Impact of Nitrogen-Containing Compounds on Secondary Metabolism in Streptomyces spp.—A Source of Metabolic Engineering Strategies
Abstract
1. Streptomyces sp. as a Source of Secondary Metabolites
1.1. Streptomycetes as Biological Natural Product Producers
1.1.1. Secondary Metabolites as a Bacterial Survival Strategy
1.1.2. Secondary Metabolite Production in Streptomycetes
1.2. Strategies for Discovery and Optimization of Secondary Metabolite Production in Stretpomyces
1.2.1. Bioinformatics-Based Approaches for Natural Product Discovery
1.2.2. Genetic Tools for Streptomyces Engineering
| Technology | Category | Feature | Reference |
|---|---|---|---|
| ClustSCAN | Bioinformatics | Semi-automatic annotation of modular BGCs and in silico prediction of new chemical structures | [71] |
| NP searcher | Bioinformatics | Automated genome mining for natural products and rapid screening for compounds with potential value | [72] |
| GNP/PRISM | Bioinformatics | Identification of biosynthetic gene clusters, prediction of genetically encoded non-ribosomal peptides and type I and II polyketides, and bio- and cheminformatic dereplication of known natural products | [73] |
| antiSMASH | Bioinformatics | Software pipeline for genome mining with a user-friendly web interface as well as prediction of the broad spectrum of BGCs | [74] |
| MultiMetEval | Bioinformatics | Genome-wide in silico metabolism reconstruction | [50,51,52] |
| Acquisition of the target BGC | Genetic manipulation strategy | Transfer from the native host genome using a genomic library of cosmids, fosmids, BAC, and PAC | [75] |
| Ligation/assembly of the BGC to the vector | Genetic manipulation strategy | Sticky/blunt end ligation, Gibson cloning, and recombination in different hosts | [46] |
| Transfer of the BGC-encoded vector to theheterologous host for expression | Genetic manipulation strategy | Conjugation and protoplast transformation | [46] |
| Target secondary metabolite production by expression of the BGC vector | Genetic manipulation strategy | Expression of integrative (pSET152, pCAP01, pESAC) or replicative (pSKC2 and pUWL201) vectors | [46] |
| PCR-targetingsystem | Genetic manipulation strategy | Nonpolar as well as in-frame deletion of genes or gene clusters in Streptomyces | [57] |
| Cre-loxP recombination system | Genetic manipulation strategy | Can be used in combination with the PCR-targeting system or can be independently used to knock out large fragments of DNA in Streptomyces | [58] |
| I-SceI promoted recombination system | Genetic manipulation strategy | I-SceI meganuclease can recognize an 18 bp unique sequence and cause DNA double-strand breaks (DSBs), which promote double-crossover recombination events | [59] |
| SpCas9-based genome editing | Genetic manipulation strategy | CRISPR/Cas-based technology does not require the pre-integration of a unique enzyme recognition sequence into the genome, but uses a transcribed synthetic guide RNA to direct Cas proteins to any site on the genome. Editing plasmids: pCRISPomyces-1/2, pKCas9dO, pCRISPR-Cas9-ScaligD, and pWHU2653 | [60,61,62,63] |
| CRISPRi-mediated gene repression for single cells | Genetic manipulation strategy | Gene repression tool based on dCas9 or ddCpf1 and the base editors (BEs) for targeted base mutagenesis based on dCas9 or Cas9n | [64] |
| FnCpf1-based genome editing and CRISPRi | Genetic manipulation strategy | Editing plasmids: pKCCpf1, pKCCpf1-MsmE, and pSETddCpf1 | [65] |
| CRISPR/Cas-based base editing tools | Genetic manipulation strategy | Editing plasmids: pCRISPR-cBEST/-aBEST, and pKC-dCas9-CDA-ULstr | [66,67] |
| Alternative CRISPR/Cas-based genome editing | Genetic manipulation strategy | Editing plasmids: pCRISPomyces-FnCpf1, pCRISPomyces-Sth1Cas9, and pCRISPomyces-SaCas9 | [68,76] |
| Synthetic promoters | Genetics parts | Constitutive ermE, SF14P, kasOP, gapdh, rpsL promoters as well as inducible tipA nitA and xylA promoters | [46] |
| Ribosome-binding sites | Genetics parts | AAAGGAGG and diverse native or synthetic RBSs | [46] |
| Terminators | Genetics parts | Fdand TD1 | [46] |
| Reporter genes | Genetics parts | Genes luxAB, amy, xylE, and gusA as well as eGFP, sfGFP, mRFP, and mCherry | [46] |
2. Importance of Nitrogen for Secondary Metabolism in Streptomyces
2.1. Nitrogen as a Key Element for Cellular Metabolism
2.1.1. Nitrogen Assimilation in Streptomyces
2.1.2. Regulation of Primary Nitrogen Metabolism in Streptomyces
2.1.3. Regulation of Secondary Metabolism in Streptomyces
2.2. Influence of Nitrogen-Containing Compounds on Antibiotic Production—Interplay between Primary and Secondary Metabolism
2.2.1. Influence of Ammonium on Antibiotic Production in Streptomyces
2.2.2. Influence of Nitrate on Antibiotic Production in Streptomyces
2.2.3. Influence of Amino Acids on Antibiotic Production in Streptomyces
2.2.4. Influence of Polyamines on Antibiotic Production in Streptomyces
| Compound | Producer | Nitrogen Source Tested | Effect on Production | Reference |
|---|---|---|---|---|
| Tylosin | Streptomyces fradiae NRRL 2702 | Ammonium (20 mM/L) | Decrease (~2-fold) | [117] |
| Leucomycin | Streptomyees kitasatoensis | Ammonium (2 mM/L) | Decrease (50%) | [145] |
| NAI-107 | Microbispora ATCC PTA-5024 | Ammonium (25 mM/L) | Increase (~0.2 fold) | [114] |
| Neomycin B | Streptomyces fradiae SF-2 | Ammonium (60 mM/L) | Increase (0.54–3.3 fold) | [122] |
| Streptonigrin | Streptomyces flocculus (ATCC 13257) | Ammonium (0.5–2 g/L) | Increase (2-fold) | [123] |
