G.; Mithieux, S.M.; Weiss, A.S. Engineered tropoelastin and elastin-based biomaterials. Adv. Protein Chem. Struct. Biol. 2009, 78, 1–24. [Google Scholar] [CrossRef]
- Fang, D.; Yuran, S.; Reches, M.; Catunda, R.; Levin, L.; Febbraio, M. A peptide coating preventing the attachment of Porphyromonas gingivalis on the surfaces of dental implants. J. Periodontal. Res. 2020, 55, 503–510. [Google Scholar] [CrossRef]
- Babaji, P.; Melkundi, M.; Bhagwat, P.; Mehta, V. An in vitro Evaluation of remineralizing capacity of self-assembling peptide (SAP) P11-4 and casein phosphopeptides-amorphous calcium phosphate (CPP-ACP) on artificial enamel. Pesqui. Bras. Odontopediatria Clínica Integr. 2019, 19. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.C.; Liu, S.; Wu, R.F.; Aparicio, C.; Wu, J.Y. In vivo osseointegration of dental implants with an antimicrobial peptide coating. J. Mater. Sci. Mater. Med. 2017, 28, 76. [Google Scholar] [CrossRef]
- Bagno, A.; Piovan, A.; Dettin, M.; Chiarion, A.; Brun, P.; Gambaretto, R.; Fontana, G.; Di Bello, C.; Palù, G.; Castagliuolo, I. Human osteoblast-like cell adhesion on titanium substrates covalently functionalized with synthetic peptides. Bone 2007, 40, 693–699. [Google Scholar] [CrossRef]
- Dettin, M.; Conconi, M.T.; Gambaretto, R.; Pasquato, A.; Folin, M.; Di Bello, C.; Parnigotto, P.P. Novel osteoblast-adhesive peptides for dental/orthopedic biomaterials. J. Biomed. Mater. Res. 2002, 60, 466–471. [Google Scholar] [CrossRef]
- Pitts, N.B.; Twetman, S.; Fisher, J.; Marsh, P.D. Understanding dental caries as a non-communicable disease. Br. Dent. J. 2021, 231, 749–753. [Google Scholar] [CrossRef]
- Mathur, V.P.; Dhillon, J.K. Dental caries: A disease which needs attention. Indian J. Pediatr. 2018, 85, 202–206. [Google Scholar] [CrossRef]
- Niu, J.Y.; Yin, I.X.; Wu, W.K.K.; Li, Q.L.; Mei, M.L.; Chu, C.H. Antimicrobial peptides for the prevention and treatment of dental caries: A concise review. Arch. Oral Biol. 2021, 122, 105022. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmana, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus mutans: Dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS ONE 2012, 7, e47722. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, L.; Haapasalo, M.; Wei, W.; Zhang, D.; Ma, J.; Shen, Y. A novel hydroxyapatite-binding antimicrobial peptide against oral biofilms. Clin. Oral Investig. 2019, 23, 2705–2712. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Goodman, S.D.; Mashburn-Warren, L.; Stafford, G.P.; Cieplik, F. The dental plaque biofilm matrix. Periodontology 2000 2021, 86, 32–56. [Google Scholar] [CrossRef]
- Da Silva, B.R.; Conrado, A.J.S.; Pereira, A.L.; Evaristo, F.F.V.; Arruda, F.V.S.; Vasconcelos, M.A.; Lorenzón, E.N.; Cilli, E.M.; Teixeira, E.H. Antibacterial activity of a novel antimicrobial peptide [W7] KR12-KAEK derived from KR-12 against Streptococcus mutans planktonic cells and biofilms. Biofouling 2017, 33, 835–846. [Google Scholar] [CrossRef]
- Da Silva, B.R.; De Freitas, V.A.A.; Carneiro, V.A.; Arruda, F.V.S.; Lorenzón, E.N.; De Aguiar, A.S.W.; Cilli, E.M.; Cavada, E.H.; Teixeira, E.H. Antimicrobial activity of the synthetic peptide Lys-a1 against oral streptococci. Peptides 2013, 42, 78–83. [Google Scholar] [CrossRef]
