Drug Discovery for Periodontitis Treatment Based on Big Data Mining, Systems Biology, and Deep Learning Methods
Abstract
1. Introduction
2. Results
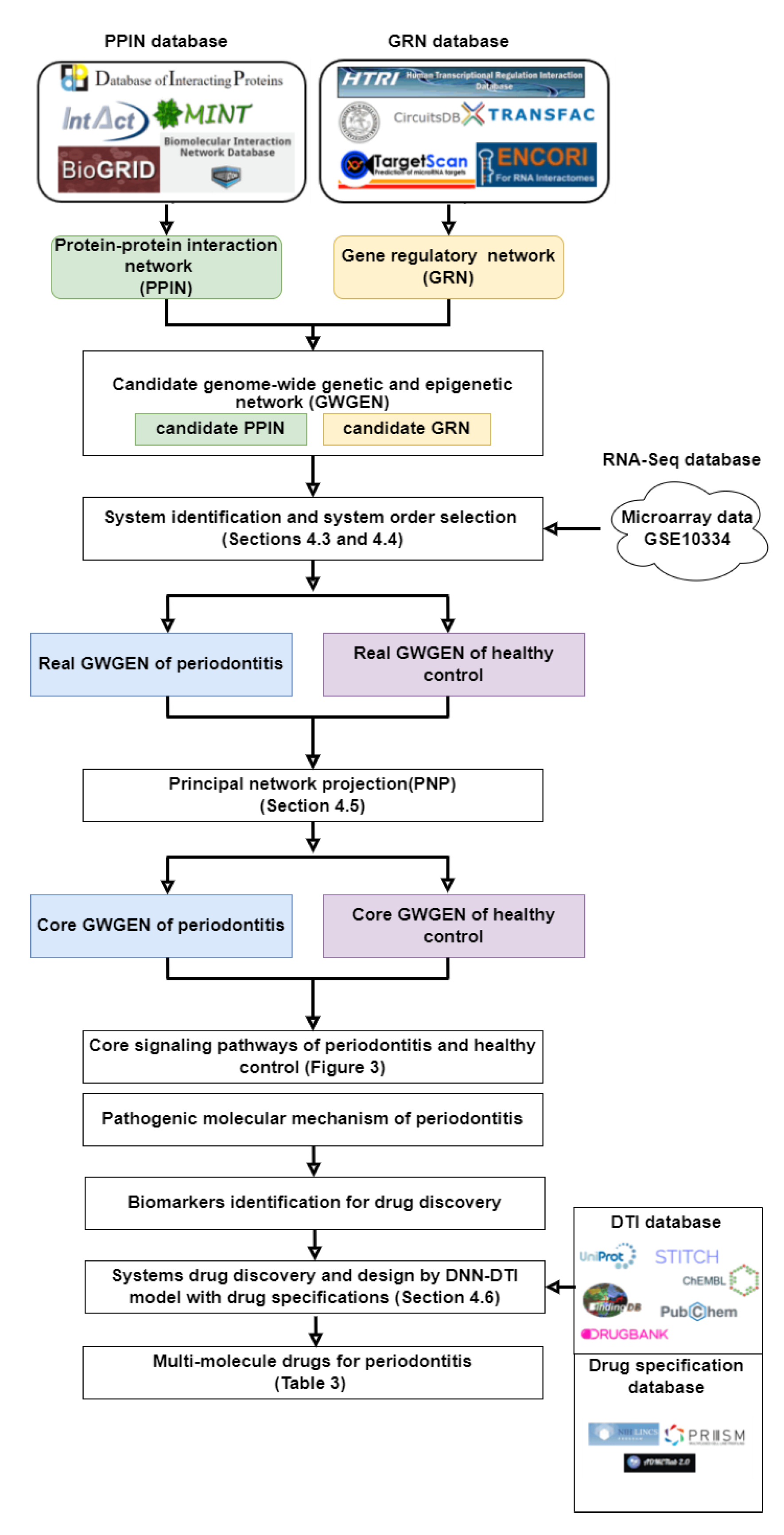
2.1. Overview of Systems Biology Method for Pathology Mechanism and Systematic Drug Discovery and Design for Periodontitis Treatment
2.2. Comparing Core Signaling Pathways of Periodontitis and Healthy Control to Identify Biomarkers of Pathological Mechanism of Periodontitis
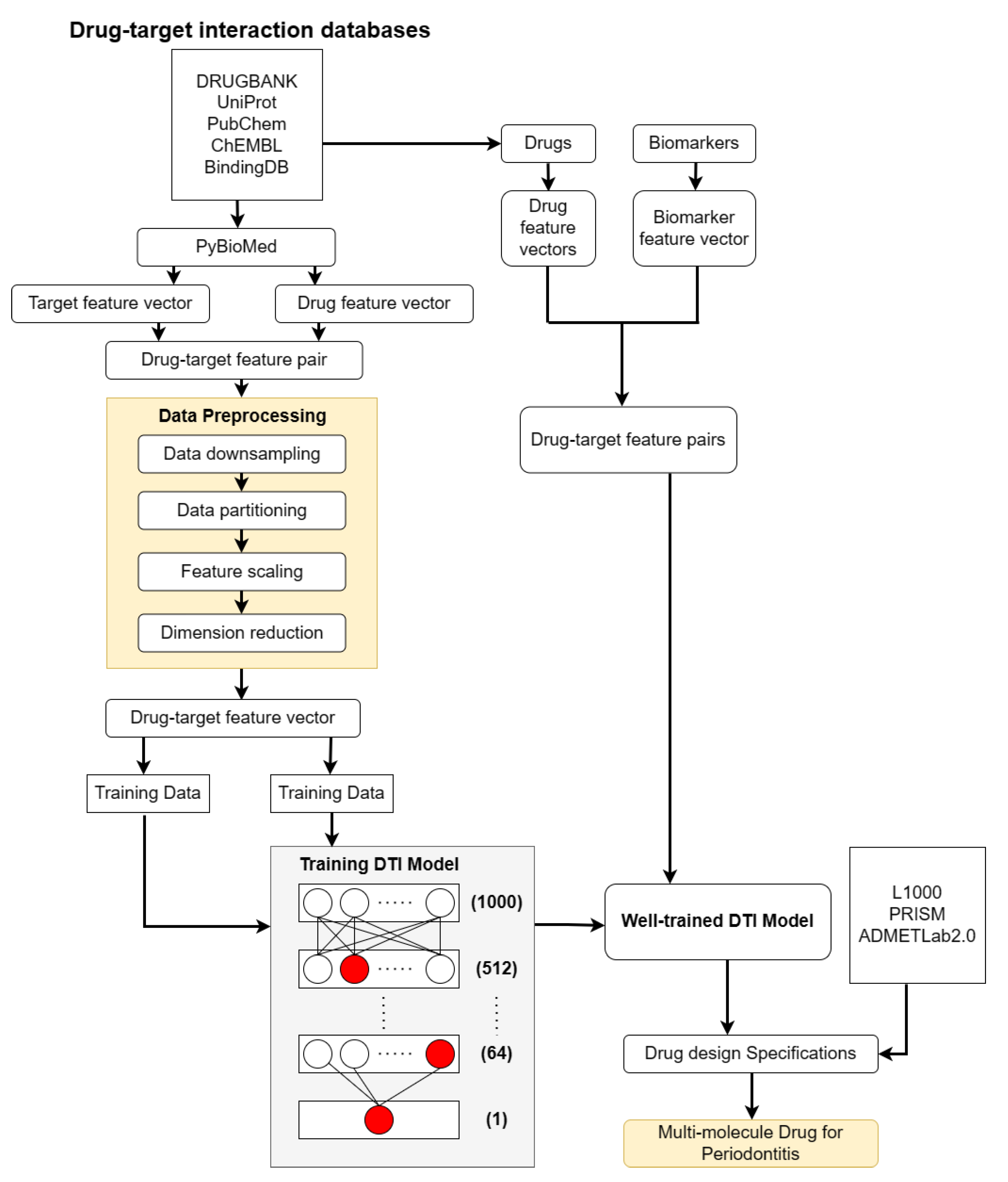
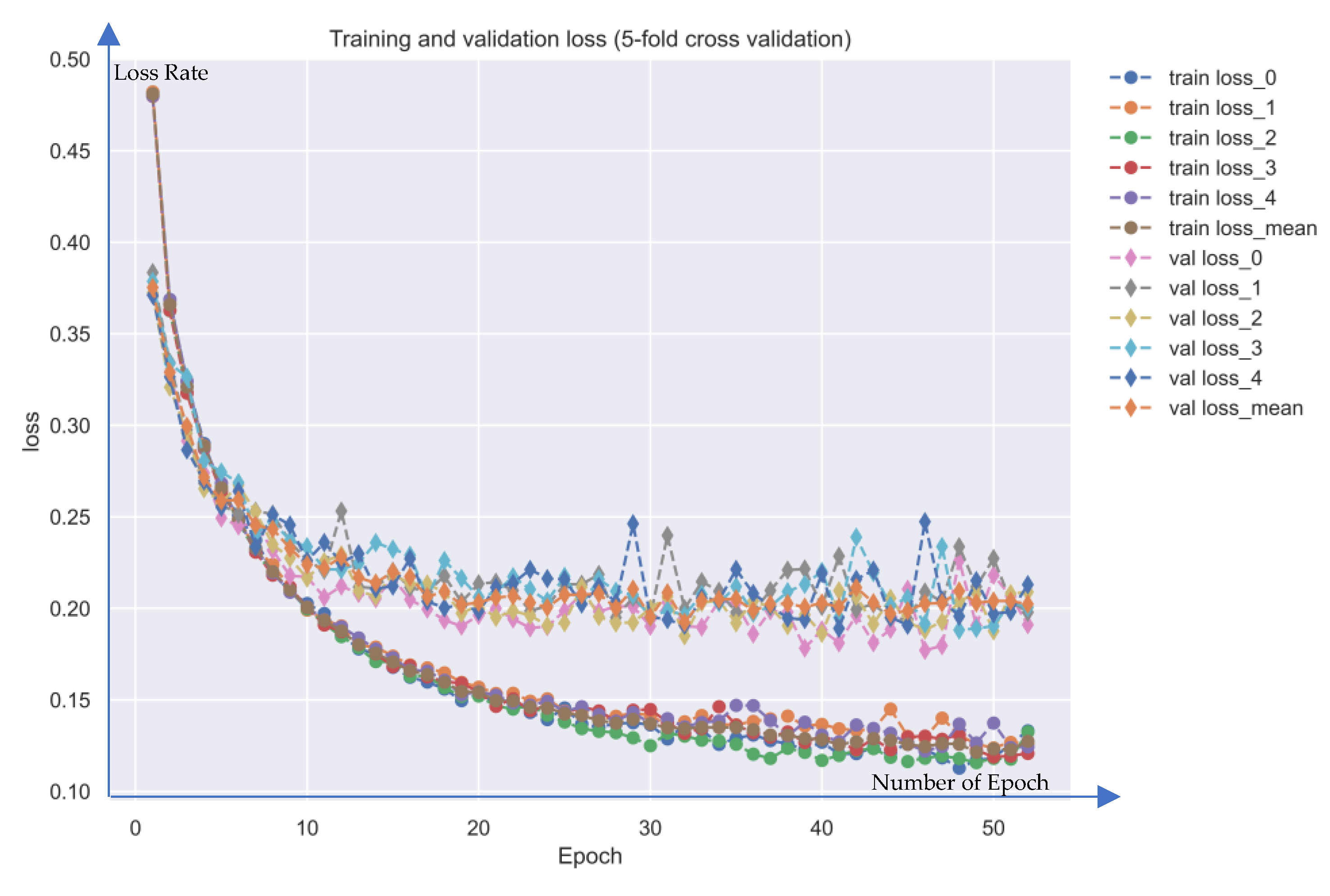
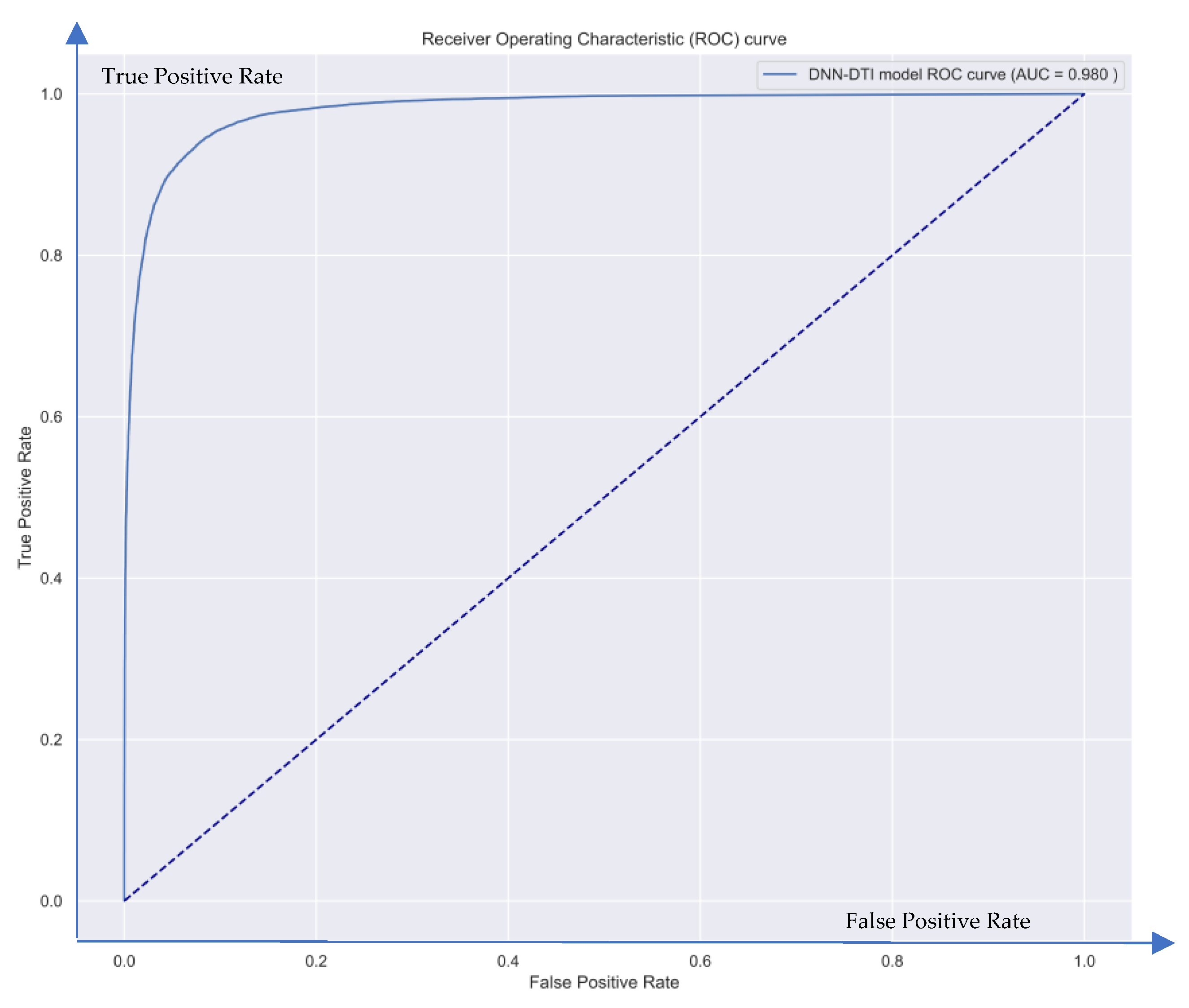
2.3. Systematic Drug Discovery Based on Deep Neural Network-Based Drug-Target Interaction Model for Periodontitis Treatment
3. Discussion
4. Material and Methods
4.1. Systems Biology Methods and Systematic Drug Design for Periodontitis Treatment: An Overview
- We obtained data from the genome-wide microarray GSE10334 dataset. For the dataset, ninety subjects with moderate to severe periodontitis (63 with chronic and 27 with aggressive periodontitis) were recruited among those referred to the Columbia University College of Dental Medicine between November 2004 and April 2007. The data is divided into a periodontitis-diseased group and a healthy control group. Next, we constructed candidate GWGEN, including candidate protein-protein interaction network (PPIN) and candidate gene/miRNA/lncRNA regulatory network (GRN), via big database mining.
- We identified the real GWGENs for both diseased and healthy control via the system identification method plus the system order detection method in Section 4.4.
- We applied the principal network projection method (PNP) to extract the core GWGEN properties, including proteins, receptors, miRNAs, TFs, and lncRNAs, from both real GWGENs to construct core GWGENs in Section 4.5.
- Based on the annotation of KEGG pathways, we built up the core signaling pathways of periodontitis as well as the common core pathways of disease and healthy control. Furthermore, we selected biomarkers that play critical roles in pathological mechanisms and lead to downstream cellular dysfunctions in periodontitis.