| AK-111-81 | Streptomyces hygroscopicus | Ammonium (0.15%) | Increase (6-fold) | [146] |
| SBR-22 | Streptomyces psammoticus BT-408 | Ammonium (2.5 g/L) | Increase (1.2-fold) | [147] |
| SA-53 | Streptomyces anandii var. Taifiensis | Ammonium (280 mg/L) | Increase (2-fold) | [148] |
| Azalomycin | Streptomyces hygroscopicus | Nitrate | Increase | [149] |
| Erythromycin | Saccharopolyspora erythraea | Nitrate (15 mM/L) | Increase | [150] |
| Lividomycin | Lividomycin poducer M814 | Nitrate | Increase | [151] |
| Lincomycin | Streptomyces lincolnensis, Streptomyces sp. MS-266 Dm4 | Nitrate (23.5 mM/L) | Increase | [152] |
| Rifamycin B and SV | Amycolatopsis mediterranei, Amycolatopsis mediterranei U32 | Nitrate (12.5–80 mM/L) | Increase (4-fold) | [126,153] |
| Meroparamycin | Streptomyces MAR01 | Nitrate (19.8 mM/L) | increase | [124] |
| Cephamycin | Streptomyces clavuligerus | Amino acids (lysine, 14.6 g L−1) | Increase (6-fold) | [144] |
| Tacrolimus (FK-506) | Streptomyces tsukubaensis | Amino acids (lysine, 2.5 g/L) | Increase (30%) | [56,154] |
| Rapamycin | Streptomyces hygroscopicus | Amino acids (lysine, 10 g/L) | Increase (150%) | [155] |
| Leucomycin | Streptomyees kitasatoensis | Amino acids (1%) | Increase (2–4 fold) | [145] |
| Prodigiosin | Streptomyces coelicolor | Polyamines (25 mM/L) | Increase | [140] |
| Actinorhodin | Streptomyces coelicolor | Polyamines (25 mM/L) | Decrease | [140] |
| Tacrolimus (FK-506) | Streptomyces tsukubaensis | Polyamines (25 mM/L) | Decrease (3-fold) | [97] |
3. Perspectives for Secondary Metabolite Discovery in Streptomyces
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Procópio, R.E.; Silva, I.R.; Martins, M.K.; Azevedo, J.L.; Araújo, J.M. Antibiotics produced by Streptomyces. Braz. J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Lacey, H.J.; Rutledge, P.J. Recently Discovered Secondary Metabolites from Streptomyces Species. Molecules 2022, 27, 887. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- Li, J.W.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Gross, H. Genomic mining—A concept for the discovery of new bioactive natural products. Curr. Opin. Drug Discov. Devel 2009, 12, 207–219. [Google Scholar] [CrossRef]
- Bachmann, B.O.; Van Lanen, S.G.; Baltz, R.H. Microbial genome mining for accelerated natural products discovery: Is a renaissance in the making? J. Ind. Microbiol. Biotechnol. 2014, 41, 175–184. [Google Scholar] [CrossRef]
- Aminov, R.I. The role of antibiotics and antibiotic resistance in nature. Environ. Microbiol. 2009, 11, 2970–2988. [Google Scholar]
- Patel, P.; Song, L.; Challis, G.L. Distinct extracytoplasmic siderophore binding proteins recognize ferrioxamines and ferricoelichelin in Streptomyces coelicolor A3. Biochemistry 2010, 49, 8033–8042. [Google Scholar] [CrossRef]
- Barona-Gómez, F.; Wong, U.; Giannakopulos, A.E.; Derrick, P.J.; Challis, G.L. Identification of a cluster of genes that directs desferrioxamine biosynthesis in Streptomyces coelicolor M145. J. Am. Chem. Soc. 2004, 126, 16282–16283. [Google Scholar] [CrossRef]
- Yamanaka, K.; Oikawa, H.; Ogawa, H.O.; Hosono, K.; Shinmachi, F.; Takano, H.; Sakuda, S.; Beppu, T.; Ueda, K. Desferrioxamine E produced by Streptomyces griseus stimulates growth and development of Streptomyces tanashiensis. Microbiology 2005, 151, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Steiger, S.; Perez-Fons, L.; Cutting, S.M.; Fraser, P.D.; Sandmann, G. Annotation and functional assignment of the genes for the C30 carotenoid pathways from the genomes of two bacteria: Bacillus indicus and Bacillus firmus. Microbiology 2015, 161, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.C.; Mothersole, D.J.; Dilbeck, P.; Niedzwiedzki, D.M.; Zhang, H.; Qian, P.; Vasilev, C.; Grayson, K.J.; Jackson, P.J.; Martin, E.C.; et al. Assembly of functional photosystem complexes in Rhodobacter sphaeroides incorporating carotenoids from the spirilloxanthin pathway. Biochim. Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Kato, F.; Hino, T.; Nakaji, A.; Tanaka, M.; Koyama, Y. Carotenoid synthesis in Streptomyces setonii ISP5395 is induced by the gene crtS, whose product is similar to a sigma factor. Mol. General. Genet. MGG 1995, 247, 387–390. [Google Scholar] [CrossRef]
- Madhusudhan, D.N.; Mazhari BB, Z.; Dastager, S.G.; Agsar, D. Production and cytotoxicity of extracellular insoluble and droplets of soluble melanin by Streptomyces lusitanus DMZ-3. BioMed Res. Int. 2014, 2014, 306895. [Google Scholar] [CrossRef]
- Huang, D.; Li, S.; Xia, M.; Wen, J.; Jia, X. Genome-scale metabolic network guided engineering of Streptomyces tsukubaensis for FK506 production improvement. Microb. Cell Factories 2013, 12, 52. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Actinobacterial melanins: Current status and perspective for the future. World J. Microbiol. Biotechnol. 2013, 29, 1737–1750. [Google Scholar] [CrossRef]
- Moody, S.C.; Zhao, B.; Lei, L.; Nelson, D.R.; Mullins, J.G.; Waterman, M.R.; Kelly, S.L.; Lamb, D.C. Investigating conservation of the albaflavenone biosynthetic pathway and CYP170 bifunctionality in streptomycetes. FEBS J. 2012, 279, 1640–1649. [Google Scholar] [CrossRef]
- Bursy, J.; Kuhlmann, A.U.; Pittelkow, M.; Hartmann, H.; Jebbar, M.; Pierik, A.J.; Bremer, E. Synthesis and uptake of the compatible solutes ectoine and 5-hydroxyectoine by Streptomyces coelicolor A3 in response to salt and heat stresses. Appl. Environ. Microbiol. 2008, 23, 7286–7296. [Google Scholar] [CrossRef]