- He, J.; Yarbrough, D.K.; Kreth, J.; Anderson, M.H.; Shi, W.; Eckert, R. Systematic approach to optimizing specifically targeted antimicrobial peptides against Streptococcus mutans. Antimicrob. Agents Chemother. 2010, 54, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Izadi, N.; Keikha, M.; Ghazvini, K.; Karbalaei, M. Oral antimicrobial peptides and new therapeutic strategies for plaque-mediated diseases. Gene Rep. 2020, 21, 100811. [Google Scholar] [CrossRef]
- Zhou, L.; Wong, H.M.; Zhang, Y.Y.; Li, Q.L. Constructing an antibiofouling and mineralizing bioactive tooth surface to protect against decay and promote self-healing. ACS Appl. Mater. Interfaces 2019, 12, 3021–3031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Fang, Z.H.; Li, Q.L.; Cao, C.Y. A tooth-binding antimicrobial peptide to prevent the formation of dental biofilm. J. Mater. Sci. Mater. Med. 2019, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, Y.; Zhou, Z.; Tu, H.; Ren, Q.; Wang, X.; Ding, L.; Zhou, X.; Zhang, L. De novo synthetic short antimicrobial peptides against cariogenic bacteria. Arch. Oral Biol. 2017, 80, 41–50. [Google Scholar] [CrossRef]
- Xu, C.; Yao, X.; Walker, M.P.; Wang, Y. Chemical/molecular structure of the dentin-enamel junction is dependent on the intratooth location. Calcif. Tissue Int. 2009, 84, 221–228. [Google Scholar] [CrossRef]
- Bartlett, J.D. Dental enamel development: Proteinases and their enamel matrix substrates. Int. Sch. Res. Not. 2013, 2013, 684607. [Google Scholar] [CrossRef]
- Farooq, I.; Bugshan, A. The role of salivary contents and modern technologies in the remineralization of dental enamel: A narrative review. F1000Research 2020, 9, 171. [Google Scholar] [CrossRef]
- Philip, N. State of the art enamel remineralization systems: The next frontier in caries management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef]
- Kastenbom, L.; Falsen, A.; Larsson, P.; Sunnegårdh-Grönberg, K.; Davidson, T. Costs and health-related quality of life in relation to caries. BMC Oral Health 2019, 19, 187. [Google Scholar] [CrossRef]
- Anand, S.; Rejula, F.; Sam, J.V.G.; Christaline, R.; Nair, M.G.; Dinakaran, S. Comparative evaluation of effect of nanohydroxyapatite and 8% arginine containing toothpastes in managing dentin hypersensitivity: Double blind randomized clinical trial. Acta. Med. 2017, 60, 114–119. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.T.; Moffa, E.B.; Crosara, K.T.B.; Xiao, Y.; de Oliveira, T.M.; Machado, M.A.D.A.M.; Siqueira, W.L. Acquired enamel pellicle engineered peptides: Effects on hydroxyapatite crystal growth. Sci. Rep. 2018, 8, 3766. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, D.K.; Hagerman, E.; Eckert, R.; He, J.; Choi, H.; Cao, N.; Le, K.; Hedger, J.; Qi, F.; Anderson, M.; et al. Specific binding and mineralization of calcified surfaces by small peptides. Calcif. Tissue Int. 2010, 86, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Bai, Q.; Wen, M.; Ma, D.; Lin, Y.; Chu, J. Recombinant amelogenin peptide TRAP promoting remineralization of early enamel caries: An in vitro study. Front. Physiol. 2023, 14, 1076265. [Google Scholar] [CrossRef] [PubMed]