- We built a deep neural network (DNN)-based drug target identification (DTI) model for drug target identification. The DNN-based DTI model is trained by the drug-target interaction database, in which the structures of the drugs and targets are converted into feature vectors. The trained DNN-based DTI model is employed to predict the interaction probability between drugs and their targets (biomarkers), i.e., predict the candidate molecular drugs for biomarkers. We then selected potential molecular drugs for each biomarker from their candidate molecular drugs to combine some potential molecular drugs as a multiple-molecule drug for therapeutic treatment of periodontitis according to drug design specifications.
4.2. Data Preprocessing and Big Data Mining for the Construction of Candidate GWGEN
4.3. Construction of the Stochastic System Model to Obtain Real GWGEN of Periodontitis by System Identification Method
4.4. Constructing Real GWGENs of Periodontitis and Healthy Control by System Identification and System Order Detection Methods
4.5. Extraction of the Core GWGEN from Real GWGEN for Core Signaling Pathways via Principal Network Projection (PNP) Method
4.6. Systematic Drug Discovery for Periodontitis Treatment via DNN-Based DTI Model Prediction and Drug Design Specifications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, C.Y. Osteoporosis and periodontal diseases—An update on their association and mechanistic links. Periodontology 2022, 89, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Boehm, T.K.; Scannapieco, F.A. The epidemiology, consequences and management of periodontal disease in older adults. J. Am. Dent. Assoc. 2007, 138, S26–S33. [Google Scholar] [CrossRef]
- Kumar, S. Evidence-based update on diagnosis and management of gingivitis and periodontitis. Dent. Clin. 2019, 63, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Abebe, G.M. Oral biofilm and its impact on oral health, psychological and social interaction. Int. J. Oral Dent. Health 2021, 7, 127–137. [Google Scholar]
- National Institute of Dental Research (US). Periodontal (Gum) Disease; National Institute of Dental Research: Washington, DC, USA, 1988. [Google Scholar]
- Singh, B.; Singh, R. Gingivitis—A silent disease. J. Dent. Med. Sci. 2013, 6, 30–33. [Google Scholar]
- Genlargements Periodontitis. Periodontal Pocket. Periodontol. Dent. Hyg.-E-Book 2015, 88. [Google Scholar]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef]
- Baelum, V.; Lopez, R. Defining and classifying periodontitis: Need for a paradigm shift? Eur. J. Oral Sci. 2003, 111, 2–6. [Google Scholar] [CrossRef]
- Darby, I. Non-surgical management of periodontal disease. Aust. Dent. J. 2009, 54, S86–S95. [Google Scholar] [CrossRef]
- Pragati, S.; Ashok, S.; Kuldeep, S. Recent advances in periodontal drug delivery systems. Int. J. Drug Deliv. 2009, 1, 1–14. [Google Scholar]
- Slots, J.; Rams, T.E. Antibiotics in periodontal therapy: Advantages and disadvantages. J. Clin. Periodontol. 1990, 17, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; He, L.; Jin, D.; Zhu, Y.; Meng, H. Effect of adjunctive systemic antibiotics on microbial populations compared with scaling and root planing alone for the treatment of periodontitis: A pilot randomized clinical trial. J. Periodontol. 2022, 93, 570–583. [Google Scholar] [CrossRef]
- Peter Brooks, M. Use and benefits of nonsteroidal anti-inflammatory drugs. Am. J. Med. 1998, 104, 9S–13S. [Google Scholar] [CrossRef] [PubMed]
- Puyathorn, N.; Senarat, S.; Lertsuphotvanit, N.; Phaechamud, T. Physicochemical and Bioactivity Characteristics of Doxycycline Hyclate-Loaded Solvent Removal-Induced Ibuprofen-Based In Situ Forming Gel. Gels 2023, 9, 128. [Google Scholar] [CrossRef]
- Lertsuphotvanit, N.; Santimaleeworagun, W.; Narakornwit, W.; Chuenbarn, T.; Mahadlek, J.; Chantadee, T.; Phaechamud, T. Borneol-based antisolvent-induced in situ forming matrix for crevicular pocket delivery of vancomycin hydrochloride. Int. J. Pharm. 2022, 617, 121603. [Google Scholar] [CrossRef]
- Lee, F.-Y.; Chen, D.W.; Hu, C.-C.; Hsieh, Y.-T.; Liu, S.-J.; Chan, E.-C. In vitro and in vivo investigation of drug-eluting implants for the treatment of periodontal disease. AAPS PharmSciTech 2011, 12, 1110–1115. [Google Scholar] [CrossRef]
- Prakasam, A.; Elavarasu, S.S.; Natarajan, R.K. Antibiotics in the management of aggressive periodontitis. J. Pharm. Bioallied Sci. 2012, 4 (Suppl. S2), S252. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Haque, M.M.; Yerex, K.; Kelekis-Cholakis, A.; Duan, K. Advances in novel therapeutic approaches for periodontal diseases. BMC Oral Health 2022, 22, 1–23. [Google Scholar] [CrossRef]