- Barth, S.; Huhn, M.; Matthey, B.; Klimka, A.; Galinski, E.A.; Engert, A. Compatible-solute-supported periplasmic expression of functional recombinant proteins under stress conditions. Appl. Environ. Microbiol. 2000, 66, 1572–1579. [Google Scholar] [CrossRef]
- Arora, A.; Ha, C.; Park, C.B. Inhibition of insulin amyloid formation by small stress molecules. FEBS Lett. 2004, 564, 121–125. [Google Scholar] [PubMed]
- Poralla, K.; Muth, G.; Härtner, T. Hopanoids are formed during transition from substrate to aerial hyphae in Streptomyces coelicolor A3. FEMS Microbiol. Lett. 2000, 189, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Waksman, S.A.; Fenner, F. Drugs of natural origin. Ann. N. Y. Acad. Sci. 1949, 52, 750–787. [Google Scholar] [CrossRef] [PubMed]
- Lancini, G.; Lorenzetti, R. Biotechnology of Antibiotics and Other Bioactive Microbial Metabolites; Plenum Publishing Corporation: London, UK, 1994. [Google Scholar]
- Yang, Y.H.; Song, E.; Lee, B.R.; Kim, E.J.; Park, S.H.; Kim, Y.G.; Lee, C.S.; Kim, B.G. Rapid functional screening of Streptomyces coelicolor regulators by use of a pH indicator and application to the MarR-like regulator AbsC. Appl. Environ. Microbiol. 2010, 76, 3645–3656. [Google Scholar] [CrossRef]
- Antoraz, S.; Santamaría, R.I.; Díaz, M.; Sanz, D.; Rodríguez, H. Toward a new focus in antibiotic and drug discovery from the Streptomyces arsenal. Front. Microbiol. 2015, 6, 461. [Google Scholar] [CrossRef] [PubMed]
- Anukool, U.; Gaze, W.H.; Wellington, E.M. In situ monitoring of streptothricin production by Streptomyces rochei F20 in soil and rhizosphere. Appl. Environ. Microbiol. 2004, 70, 5222–5228. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar]
- Johnston, C.W.; A Skinnider, M.; A Dejong, C.; Rees, P.N.; Chen, G.M.; Walker, C.G.; French, S.; Brown, E.D.; Bérdy, J.; Liu, D.Y.; et al. Assembly and clustering of natural antibiotics guides target identification. Nat. Chem. Biol. 2016, 12, 233–239. [Google Scholar] [CrossRef]
- Davies, J. Are antibiotics naturally antibiotics? J. Ind. Microbiol. Biotechnol. 2006, 33, 496–499. [Google Scholar] [CrossRef]
- Yim, G.; Wang, H.H.; Davies, J. The truth about antibiotics. Int. J. Med. Microbiol. 2006, 296, 163–170. [Google Scholar] [CrossRef]
- Davies, J. Everything depends on everything else. Clin. Microbiol. Infect. 2009, 15 (Suppl. S1), 1–4. [Google Scholar] [CrossRef] [PubMed]
- Romero, D.; Traxler, M.F.; López, D.; Kolter, R. Antibiotics as signal molecules. Chem. Rev. 2011, 111, 5492–5505. [Google Scholar] [CrossRef] [PubMed]
- Challis, G.L.; Hopwood, D.A. Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc. Natl. Acad. Sci. USA 2003, 100 (Suppl. S2), 14555–14561. [Google Scholar] [CrossRef] [PubMed]
- Bibb, M.; Hesketh, A. Analyzing the regulation of antibiotic production in streptomycetes. Methods Enzymol. 2009, 458, 93–116. [Google Scholar]
- Martin, J.F. Clusters of genes for the biosynthesis of antibiotics: Regulatory genes and overproduction of pharmaceuticals. J. Ind. Microbiol. 1992, 9, 73–90. [Google Scholar]
- Bibb, M. The regulation of antibiotic production in Streptomyces coelicolor A3. Microbiology 1996, 142, 1335–1344. [Google Scholar] [CrossRef]
- Strauch, E.; Takano, E.; Baylis, H.A.; Bibb, M.J. The stringent response in Streptomyces coelicolor A3. Mol. Microbiol. 1991, 5, 289–298. [Google Scholar] [CrossRef]
- Shapiro, R.A.; Tietje, K.M.; Subers, E.M.; Scherer, N.M.; Habecker, B.A.; Nathanson, N.M. Regulation of muscarinic acetylcholine receptor function in cardiac cells and in cells expressing cloned receptor genes. Trends Pharmacol. Sci. 1989, 43–46. [Google Scholar]
- Ishizuka, H.; Horinouchi, S.; Kieser, H.M.; Hopwood, D.A.; Beppu, T. A putative two-component regulatory system involved in secondary metabolism in regulation in Streptomyces spp. J. Bacteriol. 1992, 174, 7585–7594. [Google Scholar] [CrossRef][Green Version]
- Choi, M.H.; Lee, H.J.; Rho, J.K.; Yoon, S.C.; Nam, J.D.; Lim, D.; Lenz, R.W. Biosynthesis and local sequence specific degradation of poly(3-hydroxyvalerate-co-4-hydroxybutyrate) in Hydrogenophaga pseudoflava. Biomacromolecules 2003, 4, 38–45. [Google Scholar] [CrossRef]
- van Wezel, G.P.; McDowall, K.J. The regulation of the secondary metabolism of Streptomyces: New links and experimental advances. Nat. Prod. Rep. 2011, 28, 1311–1333. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.W.; Bai, L.; Clade, D.; Hoffmann, D.; Toelzer, S.; Trinh, K.Q.; Xu, J.; Moss, S.J.; Leistner, E.; Floss, H.G. The biosynthetic gene cluster of the maytansinoid antitumor agent ansamitocin from Actinosynnema pretiosum. Proc. Natl. Acad. Sci. USA 2002, 99, 7968–7973. [Google Scholar] [CrossRef] [PubMed]
- Bamas-Jacques, N.; Lorenzon, S.; Lacroix, P.; De Swetschin, C.; Crouzet, J. Cluster organization of the genes of Streptomyces pristinaespiralis involved in pristinamycin biosynthesis and resistance elucidated by pulsed-field gel electrophoresis. J. Appl. Microbiol. 1999, 87, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Hwang, S.; Lee, Y.; Cho, S.; Palsson, B.; Cho, B.K. Synthetic Biology Tools for Novel Secondary Metabolite Discovery in Streptomyces. J. Microbiol. Biotechnol. 2019, 29, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.; Bethe, B.; Hofs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Marmann, A.; Aly, A.H.; Lin, W.; Wang, B.; Proksch, P. Co-cultivation--a powerful emerging tool for enhancing the chemical diversity of microorganisms. Mar. Drugs 2014, 12, 1043–1065. [Google Scholar] [CrossRef]