- Marin, L.M.; Xiao, Y.; Cury, J.A.; Siqueira, W.L. Engineered salivary peptides reduce enamel demineralization provoked by cariogenic S. Mutans biofilm. Microorganisms 2022, 10, 742. [Google Scholar] [CrossRef]
- Li, Z.C.; Xi, Q.I.N.; Qian, R.E.N.; Die, H.U.; Tian, T.I.A.N.; Ting, H.E.; Li, W.; Zhang, L.L. Rational Design of β-sheet Peptides with Self-Assembly into Nanofibres on Remineralisation of Initial Caries Lesions. Chin. J. Dent. Res. 2020, 23, 131–141. [Google Scholar] [CrossRef]
- Marin, L.M.; Xiao, Y.; Cury, J.A.; Siqueira, W.L. Modulation of Streptococcus mutans Adherence to Hydroxyapatite by Engineered Salivary Peptides. Microorganisms 2022, 10, 223. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Litman, A.; Margolis, H.C.; Yamakoshi, Y.; Simmer, J.P. Biomimetic enamel regeneration mediated by leucine-rich amelogenin peptide. J. Dent. Res. 2017, 96, 524–530. [Google Scholar] [CrossRef]
- Ding, L.; Han, S.; Wang, K.; Zheng, S.; Zheng, W.; Peng, X.; Niu, Y.; Li, W.; Zhang, L. Remineralization of enamel caries by an amelogenin-derived peptide and fluoride in vitro. Regen. Biomater. 2020, 7, 283–292. [Google Scholar] [CrossRef]
- Hossein, B.G.; Sadr, A.; Espigares, J.; Hariri, I.; Nakashima, S.; Hamba, H.; Shafiei, F.; Moztarzadeh, F.; Tagami, J. Study on the influence of leucine-rich amelogenin peptide (LRAP) on the remineralization of enamel defects via micro-focus x-ray computed tomography and nanoindentation. Biomed. Mater. 2015, 10, 035007. [Google Scholar] [CrossRef]
- Dissanayake, S.S.; Ekambaram, M.; Li, K.C.; Harris, P.W.; Brimble, M.A. Identification of key functional motifs of native amelogenin protein for dental enamel remineralisation. Molecules 2020, 25, 4214. [Google Scholar] [CrossRef]
- Wang, D.; Deng, J.; Deng, X.; Fang, C.; Zhang, X.; Yang, P. Controlling enamel remineralization by amyloid-like amelogenin mimics. Adv. Mater. 2020, 32, e2002080. [Google Scholar] [CrossRef]
- Chu, J.; Feng, X.; Guo, H.; Zhang, T.; Zhao, H.; Zhang, Q. Remineralization efficacy of an amelogenin-based synthetic peptide on carious lesions. Front. Physiol. 2018, 9, 842. [Google Scholar] [CrossRef]
- Zheng, W.; Ding, L.; Wang, Y.; Han, S.; Zheng, S.; Guo, Q.; Li, W.; Zhou, X.; Zhang, L. The effects of 8DSS peptide on remineralization in a rat model of enamel caries evaluated by two nondestructive techniques. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019827798. [Google Scholar] [CrossRef]
- Han, S.; Peng, X.; Ding, L.; Lu, J.; Liu, Z.; Wang, K.; Zhang, L. TVH-19, a synthetic peptide, induces mineralization of dental pulp cells in vitro and formation of tertiary dentin in vivo. Biochem. Biophys. Res. Commun. 2021, 534, 837–842. [Google Scholar] [CrossRef]
- Machla, F.; Angelopoulos, I.; Epple, M.; Chatzinikolaidou, M.; Bakopoulou, A. Biomolecule-Mediated Therapeutics of the Dentin-Pulp Complex: A Systematic Review. Biomolecules 2022, 12, 285. [Google Scholar] [CrossRef]