- Chang, S.; Chen, J.-Y.; Chuang, Y.-J.; Chen, B.-S. Systems Approach to Pathogenic Mechanism of Type 2 Diabetes and Drug Discovery Design Based on Deep Learning and Drug Design Specifications. Int. J. Mol. Sci. 2020, 22, 166. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, B.-S. Identifying Drug Targets of Oral Squamous Cell Carcinoma through a Systems Biology Method and Genome-Wide Microarray Data for Drug Discovery by Deep Learning and Drug Design Specifications. Int. J. Mol. Sci. 2022, 23, 10409. [Google Scholar] [CrossRef]
- Ting, C.-T.; Chen, B.-S. Repurposing Multiple-Molecule Drugs for COVID-19-Associated Acute Respiratory Distress Syndrome and Non-Viral Acute Respiratory Distress Syndrome via a Systems Biology Approach and a DNN-DTI Model Based on Five Drug Design Specifications. Int. J. Mol. Sci. 2022, 23, 3649. [Google Scholar] [CrossRef]
- Yeh, S.-J.; Chung, Y.-C.; Chen, B.-S. Investigating the Role of Obesity in Prostate Cancer and Identifying Biomarkers for Drug Discovery: Systems Biology and Deep Learning Approaches. Molecules 2022, 27, 900. [Google Scholar] [CrossRef]
- Su, P.-W.; Chen, B.-S. Systems Drug Design for Muscle Invasive Bladder Cancer and Advanced Bladder Cancer by Genome-Wide Microarray Data and Deep Learning Method with Drug Design Specifications. Int. J. Mol. Sci. 2022, 23, 13869. [Google Scholar] [CrossRef]
- Wei, S.; Li, Y.l.; Gong, Q.; Liang, H.; Liu, Q.; Bernardi, R.E.; Zhang, H.T.; Chen, F.; Lawrence, A.J.; Liang, J.h. Brucine N-oxide reduces ethanol intake and preference in alcohol-preferring male Fawn-Hooded rats. Alcohol. Clin. Exp. Res. 2020, 44, 1321–1328. [Google Scholar] [CrossRef]
- Krampe, H.; Ehrenreich, H. Supervised disulfiram as adjunct to psychotherapy in alcoholism treatment. Curr. Pharm. Des. 2010, 16, 2076–2090. [Google Scholar] [CrossRef]
- Cooper-DeHoff, R.M.; Handberg, E.M.; Mancia, G.; Zhou, Q.; Champion, A.; Legler, U.F.; Pepine, C.J. INVEST revisited: Review of findings from the International Verapamil SR–Trandolapril Study. Expert Rev. Cardiovasc. Ther. 2009, 7, 1329–1340. [Google Scholar] [CrossRef]
- Passamonti, L.; Rodríguez, P.V.; Hong, Y.T.; Allinson, K.S.; Bevan-Jones, W.R.; Williamson, D.; Jones, P.S.; Arnold, R.; Borchert, R.J.; Surendranathan, A. [11C] PK11195 binding in Alzheimer disease and progressive supranuclear palsy. Neurology 2018, 90, e1989–e1996. [Google Scholar] [CrossRef]
- Stark, C.; Breitkreutz, B.-J.; Reguly, T.; Boucher, L.; Breitkreutz, A.; Tyers, M. BioGRID: A general repository for interaction datasets. Nucleic Acids Res. 2006, 34 (Suppl. S1), D535–D539. [Google Scholar] [CrossRef] [PubMed]
- Friard, O.; Re, A.; Taverna, D.; De Bortoli, M.; Corá, D. CircuitsDB: A database of mixed microRNA/transcription factor feed-forward regulatory circuits in human and mouse. BMC Bioinform. 2010, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Salwinski, L.; Miller, C.S.; Smith, A.J.; Pettit, F.K.; Bowie, J.U.; Eisenberg, D. The database of interacting proteins: 2004 update. Nucleic Acids Res. 2004, 32 (Suppl. S1), D449–D451. [Google Scholar] [CrossRef] [PubMed]
- Bovolenta, L.; Acencio, M.; Lemke, N. HTRIdb: An open-access database for experimentally verified human transcriptional regulation interactions. Nat. Preced. 2012, 13, 405. [Google Scholar]
- Orchard, S.; Ammari, M.; Aranda, B.; Breuza, L.; Briganti, L.; Broackes-Carter, F.; Campbell, N.H.; Chavali, G.; Chen, C.; Del-Toro, N. The MIntAct project—IntAct as a common curation platform for 11 molecular interaction databases. Nucleic Acids Res. 2014, 42, D358–D363. [Google Scholar] [CrossRef]
- Zheng, G.; Tu, K.; Yang, Q.; Xiong, Y.; Wei, C.; Xie, L.; Zhu, Y.; Li, Y. ITFP: An integrated platform of mammalian transcription factors. Bioinformatics 2008, 24, 2416–2417. [Google Scholar] [CrossRef]
- Zanzoni, A.; Montecchi-Palazzi, L.; Quondam, M.; Ausiello, G.; Helmer-Citterich, M.; Cesareni, G. MINT: A Molecular INTeraction database. FEBS Lett. 2002, 513, 135–140. [Google Scholar] [CrossRef]
- Li, J.-H.; Liu, S.; Zhou, H.; Qu, L.-H.; Yang, J.-H. starBase v2. 0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein–RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. elife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Wingender, E.; Chen, X.; Hehl, R.; Karas, H.; Liebich, I.; Matys, V.; Meinhardt, T.; Prüß, M.; Reuter, I.; Schacherer, F. TRANSFAC: An integrated system for gene expression regulation. Nucleic Acids Res. 2000, 28, 316–319. [Google Scholar] [CrossRef]
- Hanes, P.J.; Krishna, R. Characteristics of inflammation common to both diabetes and periodontitis: Are predictive diagnosis and targeted preventive measures possible? EPMA J. 2010, 1, 101–116. [Google Scholar] [CrossRef]
- Ding, C.; Ji, X.; Chen, X.; Xu, Y.; Zhong, L. TNF-α gene promoter polymorphisms contribute to periodontitis susceptibility: Evidence from 46 studies. J. Clin. Periodontol. 2014, 41, 748–759. [Google Scholar] [CrossRef]