- Baral, B.; Akhgari, A.; Metsa-Ketela, M. Activation of microbial secondary metabolic pathways: Avenues and challenges. Synth. Syst. Biotechnol. 2018, 3, 163–178. [Google Scholar] [CrossRef]
- Zakrzewski, P.; Medema, M.H.; Gevorgyan, A.; Kierzek, A.M.; Breitling, R.; Takano, E. MultiMetEval: Comparative and multi-objective analysis of genome-scale metabolic models. PLoS ONE 2012, 7, e51511. [Google Scholar] [CrossRef][Green Version]
- Islam, M.A.; Zengler, K.; Edwards, E.A.; Mahadevan, R.; Stephanopoulos, G. Investigating Moorella thermoacetica metabolism with a genome-scale constraint-based metabolic model. Integr. Biol. 2015, 7, 869–882. [Google Scholar] [CrossRef]
- Kim, H.U.; Charusanti, P.; Lee, S.Y.; Weber, T. Metabolic engineering with systems biology tools to optimize production of prokaryotic secondary metabolites. Nat. Prod. Rep. 2016, 33, 933–941. [Google Scholar] [CrossRef]
- Alduina, R.; De Grazia, S.; Dolce, L.; Salerno, P.; Sosio, M.; Donadio, S.; Puglia, A.M. Artificial chromosome libraries of Streptomyces coelicolor A3 and Planobispora rosea. FEMS Microbiol. Lett. 2003, 218, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Welzel, K.; Pelzer, S.; Vente, A.; Wohlleben, W. Exploiting the genetic potential of polyketide producing streptomycetes. J. Biotechnol. 2003, 106, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Cho, W.J.; Song, M.C.; Park, S.W.; Kim, K.; Kim, E.; Lee, N.; Nam, S.J.; Oh, K.H.; Yoon, Y.J. Engineered biosynthesis of milbemycins in the avermectin high-producing strain Streptomyces avermitilis. Microb. Cell Factories 2017, 16, 9. [Google Scholar] [CrossRef]
- Schulz, S.; Schall, C.; Stehle, T.; Breitmeyer, C.; Krysenko, S.; Mitulski, A.; Wohlleben, W. Optimization of the precursor supply for an enhanced FK506 production in Streptomyces tsukubaensis. Front. Bioeng. Biotechnol. 2022, 10, 1067467. [Google Scholar] [CrossRef]
- Gust, B.; Challis, G.L.; Fowler, K.; Kieser, T.; Chater, K.F. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. USA 2003, 100, 1541–1546. [Google Scholar] [CrossRef]
- Komatsu, M.; Uchiyama, T.; Ōmura, S.; Cane, D.E.; Ikeda, H. Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proc. Natl. Acad. Sci. USA 2010, 107, 2646–2651. [Google Scholar] [CrossRef]
- Lu, Z.; Xie, P.; Qin, Z. Promotion of markerless deletion of the actinorhodin biosynthetic gene cluster in Streptomyces coelicolor. Acta Biochim. Biophys. Sin. 2010, 42, 717–721. [Google Scholar] [CrossRef][Green Version]
- Cobb, R.E.; Wang, Y.; Zhao, H. High-efficiency multiplex genome editing of Streptomyces species using an engineered CRISPR/Cas system. ACS Synth. Biol. 2015, 4, 723–728. [Google Scholar] [CrossRef]
- Huang, H.; Zheng, G.; Jiang, W.; Hu, H.; Lu, Y. One-step high-efficiency CRISPR/Cas9-mediated genome editing in Streptomyces. Acta Biochim. Biophys. Sin. 2015, 47, 231–243. [Google Scholar] [CrossRef]
- Zeng, H.; Wen, S.; Xu, W.; He, Z.; Zhai, G.; Liu, Y.; Deng, Z.; Sun, Y. Highly efficient editing of the actinorhodin polyketide chain length factor gene in Streptomyces coelicolor M145 using CRISPR/Cas9-CodA (sm) combined system. Appl. Microbiol. Biotechnol. 2015, 99, 10575–10585. [Google Scholar] [CrossRef]
- Mo, J.; Wang, S.; Zhang, W.; Li, C.; Deng, Z.; Zhang, L.; Qu, X. Efficient editing DNA regions with high sequence identity in actinomycetal genomes by a CRISPR-Cas9 system. Synth. Syst. Biotechnol. 2019, 4, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Charusanti, P.; Zhang, L.; Weber, T.; Lee, S.Y. CRISPR-Cas9 based engineering of actinomycetal genomes. ACS Synth. Biol. 2015, 4, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cai, D.; Wang, Z.; He, Z.; Chen, S. Development of an efficient genome editing tool in Bacillus licheniformis using CRISPR-Cas9 nickase. Appl. Environ. Microbiol. 2018, 84, e02608–e02617. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Whitford, C.M.; Robertsen, H.L.; Blin, K.; Jørgensen, T.S.; Klitgaard, A.K.; Gren, T.; Jiang, X.; Weber, T.; Lee, S.Y. Highly efficient DSB-free base editing for streptomycetes with CRISPR-BEST. Proc. Natl. Acad. Sci. USA 2019, 116, 20366–20375. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, J.; Zheng, G.; Chen, J.; Sun, C.; Yang, Z.; Zimin, A.A.; Jiang, W.; Deng, Z.; Wang, Z. Multiplex genome editing using a dCas9-cytidine deaminase fusion in Streptomyces. Sci. China Life Sci. 2019, 63, 1053–1062. [Google Scholar] [CrossRef]
- Yeo, W.L.; Heng, E.; Tan, L.L.; Lim, Y.W.; Lim, Y.H.; Hoon, S.; Zhao, H.; Zhang, M.M.; Wong, F.T. Characterization of Cas proteins for CRISPR-Cas editing in Streptomycetes. Biotechnol. Bioeng. 2019, 116, 2330–2338. [Google Scholar] [CrossRef]
- Myronovskyi, M.; Rosenkränzer, B.; Nadmid, S.; Pujic, P.; Normand, P.; Luzhetskyy, A. Generation of a cluster-free Streptomyces albus chassis strains for improved heterologous expression of secondary metabolite clusters. Metab. Eng. 2018, 49, 316–324. [Google Scholar] [CrossRef]
- Martín, J.F.; Liras, P. The Balance Metabolism Safety Net: Integration of Stress Signals by Interacting Transcriptional Factors in Streptomyces and Related actinobacteria. Front. Microbiol. 2020, 10, 3120. [Google Scholar] [CrossRef]
- Starcevic, A.; Zucko, J.; Simunkovic, J.; Long, P.F.; Cullum, J.; Hranueli, D. ClustScan: An integrated program package for the semi-automatic annotation of modular biosynthetic gene clusters and in silico prediction of novel chemical structures. Nucleic Acids Res. 2008, 36, 6882–6892. [Google Scholar] [CrossRef]