- Zou, J.; Mao, J.; Shi, X. Influencing factors of pulp-dentin complex regeneration and related biological strategies. Zhejiang Da Xue Xue Bao Yi Xue Ban 2022, 51, 350–361. [Google Scholar] [CrossRef]
- McGuire, J.D.; Walker, M.P.; Mousa, A.; Wang, Y.; Gorski, J.P. Type VII collagen is enriched in the enamel organic matrix associated with the dentin-enamel junction of mature human teeth. Bone 2014, 63, 29–35. [Google Scholar] [CrossRef]
- Xia, K.; Chen, Z.; Chen, J.; Xu, H.; Xu, Y.; Yang, T.; Zhang, Q. RGD- and VEGF-Mimetic Peptide Epitope-Functionalized Self-Assembling Peptide Hydrogels Promote Dentin-Pulp Complex Regeneration. Int. J. Nanomed. 2020, 15, 6631–6647. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Q.; Han, S.; Ding, L.; Qin, X.; Hu, D.; He, T.; Tian, T.; Lu, Z.; Zhang, L. Promoting effect of a calcium-responsive self-assembly β-sheet peptide on collagen intrafibrillar mineralization. Regen Biomater. 2022, 9, rbac059. [Google Scholar] [CrossRef]
- Cloyd, A.K.; Boone, K.; Ye, Q.; Snead, M.L.; Spencer, P.; Tamerler, C. Engineered Peptides Enable Biomimetic Route for Collagen Intrafibrillar Mineralization. Int. J. Mol. Sci. 2023, 24, 6355. [Google Scholar] [CrossRef]
- Thalakiriyawa, D.S.; Dissanayaka, W.L. Advances in Regenerative Dentistry Approaches: An Update. Int. Dent. J. 2023. [CrossRef]
- Pountos, I.; Panteli, M.; Lampropoulos, A.; Jones, E.; Calori, G.M.; Giannoudis, P.V. The role of peptides in bone healing and regeneration: A systematic review. BMC Med. 2016, 14, 103. [Google Scholar] [CrossRef]
- Roberto-Rodrigues, M.; Fernandes, R.M.P.; Senos, R.; Scoralick, A.C.D.; Bastos, A.L.; Santos, T.M.P.; Viana, L.P.; Lima, I.; Guzman-Silva, M.A.; Kfoury-Júnior, J.R. Novel rat model of nonunion fracture with vascular deficit. Injury 2015, 46, 649–654. [Google Scholar] [CrossRef]
- Díaz-Sánchez, R.M.; Yáñez-Vico, R.M.; Fernández-Olavarría, A.; Mosquera-Pérez, R.; Iglesias-Linares, A.; Torres-Lagares, D. Current approaches of bone morphogenetic proteins in dentistry. J. Oral Implantol. 2015, 41, 337–342. [Google Scholar] [CrossRef]
- Schwarz, F.; Ferrari, D.; Sager, M.; Herten, M.; Hartig, B.; Becker, J. Guided bone regeneration using rhGDF-5-and rhBMP-2-coated natural bone mineral in rat calvarial defects. Clin. Oral Implants Res. 2009, 20, 1219–1230. [Google Scholar] [CrossRef]
- Dupoirieux, L.; Pohl, J.; Hanke, M.; Pourquier, D. A preliminary report on the effect of dimeric rhGDF-5 and its monomeric form rhGDF-5C465A on bone healing of rat cranial defects. J. Craniomaxillofac. Surg. 2009, 37, 30–35. [Google Scholar] [CrossRef]
- Lee, J.S.; Wikesjö, U.M.E.; Jung, U.W.; Choi, S.H.; Pippig, S.; Siedler, M.; Kim, C.K. Periodontal wound healing/regeneration following implantation of recombinant human growth/differentiation factor-5 in a β-tricalcium phosphate carrier into one-wall intrabony defects in dogs. J. Clin. Periodontol. 2010, 37, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.K.C.; Frcd, C.; Peel, S.A.F.; Hu, Z.M. Augmentation of the maxillary sinus: Comparison of bioimplants containing bone morphogenetic protein and autogenous bone in a rabbit model. J. Can. Dent. Assoc. 2010, 76, a108. [Google Scholar]