- Aung, K.T.; Akiyama, K.; Kunitomo, M.; Mun, A.Y.; Tosa, I.; Nguyen, H.T.T.; Zhang, J.; Kohno, T.; Ono, M.; Hara, E.S. Aging-affected MSC functions and severity of periodontal tissue destruction in a ligature-induced mouse periodontitis model. Int. J. Mol. Sci. 2020, 21, 8103. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.S. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell 2021, 184, 149–168.e117. [Google Scholar] [CrossRef]
- Kibune, R.; Muraoka, K.; Morishita, M.; Ariyoshi, W.; Awano, S. Relationship between Dynamics of TNF-α and Its Soluble Receptors in Saliva and Periodontal Health State. Dent. J. 2022, 10, 25. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jung, J.-Y.; Jeong, Y.-J.; Park, J.-H.; Yang, K.-H.; Choi, N.-K.; Kim, S.-H.; Kim, W.-J. Involvement of both mitochondrial-and death receptor-dependent apoptotic pathways regulated by Bcl-2 family in sodium fluoride-induced apoptosis of the human gingival fibroblasts. Toxicology 2008, 243, 340–347. [Google Scholar] [CrossRef]
- Canakci, C.; Cicek, Y.; Canakci, V. Reactive oxygen species and human inflammatory periodontal diseases. Biochemistry 2005, 70, 619–628. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Xu, J.; Liu, F.; Hu, R.; Deng, H. Effects of Porphyromonas gingivalis lipopolysaccharide on the expression of key genes involved in cholesterol metabolism in macrophages. Arch. Med. Sci. 2016, 12, 959–967. [Google Scholar] [CrossRef]
- Waddington, R.J.; Moseley, R.; Embery, G. Periodontal Disease Mechanisms: Reactive oxygen species: A potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000, 6, 138–151. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Merritt, A.J.; Allen, T.D.; Potten, C.S.; Hickman, J.A. Apoptosis in small intestinal epithelia from p53-null mice: Evidence for a delayed, p53-indepdendent G2/M-associated cell death after γ-irradiation. Oncogene 1997, 14, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Nakajima, A.; Sakon-Komazawa, S.; Piao, J.; Xue, X.; Okumura, K. Reactive oxygen species mediate crosstalk between NF-κB and JNK. Cell Death Differ. 2006, 13, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Soory, M. Actions of Glutathione in Chronic Inflammatory Diseases, Including Periodontitis: Dietary Agonists. In Glutathione: Dietary Sources, Role in Cellular Functions and Therapeutic Effects; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2014. [Google Scholar]
- Ni, Z.; Wang, B.; Dai, X.; Ding, W.; Yang, T.; Li, X.; Lewin, S.; Xu, L.; Lian, J.; He, F. HCC cells with high levels of Bcl-2 are resistant to ABT-737 via activation of the ROS–JNK–autophagy pathway. Free Radic. Biol. Med. 2014, 70, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Feng, J.; Wang, W. Expression of miR-155 and miR-146a in the saliva of patients with periodontitis and its clinical value. Am. J. Transl. Res. 2021, 13, 6670. [Google Scholar] [PubMed]
- Zhang, J.; Kim, J.; Alexander, A.; Cai, S.; Tripathi, D.N.; Dere, R.; Tee, A.R.; Tait-Mulder, J.; Di Nardo, A.; Han, J.M. A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS. Nat. Cell Biol. 2013, 15, 1186–1196. [Google Scholar] [CrossRef]
- Bezamat, M.; Deeley, K.; Khaliq, S.; Letra, A.; Scariot, R.; Silva, R.M.; Weber, M.L.; Bussaneli, D.G.; Trevilatto, P.C.; Almarza, A.J. Are mTOR and endoplasmic reticulum stress pathway genes associated with oral and bone diseases? Caries Res. 2019, 53, 235–241. [Google Scholar] [CrossRef]
- Gross, D.; Van Den Heuvel, A.; Birnbaum, M. The role of FoxO in the regulation of metabolism. Oncogene 2008, 27, 2320–2336. [Google Scholar] [CrossRef]
- Hu, W.; Spaink, H.P. The role of TLR2 in infectious diseases caused by mycobacteria: From cell biology to therapeutic target. Biology 2022, 11, 246. [Google Scholar] [CrossRef]
- Bulut, Ş.; Uslu, H.; Özdemir, B.H.; Bulut, Ö.E. Expression of caspase-3, p53 and Bcl-2 in generalized aggressive periodontitis. Head Face Med. 2006, 2, 1–7. [Google Scholar] [CrossRef]
- Sattler, M.; Liang, H.; Nettesheim, D.; Meadows, R.P.; Harlan, J.E.; Eberstadt, M.; Yoon, H.S.; Shuker, S.B.; Chang, B.S.; Minn, A.J. Structure of Bcl-xL-Bak peptide complex: Recognition between regulators of apoptosis. Science 1997, 275, 983–986. [Google Scholar] [CrossRef]
- Liu, C.; Mo, L.; Niu, Y.; Li, X.; Zhou, X.; Xu, X. The role of reactive oxygen species and autophagy in periodontitis and their potential linkage. Front. Physiol. 2017, 8, 439. [Google Scholar] [CrossRef]
- Ishida, K.; Yuge, Y.; Hanaoka, M.; Yasukawa, M.; Minami, Y.; Ogawa, M.; Masumoto, K.h.; Shigeyoshi, Y.; Saito, M.; Tsuji, T. Gadd45g regulates dental epithelial cell proliferation through p38 MAPK-mediated p21 expression. Genes Cells 2013, 18, 660–671. [Google Scholar] [CrossRef]