- Li, M.H.; Ung, P.M.; Zajkowski, J.; Garneau-Tsodikova, S.; Sherman, D.H. Automated genome mining for natural products. BMC Bioinform. 2009, 10, 185. [Google Scholar] [CrossRef]
- Skinnider, M.A.; Dejong, C.A.; Rees, P.N.; Johnston, C.W.; Li, H.; Webster, A.L.H.; Wyatt, M.A.; Magarvey, N.A. Genomes to natural products prediction informatics for secondary metabolomes (PRISM). Nucleic Acids Res. 2015, 43, 9645–9662. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Wolf, T.; Chevrette, M.G.; Lu, X.; Schwalen, C.J.; Kautsar, S.A.; Duran, H.G.S.; de los Santos, E.L.C.; Kim, H.U.; Nave, M.; et al. antiSMASH 4.0-improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 2017, 45, W36–W41. [Google Scholar] [CrossRef] [PubMed]
- Miao, V.; Coeffet-LeGal, M.F.; Brian, P.; Brost, R.; Penn, J.; Whiting, A.; Martin, S.; Ford, R.; Parr, I.; Bouchard, M.; et al. Daptomycin biosynthesis in Streptomyces roseosporus: Cloning and analysis of the gene cluster andrevision of peptide stereochemistry. Microbiology 2005, 151, 1507–1523. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, F.; Tong, Y.; Habisch, R.; Yang, B.; Zhang, L.; Müller, R.; Fu, C. Dual-function chromogenic screening-based CRISPR/Cas9 genome editing system for actinomycetes. Appl. Microbiol. Biotechnol. 2020, 104, 225–239. [Google Scholar] [CrossRef]
- Fuchs, G. Allgemeine Mikrobiologie. 8. Auflage; Georg Thieme Verlag Stuttgart: New York, NY, USA, 2007. [Google Scholar]
- Hodgson, D.A. Primary metabolism and its control in streptomycetes: A most unusual group of bacteria. Adv. Microb. Physiol. 2000, 42, 47–238. [Google Scholar]
- Champness, W.C.; Chater, K.F. Regulation and integration of antibiotic production and morphological differentiation in Streptomyces spp. In Regulation of Bacterial Differentiation; Piggot, P.J., Moran, C.P., Jr., Youngman, P., Hrsg, Eds.; American Society for Microbiology Press: Washington, DC, USA, 1994; pp. 61–93. [Google Scholar]
- Karandikar, A.; Sharples, G.P.; Hobbs, G. Differentiation of Streptomyces coelicolor A3 under nitrate-limited conditions. Microbiology 1997, 143, 3581–3590. [Google Scholar] [CrossRef]
- Brana, A.F.; und Demain, A.L.S. Nitrogen Control of Antibiotic Biosynthesis in Actinomycetes Nitrogen Source Control of Microbial Processes; CRC Press: Boca Raton, FL, USA, 1988; pp. 99–119. [Google Scholar]
- Hobbs, G.; Obanye, A.I.; Petty, J.; Mason, J.C.; Barratt, E.; Garnder, D.C.; Flett, F.; Smith, C.P.; Broda, P.; Hodgson, D.A. An integrated approach to studying regulation of production of the antibiotic methylenomycin by Streptomyces coelicolor A3. J. Bacteriol. 1992, 174, 1487–1497. [Google Scholar] [CrossRef]
- Chater, K.F. Regulation of sporulation in Streptomyces coelicolor A3: A checkpoint multiplex? Curr. Opin. Microbiol. 2001, 4, 667–673. [Google Scholar] [CrossRef]
- Voelker, F.; Altaba, S. Nitrogen source governs the patterns of growth and pristinamycin production in ‘Streptomyces pristinaespiralis’. Microbiology 2001, 147, 2447–2459. [Google Scholar] [CrossRef][Green Version]
- Magasanik, B. Genetic control of nitrogen assimilation in bacteria. Annu. Rev. Genet. 1982, 16, 135–168. [Google Scholar] [CrossRef]
- Krysenko, S.; Wohlleben, W. Polyamine and Ethanolamine Metabolism in Bacteria as an Important Component of Nitrogen Assimilation for Survival and Pathogenicity. Med. Sci. 2022, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Merrick, M.J.; Edwards, R.A. Nitrogen control in bacteria. Microbiol. Rev. 1995, 59, 604–622. [Google Scholar] [CrossRef] [PubMed]
- Krysenko, S.; Wohlleben, W. Überlebenswichtig: Glutaminsynthetasehomologe Proteine in Streptomyceten. Biospektrum 2022, 28, 23–26. [Google Scholar] [CrossRef]
- Fink, D.; Falke, D.; Wohlleben, W.; Engels, A. Nitrogen metabolism in Streptomyces coelicolor A3: Modification of glutamine synthetase I by an adenylyltransferase. Microbiology 1999, 145, 2313–2322. [Google Scholar] [CrossRef]
- Hesketh, A.; Fink, D.; Gust, B.; Rexer, H.U.; Scheel, B.; Chater, K.; Wohlleben, W.; Engels, A. The GlnD and GlnK homologues of Streptomyces coelicolor A3 are functionally dissimilar to their nitrogen regulatory system counterparts from enteric bacteria. Mol. Microbiol. 2002, 46, 319–330. [Google Scholar] [CrossRef]
- Strösser, J.; Lüdke, A.; Schaffer, S.; Krämer, R.; Burkovski, A. Regulation of GlnK activity: Modification, membrane sequestration, and proteolysis as regulatory principles in the network of nitrogen control in Corynebacterium glutamicum. Mol. Microbiol. 2004, 54, 132–147. [Google Scholar] [CrossRef]
- Reuther, J.; Wohlleben, W. Nitrogen metabolism in Streptomyces coelicolor: Transcriptional and post-translational regulation. J. Mol. Microbiol. Biotechnol. 2007, 12, 139–146. [Google Scholar] [CrossRef]
- Waldvogel, E.; Herbig, A.; Battke, F.; Amin, R.; Nentwich, M.; Nieselt, K.; Ellingsen, T.E.; Wentzel, A.; Hodgson, D.A.; Wohlleben, W.; et al. The PII protein GlnK is a pleiotropic regulator for morphological differentiation and secondary metabolism in Streptomyces coelicolor. Appl. Microbiol. Biotechnol. 2011, 92, 1219–1236. [Google Scholar] [CrossRef]
- Wray, L.V., Jr.; Atkinson, M.R.; Fisher, S.H. Identification and cloning of the glnR locus, which is required for transcription of the glnA gene in Streptomyces coelicolor A3. J. Bacteriol. 1991, 173, 7351–7360. [Google Scholar] [CrossRef]