- Ayoub, A.; Challa, S.R.R.; Abu-Serriah, M.; McMahon, J.; Moos, K.; Creanor, S.; Odell, E. Use of a composite pedicled muscle flap and rhBMP-7 for mandibular reconstruction. Int. J. Oral Maxillofac. Surg. 2007, 36, 1183–1192. [Google Scholar] [CrossRef]
- Correa, R.; Arenas, J.; Montoya, G.; Hoz, L.; Lopez, S.; Salgado, F.; Arroyo, R.; Salmeron, N.; Romo, E.; Zeichner-David, M.; et al. Synthetic cementum protein 1-derived peptide regulates mineralization in vitro and promotes bone regeneration in vivo. FASEB J. 2019, 33, 1167–1178. [Google Scholar] [CrossRef]
- Whitfield, J.F.; Morley, P.; Willick, G.E. Parathyroid hormone, its fragments and their analogs for the treatment of osteoporosis. Treat. Endocrinol. 2002, 1, 175–190. [Google Scholar] [CrossRef]
- Qin, L.; Raggatt, L.J.; Partridge, N.C. Parathyroid hormone: A double-edged sword for bone metabolism. Trends Endocrinol. Metab. 2004, 15, 60–65. [Google Scholar] [CrossRef]
- Hsu, C.; He, Z.; Le Henaff, C.; Partridge, N.C. Differential effects of parathyroid hormone, parathyroid hormone-related protein, and abaloparatide on collagen 1 expression by mouse cementoblasts and mouse tooth root density. Am. J. Orthod. Dentofac. Orthop. 2023, 163, 378–388.e1. [Google Scholar] [CrossRef]
- Safaei, M.; Mobini, G.R.; Abiri, A.; Shojaeian, A. Synthetic biology in various cellular and molecular fields: Applications, limitations, and perspective. Mol. Biol. Rep. 2020, 47, 6207–6216. [Google Scholar] [CrossRef]
- Trump, B.D.; Cegan, J.C.; Wells, E.; Keisler, J.; Linkov, I. A critical juncture for synthetic biology: Lessons from nanotechnology could inform public discourse and further development of synthetic biology. EMBO Rep. 2018, 19, e46153. [Google Scholar] [CrossRef]
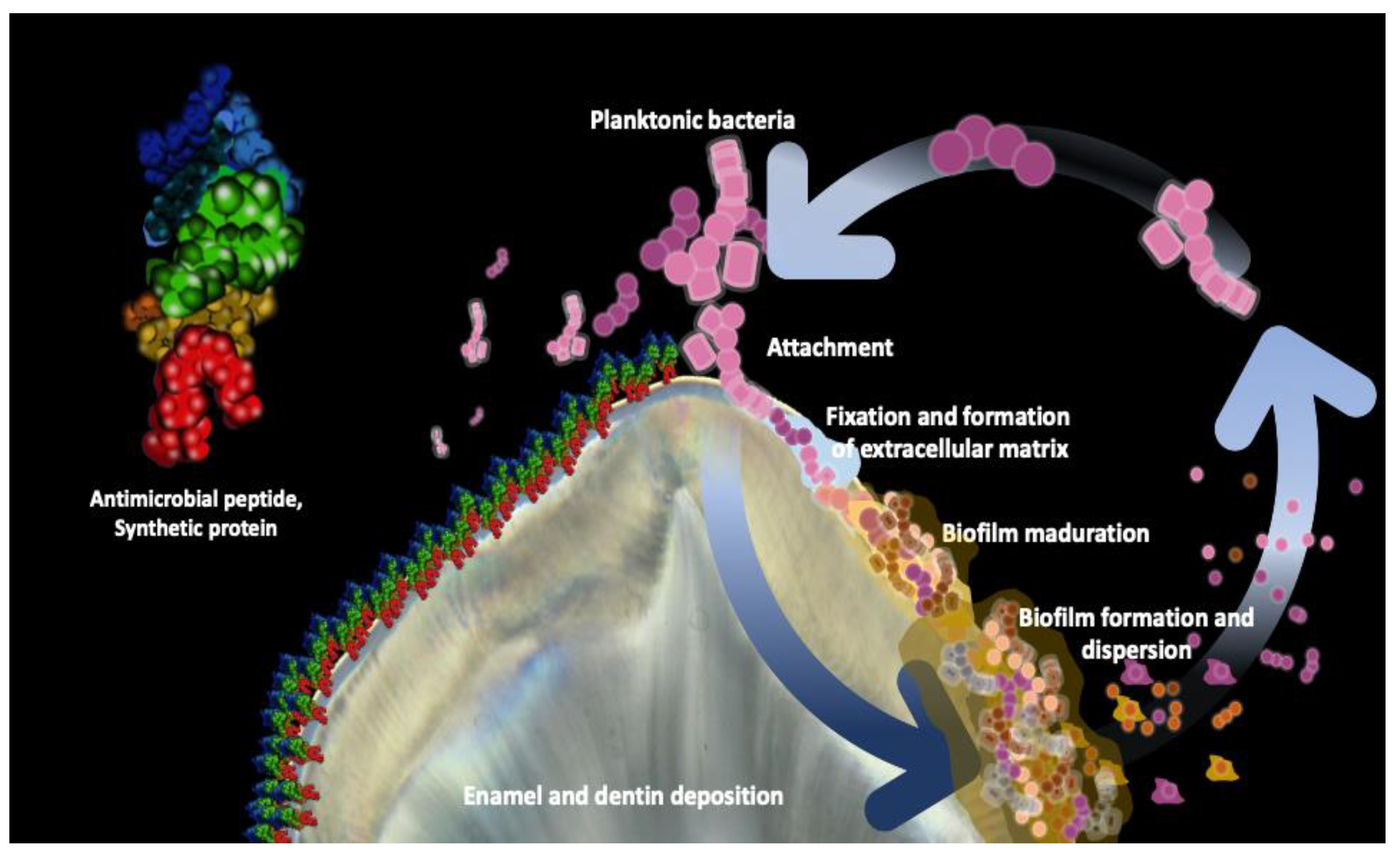
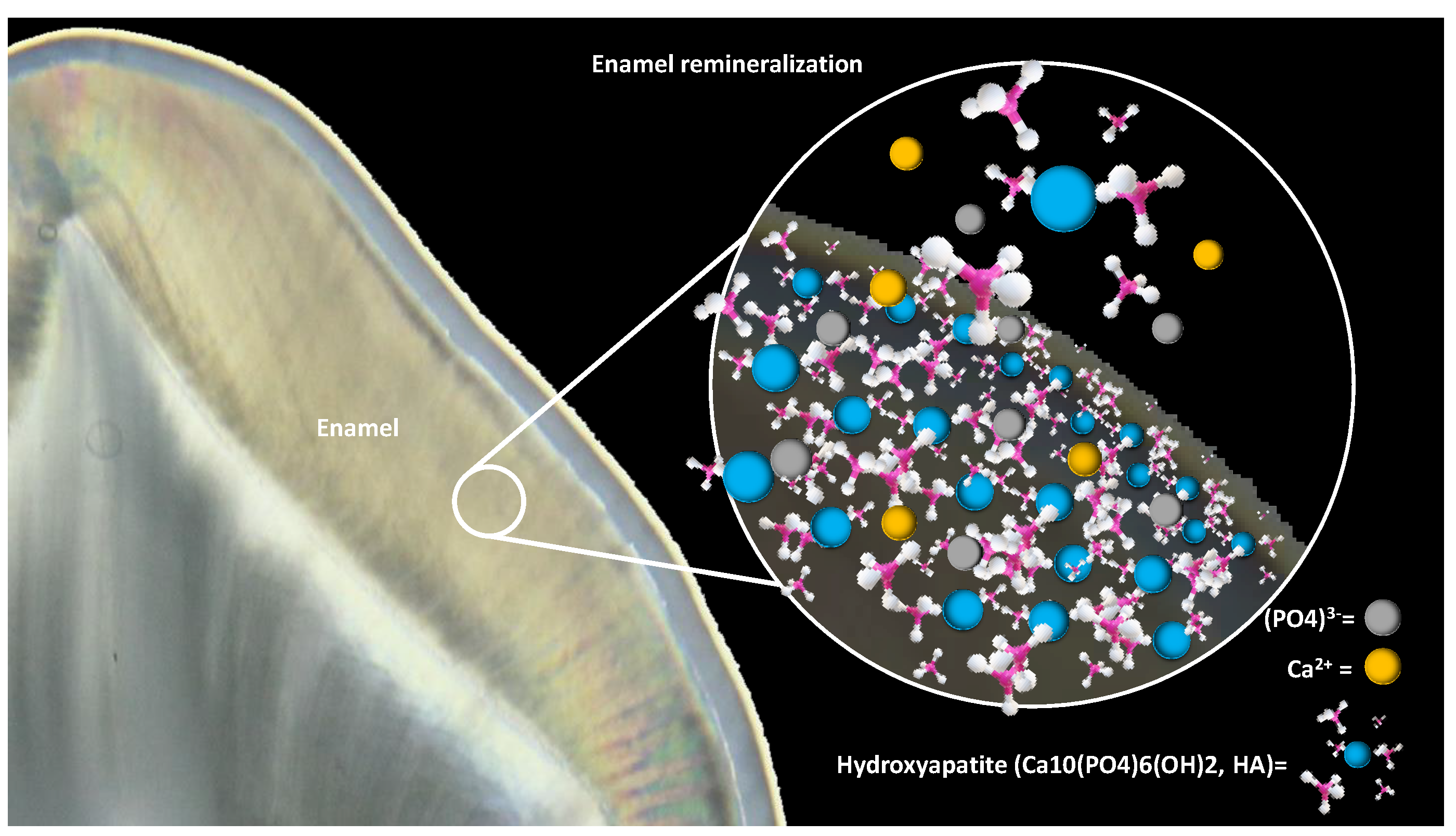

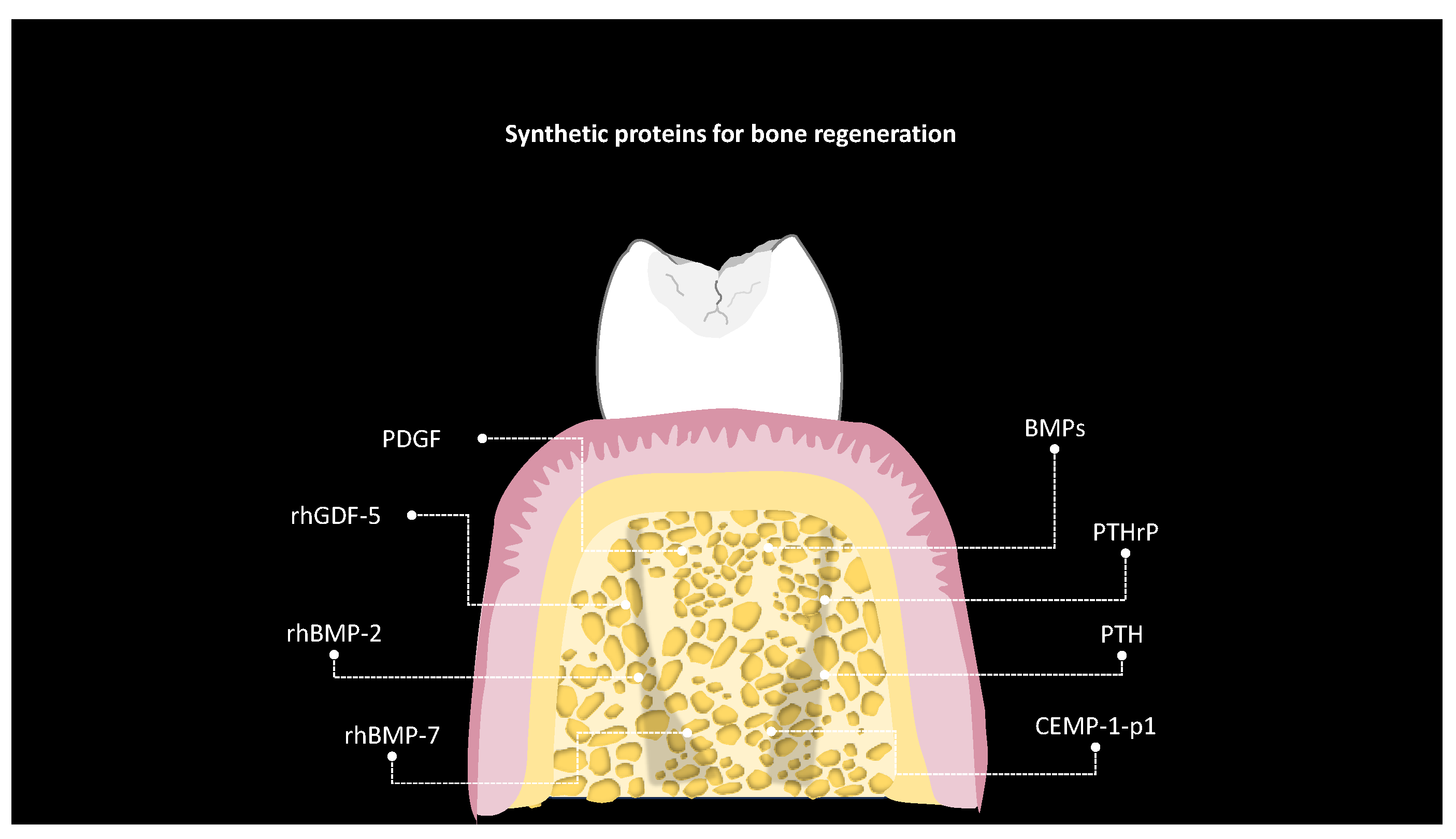
| Study and Year [Reference] | Pepetide | Sequence | Structure | Type of Study | Antimicrobial Activity | Bacteria |
|---|---|---|---|---|---|---|
| da Silva 2017 [29] | [W7]KR12-KAEK | KRIVQRWKDFLRKAEK-NH2 | α-helical | In vitro | Antimicrobial effect by determining the minimum inhibitory concentration (MIC) ranging from 7.8 to 31.25 μg mL−1 and minimum bactericidal concentration (MBC), 15.6 to 62.5 μg mL−1 On biofilm the cell viability decreased of biofilms from all strains evaluated, with biomass reduction ranging from 48 to 96%. | S. mutans ATCC 25175, UA 159, UA 130. |
| da Silva 2013 [30] | LYS-[TRP6]-Hy-A1 (Lys-a1) | KIFGAIWPLALGALKNLIK-NH2 | α-helical | In vitro | Antimicrobial activity on the planktonic and biofilm growth. The MIC values ranged from 3.9 to 125 μg mL−1. The MBC values ranged from 3.9 to 500 μg mL−1. S. mutans was more resistant to the biofilm inhibiting activity of the peptide. At concentrations from 7.8 to 62 μg mL−1, interference in biofilm formation, with biomass reductions ranging from 10 to 88%. | S. oralis ATCC 10557, S. sanguinis ATCC 10556, S. parasanguinis ATCC 903, S. salivarius ATCC 7073, S. mutans ATCC 25175 and S. sobrinus ATCC 6715. |