- Li, W.; Xu, L.; Lu, R.; Meng, H. The association of EGF rs2237051 variant, serum EGF levels and generalized aggressive periodontitis: A preliminary study. PeerJ 2020, 8, e9212. [Google Scholar]
- Birkedal-Hansen, H. Role of matrix metalloproteinases in human periodontal diseases. J. Periodontol. 1993, 64, 474–484. [Google Scholar]
- Pietruska, M.; Pietruski, J.; Stokowska, W. Polypeptide growth factors in the course of surgical periodontal treatment. Rocz. Akad. Med. W Bialymst. (1995) 2000, 45, 199–210. [Google Scholar]
- Niu, C.; Yuan, K.; Ma, R.; Gao, L.; Jiang, W.; Hu, X.; Lin, W.; Zhang, X.; Huang, Z. Gold nanoparticles promote osteogenic differentiation of human periodontal ligament stem cells via the p38 MAPK signaling pathway. Mol. Med. Rep. 2017, 16, 4879–4886. [Google Scholar] [CrossRef]
- Cui, S.; Guo, W.; Chen, C.; Tang, X.; Zhao, J.; Mao, B.; Zhang, H. Metagenomic analysis of the effects of Lactiplantibacillus plantarum and fructooligosaccharides (FOS) on the fecal microbiota structure in mice. Foods 2022, 11, 1187. [Google Scholar] [CrossRef]
- Xiao, G. Autophagy and NF-κB: Fight for fate. Cytokine Growth Factor Rev. 2007, 18, 233–243. [Google Scholar] [CrossRef]
- Kizilirmak, C.; Bianchi, M.E.; Zambrano, S. Insights on the NF-κB system using live cell imaging: Recent developments and future perspectives. Front. Immunol. 2022, 13, 886127. [Google Scholar] [CrossRef]
- Peng, S.; Li, W.; Hou, N.; Huang, N. A review of FoxO1-regulated metabolic diseases and related drug discoveries. Cells 2020, 9, 184. [Google Scholar] [CrossRef]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K. A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell 2017, 171, 1437–1452.e1417. [Google Scholar] [CrossRef]
- Corsello, S.M.; Nagari, R.T.; Spangler, R.D.; Rossen, J.; Kocak, M.; Bryan, J.G.; Humeidi, R.; Peck, D.; Wu, X.; Tang, A.A. Discovering the anticancer potential of non-oncology drugs by systematic viability profiling. Nat. Cancer 2020, 1, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Wikesjö, U.M.; Nilvéus, R.E.; Selvig, K.A. Significance of early healing events on periodontal repair: A review. J. Periodontol. 1992, 63, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Shafqat, S.S.; Niaz, M.O.; Raza, A.; Naseem, M. Clinical efficacy of photodynamic therapy and laser irradiation as an adjunct to open flap debridement in the treatment of chronic periodontitis: A systematic review and meta-analysis. Photodermatol. Photoimmunol. Photomed. 2020, 36, 3–13. [Google Scholar] [CrossRef]
- Lee, C.; Huang, H.; Sun, T.; Karimbux, N. Impact of patient compliance on tooth loss during supportive periodontal therapy: A systematic review and meta-analysis. J. Dent. Res. 2015, 94, 777–786. [Google Scholar] [CrossRef]
- Mohd-Dom, T.; Ayob, R.; Mohd-Nur, A.; Abdul-Manaf, M.R.; Ishak, N.; Abdul-Muttalib, K.; Aljunid, S.M.; Ahmad-Yaziz, Y.; Abdul-Aziz, H.; Kasan, N. Cost analysis of periodontitis management in public sector specialist dental clinics. BMC Oral Health 2014, 14, 56. [Google Scholar] [CrossRef]
- Mehra, N.; Varmeziar, A.; Chen, X.; Kronick, O.; Fisher, R.; Kota, V.; Mitchell, C.S. Cross-Domain Text Mining to Predict Adverse Events from Tyrosine Kinase Inhibitors for Chronic Myeloid Leukemia. Cancers 2022, 14, 4686. [Google Scholar] [CrossRef]
- Serasanambati, M.; Chilakapati, S.R.; Vangavaragu, J.R.; Chilakapati, D.R. Inhibitory effect of gemcitabine and brucine on MDA MB-231human breast cancer cells. Int. J. Drug Deliv. 2014, 6, 133. [Google Scholar]
- Qin, J.-M.; Yin, P.-H.; Li, Q.; Sa, Z.-Q.; Sheng, X.; Yang, L.; Huang, T.; Zhang, M.; Gao, K.-P.; Chen, Q.; et al. Anti-tumor effects of brucine immuno-nanoparticles on hepatocellular carcinoma. Int. J. Nanomed. 2012, 7, 369–379. [Google Scholar] [CrossRef]
- Lu, L.; Huang, R.; Wu, Y.; Jin, J.-M.; Chen, H.-Z.; Zhang, L.-J.; Luan, X. Brucine: A review of phytochemistry, pharmacology, and toxicology. Front. Pharmacol. 2020, 11, 377. [Google Scholar] [CrossRef]
- Yin, W.; Wang, T.-S.; Yin, F.-Z.; Cai, B.-C. Analgesic and anti-inflammatory properties of brucine and brucine N-oxide extracted from seeds of Strychnos nux-vomica. J. Ethnopharmacol. 2003, 88, 205–214. [Google Scholar] [CrossRef]
- Xie, C.; Yan, J.; Cao, S.; Liu, R.; Sun, B.; Xie, Y.; Qu, K.; Zhang, W.; Weng, Z.; Wang, Z. Bi-layered disulfiram-loaded fiber membranes with antibacterial properties for wound dressing. Appl. Biochem. Biotechnol. 2022, 194, 1359–1372. [Google Scholar] [CrossRef]
- Elliott, J.H.; McMahon, J.; Chang, C.C.; Lee, S.A.; Hartogensis, W.; Bumpus, N.; Savic, R.; Roney, J.; Hoh, R.; Solomon, A.; et al. Short-term administration of disulfiram for reversal of latent HIV infection: A phase 2 dose-escalation study. Lancet HIV 2015, 2, e520–e529. [Google Scholar] [CrossRef]