- Tiffert, Y.; Supra, P.; Wurm, R.; Wohlleben, W.; Wagner, R.; Reuther, J. The Streptomyces coelicolor GlnR regulon: Identification of new GlnR targets and evidence for a central role of GlnR in nitrogen metabolism in Actinomycetes. Mol. Microbiol. 2008, 67, 861–880. [Google Scholar] [CrossRef]
- Amin, R.; Franz-Wachtel, M.; Tiffert, Y.; Heberer, M.; Meky, M.; Ahmed, Y.; Matthews, A.; Krysenko, S.; Jakobi, M.; Hinder, M.; et al. Post-translational Serine/Threonine Phosphorylation and Lysine Acetylation: A Novel Regulatory Aspect of the Global Nitrogen Response Regulator GlnR in S. coelicolor M145. Front. Mol. Biosci. 2016, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Krysenko, S.; Okoniewski, N.; Nentwich, M.; Matthews, A.; Bäuerle, M.; Zinser, A.; Busche, T.; Kulik, A.; Gursch, S.; Kemeny, A.; et al. A Second Gamma-Glutamylpolyamine Synthetase, GlnA2, Is Involved in Polyamine Catabolism in Streptomyces coelicolor. Int. J. Mol. Sci. 2022, 23, 3752. [Google Scholar] [CrossRef] [PubMed]
- Wray, L.V., Jr.; Fisher, S.H. The Streptomyces coelicolor glnR gene encodes a protein similar to other bacterial response regulators. Gene 1993, 130, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Hindra Mulder, D.; Yin, C.; Elliot, M.A. Crp is a global regulator of antibiotic production in streptomyces. mBio 2012, 3, e00407–e00412. [Google Scholar] [CrossRef]
- Schulz, S.; Sletta, H.; Degnes, K.F.; Krysenko, S.; Williams, A.; Olsen, S.M.; Vernstad, K.; Mitulski, A.; Wohlleben, W. Optimization of FK-506 production in Streptomyces tsukubaensis by modulation of Crp-mediated regulation. Appl. Microbiol. Biotechnol. 2023, 107, 2871–2886. [Google Scholar] [CrossRef]
- Perez-Redondo, R.; Rodriguez-Garcia, A.; Botas, A.; Santamarta, I.; Martin, J.F.; Liras, P. ArgR of Streptomyces coelicolor is a versatile regulator. PLoS ONE 2012, 7, e32697. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; Sola-Landa, A.; Apel, K.; Santos-Beneit, F.; Martín, J.F. Phosphate control over nitrogen metabolism in Streptomyces coelicolor: Direct and indirect negative control of glnR, glnA, glnII and amtB expression by the response regulator PhoP. Nucleic Acids Res. 2009, 37, 3230–3242. [Google Scholar] [CrossRef]
- Santos-Beneit, F.; Rodriguez-Garcia, A.; Martin, J.F. Overlapping binding of PhoP and AfsR to the promoter region of glnR in Streptomyces coelicolor. Microbiol. Res. 2012, 167, 532–535. [Google Scholar] [CrossRef]
- Rigali, S.; Titgemeyer, F.; Barends, S.; Mulder, S.; Thomae, A.W.; Hopwood, D.A.; van Wezel, G.P. Feast or famine: The global regulator DasR links nutrient stress to antibiotic production by Streptomyces. EMBO Rep. 2008, 9, 670–675. [Google Scholar] [CrossRef]
- Wang, R.; Mast, Y.; Wang, J.; Zhang, W.; Zhao, G.; Wohlleben, W.; Lu, Y.; Jiang, W. Identification of two-component system AfsQ1/Q2 regulon and its cross-regulation with GlnR in Streptomyces coelicolor. Mol. Microbiol. 2013, 87, 30–48. [Google Scholar] [CrossRef]
- Lopez, P.; Hornung, A.; Welzel, K.; Unsin, C.; Wohlleben, W.; Weber, T.; Pelzer, S. Isolation of the lysolipin gene cluster of Streptomyces tendae Tü 4042. Gene 2010, 461, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Finking, R.; Marahiel, M.A. Biosynthesis of nonribosomal peptides1. Annu. Rev. Microbiol. 2004, 58, 453–488. [Google Scholar] [CrossRef] [PubMed]
- Staunton, J.; Weissman, K.J. Polyketide biosynthesis: A millennium review. Nat. Prod. Rep. 2001, 18, 380–416. [Google Scholar] [CrossRef] [PubMed]
- Caboche, S.; Pupin, M.; Leclère, V.; Fontaine, A.; Jacques, P.; Kucherov, G. NORINE: A database of nonribosomal peptides. Nucleic Acids Res. 2008, 36 (Suppl. S1), D326–D331. [Google Scholar] [CrossRef]
- Spížek, J.; Tichý, P. Some aspects of overproduction of secondary metabolites. Folia Microbiol. 1995, 40, 43–50. [Google Scholar] [CrossRef]
- Ungermann, V.; Fiedler, H.-P.; Utz, R.; Kellner, R.; Hörner, T.; Zähner, H.; Jung, G. Comparative studies on the fermentative production of lantibiotics by staphylococci. Appl. Microbiol. Biotechnol. 1990, 32, 511–517. [Google Scholar]
- De Vuyst, L.; Vandamme, E.J. Influence of the carbon source on nisin production in Lactococcus lactis subsp. lactis batch fermentations. J. General. Microbiol. 1992, 138, 571–578. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, D.K.; Jani, R.H.; Blumbach, J.; Ganguli, B.N.; Klesel, N.; Limbert, M.; Seibert, G. Mersacidin, a new antibiotic from Bacillus. In vitro and in vivo antibacterial activity. J. Antibiot. 1992, 45, 839–845. [Google Scholar] [CrossRef]
- Münch, D.; Müller, A.; Schneider, T.; Kohl, B.; Wenzel, M.; Bandow, J.E.; Maffioli, S.; Sosio, M.; Donadio, S.; Wimmer, R.; et al. The lantibiotic NAI-107 binds to bactoprenol-bound cell wall precursors and impairs membrane functions. J. Biol. Chem. 2014, 289, 12063–12076. [Google Scholar] [CrossRef]
- Giardina, A.; Alduina, R.; Gallo, G.; Monciardini, P.; Sosio, M.; Puglia, A.M. Inorganic phosphate is a trigger factor for Microbispora sp. ATCC-PTA-5024 growth and NAI-107 production. Microb. Cell Factories 2014, 13, 133. [Google Scholar] [CrossRef]
- Aharonowitz, Y. Nitrogen metabolite regulation of antibiotic biosynthesis. Annu. Rev. Microbiol. 1980, 34, 209–233. [Google Scholar] [CrossRef] [PubMed]
- Vu-Trong, K.; Gray, P. Influence of ammonium on the biosynthesis of the macrolide antibiotic tylosin. Enzym. Microb. Technol. 1987, 9, 590–593. [Google Scholar] [CrossRef]