| Zhou 2019 [33] | Sp−H5 | Phosphoserine-DSHAKRHHGYKRKF HEKHHSHRGY | Not specified | In vitro | The MIC was 2 μmol/mL. Sp−H5 kills S. mutans biofilm from 16× MIC. After coating on the enamel surface, Sp−H5 inhibits S. mutans adhesion from 2× MIC. | S. mutans ATCC 35668 |
| Zhang 2019 [34] | Poly-phemusin I (PI) and tooth-binding AMP (DPS-PI) | PI (Arg-Arg-Trp-Cys-Phe-Arg-Val-Cys-Tyr-Arg-Gly-Phe-Cys-Tyr-Arg-Lys-Cys-Arg) and DPS-PI (Ser(p)-Ser(p)-Arg-Arg-Trp-Cys-Phe-Arg-Val-Cys-Tyr-Arg-Gly-Phe-Cys-Tyr-Arg-Lys-Cys-Arg) | α-helical | In vitro and in vivo (biofilm formation on rabbit incisor surfaces) | The MIC was PI = 40 μg/mL, DPS-PI = 80 μg/mL. Antibiofilm: delay in the formation of biofilm and maintaining the inhibitory effect after a diet rich in sucrose only with DPS-PI at 2× MIC. | S. mutans ATCC 35668 |
| Wang 2017 [35] | GH8 GH12 GH16 | GH8, GLLWHLLH-NH2; GH12, GLLWHLLHHLLH-NH2; and GH16, GLLWHLLHHLLHLLHH-NH2 | α-helical | In vitro | GH12 showed the most potent inhibiting with MIC of 4.0–8.0 μg/mL and MBC of 8.0–32.0 μg/mL. GH8 were 16 to 32 times higher than GH12, and GH16 showed antimicrobial activity only against Lactobacillus species. GH12 exhibited an inhibitory effect on biofilm formation of S. mutans, with MBIC50 values of 8.0 μg/mL. | S. mutans UA159, S. gordonii DL1, S. sanguinis ATCC10556, L. acidophilus ATCC14931, L. casei ATCC393, L. fermentium ATCC9338, A. viscosus ATCC15987, and A. naeslundii ATCC12104 |
| Study and Year [Reference] | Peptide | Sequence | Structure | Type of Study | Remineralization effect |
|---|---|---|---|---|---|
| Valente 2018 [43] | DR9-DR9, DR9-DR14 | DSpSpEEKFLRDSpSpEEKFLR (DR9-DR9) DSpSpEEKFLRRKFHEKHHSHRGYR (DR9-RR14) | Not specified | In vitro | The effect on hydroxyapatite (HA) crystal growth inhibition that promotes enamel remineralization. The presence of phosphoserine at positions 2 and 3 in DR9 resulted in a higher degree of HA inhibition. The presence of 4 phosphorylated sites in the DR9-DR9 suggests a more stable and strong binding conformation to the HA crystal. |
| Yarbrough 2010 [44] | 2DSS, 6 DSS, 8DSS, 4ESS, 4NSS, 4DTT, 4ETT, 4NTT, 8DAA, 8NAA, 8ASS. | 2DSS (DSSDSS), 4DSS (DSSDSSDSSDSS), 6DSS (DSSDS SDSSDSSDSSDSS), 8DSS (DSSDSSDSSDSSDSSDSSDSSDSS), 4ESS (ESSESSESSESS), 4NSS (NSSNSSNSSN SS), 4DTT (DTTDTTDTTDTT), 4ETT (ETTETTETTETT), 4NTT (NTTNTTNTTNTT), 8DAA (DAADAADA-ADAADAADAADAADAA), 8NAA (NAANAANAANAA NAANAANAANAA), 8ASS (ASSASSASSASSASSASSASSASS) | Not specified | In vitro | Binding of DSS-containing peptides to defined HA substrates depends strongly on the length of the peptides, additional increase in affinity seen in peptides with eight repeats; the HA binding affinity of the 8DSS peptide was 290,000 M-1, It compares favorably with measured values for histatins (K = 353,000–1,903,000 M-1), a class of small antimicrobial peptides that are known to bind HA with high affinity. With these high affinities the peptides can bind to mineralized tissues and recruit calcium phosphate to demineralized surfaces. |
| Li 2023 [45] | Peptide 1. N- and C-termini of porcine amelogenin. | Peptide 1. MPLPPHPGHPGYINF(p-S)YEVLTPLKWYONMIRHPYTSYGYEPMGGWATDKTKREEVD | Not specified | In vitro | The results of this study indicated the potential of the recombinant amelogenin peptide TRAP to promote the remineralization of incipient enamel caries. |