- Rokem, J.S.; Lantz, A.E.; Nielsen, J. Systems biology of antibiotic production by microorganisms. Nat. Prod. Rep. 2007, 24, 1262–1287. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Cong, W.; Cai, Z. Nisin Production by Lactococcus lactis subsp. lactis under Nutritional Limitation in Fed-Batch Culture. Biotechnol. Lett. 2004, 26, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Guerra, N.P.; Rua, M.; Pastrana, L. Nutritional factors affecting the production of two bacteriocins from lactic acid bacteria on whey. Int. J. Food Microbiol. 2001, 70, 267–281. [Google Scholar] [CrossRef]
- Bascaran, V.; Hardisson, C.; Brana, A.F. Regulation of nitrogen catabolic enzymes in Streptomyces clavuligerus. J. Gen. Microbiol. 1989, 135, 2465–2474. [Google Scholar] [PubMed]
- Li, X.; Yu, F.; Liu, K.; Zhang, M.; Cheng, Y.; Wang, F.; Wang, S.; Han, R.; Xue, Z. Uncovering the Effects of Ammonium Sulfate on Neomycin B Biosynthesis in Streptomyces fradiae SF-2. Fermentation 2022, 8, 678. [Google Scholar] [CrossRef]
- Wallace, K.K.; Payne, G.F.; Speedie, M.K. Ammonium effects on streptonigrin biosynthesis by Streptomyces flocculus. J. Ind. Microbiol. 1990, 6, 43–48. [Google Scholar] [CrossRef]
- El-Naggar, M.Y.; El-Assar, S.A.; Abdul-Gawad, S.M. Meroparamycin production by newly isolated Streptomyces sp. strain MAR01: Taxonomy, fermentation, purification and structural elucidation. J. Microbiol. 2006, 44, 432–438. [Google Scholar]
- El Hassan, A.M.; Fahal, A.H.; Ahmed, A.O.; Ismail, A.; Veress, B. The immunopathology of actinomycetoma lesions caused by Streptomyces somaliensis. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 89–92. [Google Scholar] [CrossRef]
- Shao, Z.H.; Ren, S.X.; Liu, X.Q.; Xu, J.; Yan, H.; Zhao, G.P.; Wang, J. A preliminary study of the mechanism of nitrate-stimulated remarkable increase of rifamycin production in Amycolatopsis mediterranei U32 by RNA-seq. Microb. Cell Fact. 2015, 14, 75. [Google Scholar] [CrossRef]
- Yu, H.; Yao, Y.; Liu, Y.; Jiao, R.; Jiang, W.; Zhao, G.P. A complex role of Amycolatopsis mediterranei GlnR in nitrogen metabolism and related antibiotics production. Arch. Microbiol. 2007, 188, 89–96. [Google Scholar] [CrossRef]
- Yao, L.L.; Liao, C.H.; Huang, G.; Zhou, Y.; Rigali, S.; Zhang, B.; Ye, B.C. GlnR-mediated regulation of nitrogen metabolism in the actinomycete Saccharopolyspora erythraea. Appl. Microbiol. Biotechnol. 2014, 98, 7935–7948. [Google Scholar] [CrossRef]
- Neubauer, H.; Götz, F. Physiology and interaction of nitrate and nitrite reduction in Staphylococcus carnosus. J. Bacteriol. 1996, 178, 2005–2009. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, R.R.; Schneider, T.; Sahl, H.-G.; Wiedemann, I. Insights into in vivo activities of lantibiotics from gallidermin and epidermin mode-of-action studies. Antimicrob. Agents Chemother. 2006, 50, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Theobald, U.; Fiedler, H.-P. Correlation between the consumption of amino acids and the production of the antibiotic gallidermin by Staphylococcus gallinarum. Biotechnol. Lett. 1999, 21, 959–963. [Google Scholar] [CrossRef]
- Hu, D.S.; Hood, D.W.; Heidstra, R.; Hodgson, D.A. The expression of the trpD, trpC and trpBA genes of Streptomyces coelicolor A3 is regulated by growth rate and growth phase but not by feedback repression. Mol. Microbiol. 1999, 32, 869–880. [Google Scholar] [CrossRef]
- Mast, Y.; Weber, T.; Gölz, M.; Ort-Winklbauer, R.; Gondran, A.; Wohlleben, W.; Schinko, E. Characterization of the ‘pristinamycin supercluster’of Streptomyces pristinaespiralis. Microb. Biotechnol. 2011, 4, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.A.; Cavallieri, A.P.; Araujo, M.L. Enhancing effect of lysine combined with other compounds on cephamycin C production in Streptomyces clavuligerus. BMC Microbiol. 2013, 13, 296. [Google Scholar] [CrossRef]
- Yatin, M. Polyamines in living organisms. Mol. Cell. Biol. 2002, 1, 57–67. [Google Scholar]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef] [PubMed]
- Young, C.C.; Chen, L.F. Polyamines in humic acid and their effect on radical growth of lettuce seedlings. Plant Soil 1997, 195, 143–149. [Google Scholar] [CrossRef]
- Burrell, M.; Hanfrey, C.C.; Kinch, L.N.; Elliot, K.A.; Michael, J.A. Evolution of a novel lysine decarboxylase in siderophore biosynthesis. Mol. Microbiol. 2012, 86, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kurihara, S. Polyamine Catabolism in Prokaryotes. In Polyamines: A Universal Molecular Nexus for Growth, Survival, and Specialized Metabolism; Kusano, T., Suzuki, H., Eds.; Springer: Tokyo, Japan, 2015; pp. 47–59. [Google Scholar]
- Krysenko, S.; Okoniewski, N.; Kulik, A.; Matthews, A.; Grimpo, J.; Wohlleben, W.; Bera, A. Gamma-Glutamylpolyamine Synthetase GlnA3 is involved in the first step of polyamine degradation pathway in Streptomyces coelicolor M145. Front. Microbiol. 2017, 8, 726. [Google Scholar] [CrossRef]
- Krysenko, S.; Lopez, M.; Meyners, C.; Purder, P.L.; Zinser, A.; Hausch, F.; Wohlleben, W. A novel synthetic inhibitor of polyamine utilization in Streptomyces coelicolor. FEMS Microbiol. Lett. 2023, 370, fnad096. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Martín, J.; Garcia-Estrada, C.; Kosalkova, K.; Ullán, R.V.; Albillos, S.; Martín, J.-F. The inducers 1,3-diaminopropane and spermidine produce a drastic increase in the expression of the penicillin biosynthetic genes for prolonged time, mediated by the LaeA regulator. Fungal Genet. Biol. 2012, 49, 1004–1013. [Google Scholar] [CrossRef]