| Peptide 2. TRAP | Peptide 2. MPLPPHPGHPGYINF(p-S)YEVLTPLKWYONMIRHPYTSYGYEPMGGW | ||||
| Marin 2022 [46] | DR9-DR9 and DR9-RR14 | Not specified | Not specified | In vitro | DR9-RR14 peptide displayed a potential protective effect against enamel demineralization but did not have a significant effect on S. mutans biofilm biomass. |
| Li 2020 [47] | ID4 and ID8 | ID4 (Ac-Ile-Asp-Ile-Asp) ID8 (Ac-Ile-Asp-Ile-Asp-Ile-Asp-Ile-Asp) | β-sheet | In vitro | ID8 showed better potential than ID4 for remineralization of initial caries lesions. |
| Kwak 2017 [49] | Leucine-rich amelogenin peptide (LRAP) | Not specified | Not specified | In vitro | LRAP has the capacity to promote the linear growth of mature enamel crystals along the c-axis and regulate the size, shape, and orientation, demonstrating a potential for the development of a new approach to regenerate enamel structure. |
| Ding 2020 [50] | QP5 | QPYQPVQPHQPMQPQTKREEVD | Not specified | In vitro | QP5 peptide binds to demineralized enamel and HA, increasing the surface microhardness of dental enamel and favoring a lower loss of minerals. |
| Wang 2020 [53] | C-AMG | Not specified | β-sheet | In vitro and in vivo | C-AMG facilitated the oriented arrangement of amorphous calcium phosphate (ACP) nanoparticles and their transformation to ordered enamel-like HA crystals and recovered the highly oriented structure and mechanical properties to levels close to natural enamel. |
| Zheng 2019 [55] | 8DSS | DSSDSSDSSDSSDSSDSSDSSDSS | Not specified | In vivo | 8DSS demonstrates the regression of enamel demineralization and boosts enamel remineralization in a rat model with a potential comparable to NaF effects. |
| Study and Year [Reference] | Peptide | Sequence | Structure | Type of Study | Dentin-Pulp Complex Effect |
|---|---|---|---|---|---|
| Han 2021 [56] | TVH-19 | TKRQQVVGLLWHLLHHLLH-NH2 | Not specified | In vivo | Amounts of 10 to 200 μg/mL of TVH-10 did not show cytotoxic features or any difference in the proliferation when compared with the untreated hDPCs, after 1, 2, and 4 days of incubation. TVH-19 induces differentiation of hDPSCs, promotes tertiary dentin formation, relieves inflammation, and reduces apoptosis, indicating the potential applications in indirect pulp capping. |
| Xia 2020 [60] | RGD and VEGF | Not specified | β-sheet | In vivo | The results of this study show the survival and differentiation of dental pulp stem cells (hDPSCs) in promoting regeneration of the dentin-pulp complex in partially pulpotomized rat molars over a period of 28 days. RGD and VEGF mimetic peptide epitopes provided a 3D microenvironment for hDPSCs which enhanced angiogenic and odontogenic differentiation. |
| Li 2022 [62] | ID8 | Ile-Asp-Ile-Asp-Ile-Asp-Ile-Asp | β-sheet | In vitro | The calcium-sensitive self-assembly ability of ID8 gives it the inherent advantage of forming polyelectrolyte-calcium complexes easily, these peptides are expected to be potential tools for biomimetic mineralization of collagen. |
| Cloyd 2023 [63] | HABP1 CBP | TKKLTLRT | Not specified | In vitro | The engineered peptide demonstrated intermolecular interactions that enhanced nanomechanical properties and offer a promising route for collagen intrafibrillar remineralization. |
| Study and Year [Reference] | Peptide | Sequence | Structure | Type of Study | Bone Regeneration Effect |
|---|---|---|---|---|---|
| Schwarz 2009 [68] | rhBMP-2 | Not specified | Not specified | In vivo | All treatment procedures investigated supported bone regeneration at 24 weeks; rhBMP-2 could have the potential to improve healing outcome, particularly during the early stages, and could therefore be considered as a potential candidate for guided tissue regeneration. |
| Dupoirieux 2009 [60] | rhGDF-5 | Not specified | Not specified | In vivo | The data of this study show that dimeric GDF-5 and its monomeric form rhGDF-5C456A have a positive effect on membranous bone growth in vivo. The newly formed bone in the specimens was composed of trabecular bone with abundant vascularization and bone marrow. |
| Lee 2010 [70] | rhGDF-5 | Not specified | Not specified | In vivo | Sites implanted with rHGDF-5/β-TCP exhibited greater enhanced cementum and bone formation compared with β-TCP and sham-surgery controls in one-wall intrabony defects in dogs. rHGDF-5/β-TCP has a greater potential to support periondontal regeneraton. |
| Ayoub 2007 [72] | rhBMP-7 | Not specified | Not specified | In vivo | Histologically, the induced bone regenerate showed maturation from woven to lamellar bone. This study confirms that bone can be formed within a muscular ‘scaffolding’ at the site of a created defect. |
| Correa 2019 [73] | CEMP-1-p1 | MGTSSTDSQQAGHRRCSTSN | Not specified | In vitro and in vivo | An amount of 5 μg/mL of CEMP-1p1 was an optimal concentration to promote cell proliferation. Histomorphometry evaluation indicated that the peptide promoted new bone formation at 30 and 60 days. The bone formation in vivo was demonstrated with a rat model, which is defined as the area of bone that naturally regenerates throughout the life of the animal and is <10% of the initial defect size. These results show osteoinductive properties which enhanced the physiologic formation and maturation of newly formed bone. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Ayuso, C.A.; Aranda-Herrera, B.; Guzman-Rocha, D.; Chavez-Granados, P.A.; Garcia-Contreras, R. Synthetic Proteins in Dental Applications. SynBio 2024, 2, 1-20. https://doi.org/10.3390/synbio2010001
Lopez-Ayuso CA, Aranda-Herrera B, Guzman-Rocha D, Chavez-Granados PA, Garcia-Contreras R. Synthetic Proteins in Dental Applications. SynBio. 2024; 2(1):1-20. https://doi.org/10.3390/synbio2010001
Chicago/Turabian StyleLopez-Ayuso, Christian Andrea, Benjamin Aranda-Herrera, Dulce Guzman-Rocha, Patricia Alejandra Chavez-Granados, and Rene Garcia-Contreras. 2024. "Synthetic Proteins in Dental Applications" SynBio 2, no. 1: 1-20. https://doi.org/10.3390/synbio2010001
APA StyleLopez-Ayuso, C. A., Aranda-Herrera, B., Guzman-Rocha, D., Chavez-Granados, P. A., & Garcia-Contreras, R. (2024). Synthetic Proteins in Dental Applications. SynBio, 2(1), 1-20. https://doi.org/10.3390/synbio2010001