- Leitão, A.L.; Enguita, F.J.; De La Fuente, J.L.; Liras, P.; Martin, J.F. Inducing Effect of Diamines on Transcription of the Cephamycin C Genes from the lat and pcbAB Promoters in Nocardia lactamdurans. J. Bacteriol. 1999, 181, 2379–2384. [Google Scholar] [CrossRef]
- Omura, S.; Tanaka, Y.; Kitao, C.; Tanaka, H.; Iwai, Y. Stimulation of leucomycin production by magnesium phosphate and its relevance to nitrogen catabolite regulation. Antimicrob. Agents Chemother. 1980, 18, 691–695. [Google Scholar] [CrossRef]
- Gesheva, V.; Ivanova, V.; Gesheva, R. Effects of nutrients on the production of AK-111-81 macrolide antibiotic by Streptomyces hygroscopicus. Microbiol. Res. 2005, 160, 243–248. [Google Scholar] [CrossRef]
- Sujatha, P.; Bapi Raju, K.V.; Ramana, T. Studies on a new marine streptomycete BT-408 producing polyketide antibiotic SBR-22 effective against methicillin resistant Staphylococcus aureus. Microbiol. Res. 2005, 160, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kamel, Z.; Al-Zahrani, S.H. Optimization of a Growth Medium for Antibiotic Production by Streptomyces anandii var. Taifiensis. In Perspectives in Biotechnology and Applied Microbiology; Alani, D.I., Moo-Young, M., Eds.; Springer: Dordrecht, The Netherlands, 1986. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, W.Y. Improvement of fermentation conditions for azalomycin B produced by Streptomyces hygroscopicus. Chin. J. Bioprocess. Eng. 2004, 2, 53–57. [Google Scholar]
- Stocks, S.M.; Thomas, C.R. Strength of mid-logarithmic and stationary phase Saccharopolyspora erythraea hyphae during a batch fermentation in defined nitrate-limited medium. Biotechnol. Bioeng. 2001, 73, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Huang, W.Y. Study of lividomycin produced by lividomycin producer M814. J. Zhejiang Univ. Technol. 1995, 23, 67–72. [Google Scholar]
- Ababutain, I.M.; Abdul Aziz, Z.K.; AL-Meshhen, N.A. Optimization of environmental and nutritional conditions to improve growth and antibiotic productions by Streptomyces sp. Isolated from Saudi Arabia Soil. Int. Res. J. Microbiol. 2013, 4, 179–187. [Google Scholar]
- Jiao, R.S.; Chen, Y.M.; Wu, M.G.; Gu, W.L. Studies on the metabolic regulation of biosynthesis of rifamycin by Norcadia (Amycolatopsis) mediterranei I. The stimulative effect of nitrate on biosynthesis of rifamycin SV by Nocardia mediterranei. Acta Phytophysiol. Sin. 1979, 5, 395–402. [Google Scholar]
- Martínez-Castro, M.; Salehi-Najafabadi, Z.; Romero, F.; Pérez-Sanchiz, R.; Fernández-Chimeno, R.I.; Martín, J.F.; Barreiro, C. Taxonomy and chemically semi-defined media for the analysis of the tacrolimus producer ‘Streptomyces tsukubaensis’. Appl. Microbiol. Biotechnol. 2013, 97, 2139–2152. [Google Scholar] [CrossRef]
- Cheng, Y.R.; Fang, A.; Demain, A.L. Effect of amino acids on rapamycin biosynthesis by Streptomyces hygroscopicus. Appl. Microbiol. Biotechnol. 1995, 43, 1096–1098. [Google Scholar] [CrossRef]
- Machado, H.; Tuttle, R.N.; Jensen, P.R. Omics-based natural product discovery and the lexicon of genome mining. Curr. Opin. Microbiol. 2017, 39, 136–142. [Google Scholar] [CrossRef]
- Jose, P.A.; Maharshi, A.; Jha, B. Actinobacteria in natural products research: Progress and prospects. Microbiol. Res. 2021, 246, 126708. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, L.; Luo, Y. Recent advances in natural products exploitation in Streptomyces via synthetic biology. Eng. Life Sci. 2019, 19, 452–462. [Google Scholar] [CrossRef] [PubMed]
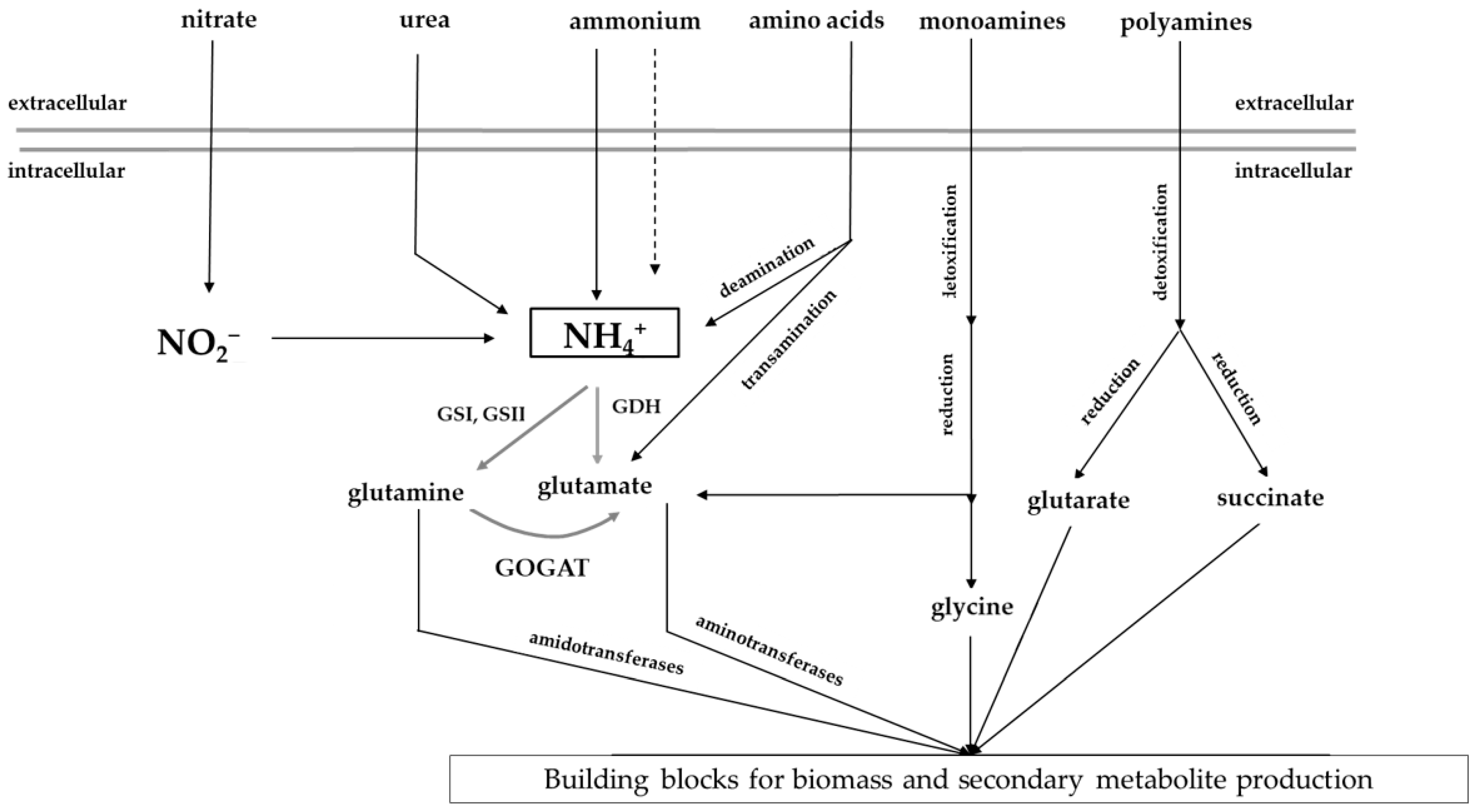
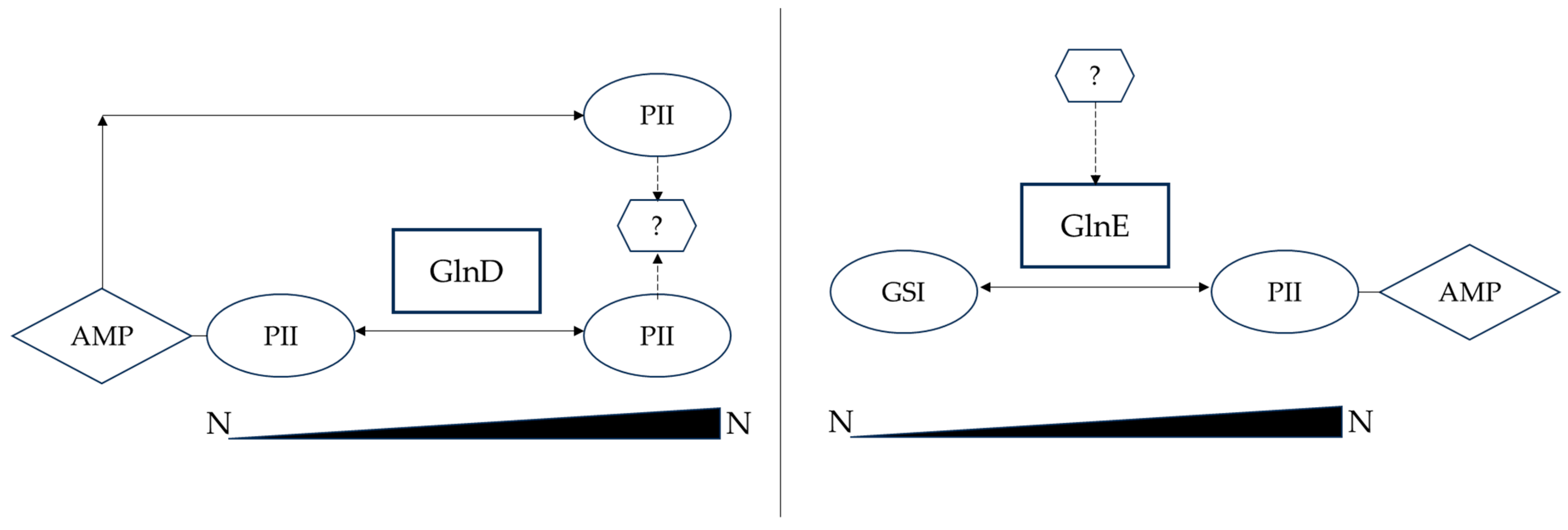
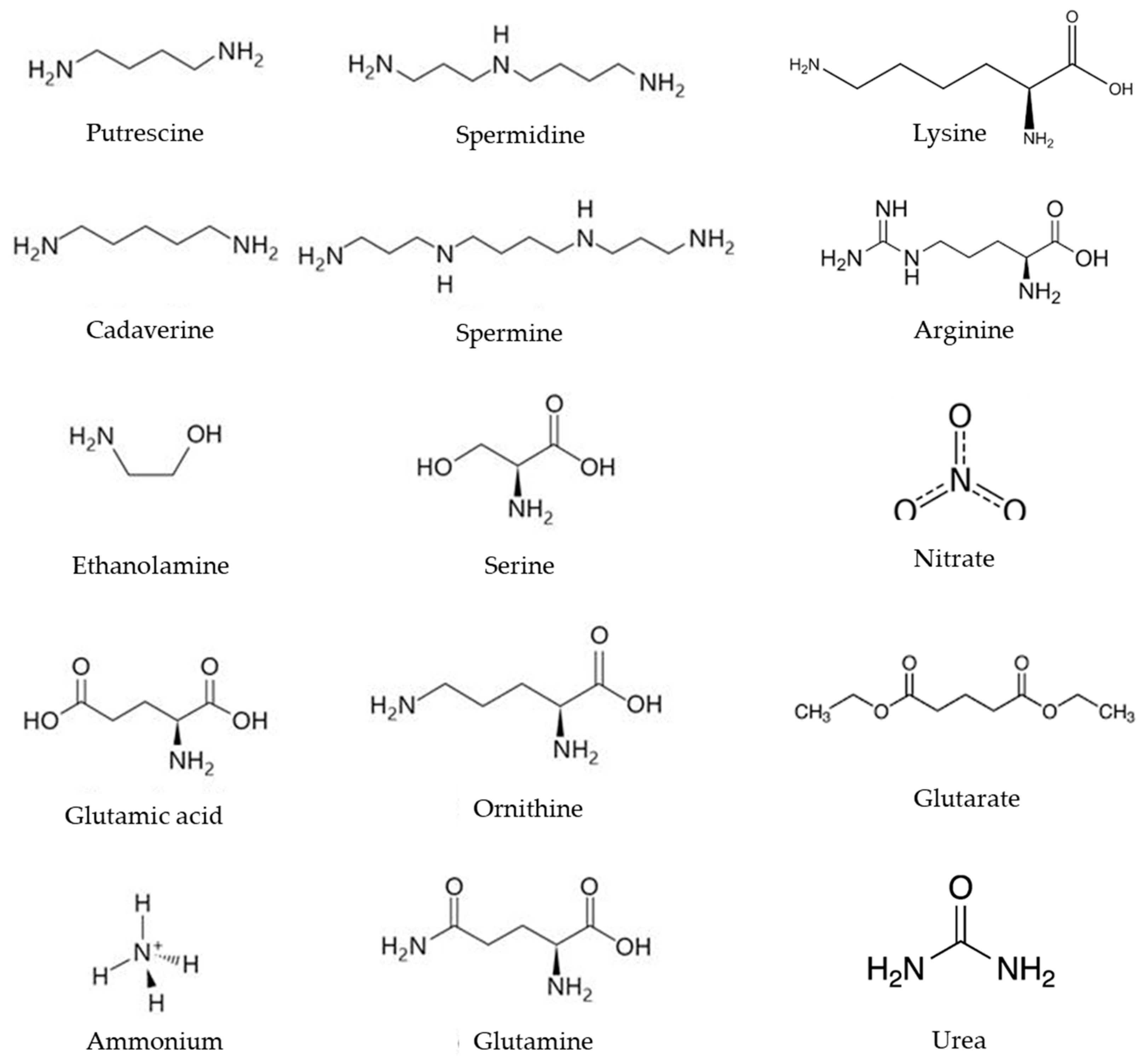

| Regulator Name | Function | Reference |
|---|---|---|
| GlnR | Central regulator of nitrogen metabolism regulating glnA, glnII, gdhA, nirB, ureA, and amtB-glnK-glnD | [87] |
| GlnRII | A GlnR homologue that recognizes glnA, amtB, and glnII | [82] |
| Crp | Regulates the interplay of primary and secondary metabolism, activating glnA, glnII, and amtB-glnK-glnD | [99] |
| ArgR | Controls the expression of glnR in response to nutrient-stress stimuli | [101] |
| PhoP | Represses the amtB-glnK-glnD operon and glnA, glnII, and glnR under conditions of phosphate limitation | [102] |
| AfsR | Controls expression of glnR in response to unknown nutrient stress stimulus | [103] |
| DasR | Links nutrient stress to antibiotic production | [104] |
| AfsQ1 | Required for regulation of carbon, nitrogen, and phosphate metabolism in the presence of glutamate | [105] |
| MtrA | Activates antibiotic biosynthetic gene clusters | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krysenko, S. Impact of Nitrogen-Containing Compounds on Secondary Metabolism in Streptomyces spp.—A Source of Metabolic Engineering Strategies. SynBio 2023, 1, 204-225. https://doi.org/10.3390/synbio1030015
Krysenko S. Impact of Nitrogen-Containing Compounds on Secondary Metabolism in Streptomyces spp.—A Source of Metabolic Engineering Strategies. SynBio. 2023; 1(3):204-225. https://doi.org/10.3390/synbio1030015
Chicago/Turabian StyleKrysenko, Sergii. 2023. "Impact of Nitrogen-Containing Compounds on Secondary Metabolism in Streptomyces spp.—A Source of Metabolic Engineering Strategies" SynBio 1, no. 3: 204-225. https://doi.org/10.3390/synbio1030015
APA StyleKrysenko, S. (2023). Impact of Nitrogen-Containing Compounds on Secondary Metabolism in Streptomyces spp.—A Source of Metabolic Engineering Strategies. SynBio, 1(3), 204-225. https://doi.org/10.3390/synbio1030015