- Meneguello, J.E.; Murase, L.S.; de Souza, J.V.P.; de Oliveira, C.G.; Ghiraldi-Lopes, L.D.; Teixeira, J.J.V.; de Lima Scodro, R.B.; Ferracioli, K.R.C.; Siqueira, V.L.D.; Campanerut-Sá, P.A.Z. A systematic review of disulfiram as an antibacterial agent: What is the evidence? Int. J. Antimicrob. Agents 2022, 59, 106578. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Chiba, M.; Mitani, H. The induction of c-fos mRNA expression by mechanical stress in human periodontal ligament cells. Arch. Oral Biol. 2002, 47, 465–471. [Google Scholar] [CrossRef]
- Singh, B.N.; Ellrodt, G.; Peter, C.T. Verapamil: A review of its pharmacological properties and therapeutic use. Drugs 1978, 15, 169–197. [Google Scholar] [CrossRef]
- Surma, S.; Romańczyk, M.; Witalińska-Łabuzek, J.; Czerniuk, M.R.; Łabuzek, K.; Filipiak, K.J. Periodontitis, blood pressure, and the risk and control of arterial hypertension: Epidemiological, clinical, and pathophysiological aspects—Review of the literature and clinical trials. Curr. Hypertens. Rep. 2021, 23, 27. [Google Scholar] [CrossRef]
- Muñoz Aguilera, E.; Suvan, J.; Orlandi, M.; Miró Catalina, Q.; Nart, J.; D’Aiuto, F. Association between periodontitis and blood pressure highlighted in systemically healthy individuals: Results from a nested case-control study. Hypertension 2021, 77, 1765–1774. [Google Scholar] [CrossRef]
- Kreisl, W.C.; Fujita, M.; Fujimura, Y.; Kimura, N.; Jenko, K.J.; Kannan, P.; Hong, J.; Morse, C.L.; Zoghbi, S.S.; Gladding, R.L. Comparison of [11C]-(R)-PK 11195 and [11C] PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: Implications for positron emission tomographic imaging of this inflammation biomarker. Neuroimage 2010, 49, 2924–2932. [Google Scholar] [CrossRef]
- Walter, R.B.; Pirga, J.L.; Cronk, M.R.; Mayer, S.; Appelbaum, F.R.; Banker, D.E. PK11195, a peripheral benzodiazepine receptor (pBR) ligand, broadly blocks drug efflux to chemosensitize leukemia and myeloma cells by a pBR-independent, direct transporter-modulating mechanism. Blood 2005, 106, 3584–3593. [Google Scholar] [CrossRef] [PubMed]
- Hazini, Z. Prevalence of Periodontal Disease in Patients with Leukemia: A Systematic Review. 2022. Available online: https://titula.universidadeuropea.com/bitstream/handle/20.500.12880/1833/tfg_ZahraHazini.pdf?sequence=1&isAllowed=y (accessed on 10 May 2023).
- Javed, F.; Utreja, A.; Correa, F.O.B.; Al-Askar, M.; Hudieb, M.; Qayyum, F.; Al-Rasheed, A.; Almas, K.; Al-Hezaimi, K. Oral health status in children with acute lymphoblastic leukemia. Crit. Rev. Oncol. /Hematol. 2012, 83, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-S.; Wu, C.-C. Systems biology as an integrated platform for bioinformatics, systems synthetic biology, and systems metabolic engineering. Cells 2013, 2, 635–688. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://github.com/Charles871129/Systems-biology-and-DNN (accessed on 16 May 2023).
- Wang, Y.; Zhang, S.; Li, F.; Zhou, Y.; Zhang, Y.; Wang, Z.; Zhang, R.; Zhu, J.; Ren, Y.; Tan, Y. Therapeutic target database 2020: Enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020, 48, D1031–D1041. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, A.; Hersey, A.; Nowotka, M.; Bento, A.P.; Chambers, J.; Mendez, D.; Mutowo, P.; Atkinson, F.; Bellis, L.J.; Cibrián-Uhalte, E. The ChEMBL database in 2017. Nucleic Acids Res. 2017, 45, D945–D954. [Google Scholar] [CrossRef]
- Kuhn, M.; von Mering, C.; Campillos, M.; Jensen, L.J.; Bork, P. STITCH: Interaction networks of chemicals and proteins. Nucleic Acids Res. 2007, 36 (Suppl. S1), D684–D688. [Google Scholar] [CrossRef]
- Kanehisa, M.; Susumu, G. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Consortium, U. UniProt: A hub for protein information. Nucleic Acids Res. 2015, 43, D204–D212. [Google Scholar] [CrossRef]
- Frazier, K.; Moore, J.; Long, T. Antibacterial activity of disulfiram and its metabolites. J. Appl. Microbiol. 2019, 126, 79–86. [Google Scholar] [CrossRef]
- Mancia, G.; Messerli, F.; Bakris, G.; Zhou, Q.; Champion, A.; Pepine, C.J. Blood pressure control and improved cardiovascular outcomes in the International Verapamil SR-Trandolapril Study. Hypertension 2007, 50, 299–305. [Google Scholar] [CrossRef]
- Martin-Cabezas, R.; Seelam, N.; Petit, C.; Agossa, K.; Gaertner, S.; Tenenbaum, H.; Davideau, J.-L.; Huck, O. Association between periodontitis and arterial hypertension: A systematic review and meta-analysis. Am. Heart J. 2016, 180, 98–112. [Google Scholar] [CrossRef]
- Corsi, F.; Baglini, E.; Barresi, E.; Salerno, S.; Cerri, C.; Martini, C.; Da Settimo Passetti, F.; Taliani, S.; Gargini, C.; Piano, I. Targeting TSPO Reduces Inflammation and Apoptosis in an In Vitro Photoreceptor-Like Model of Retinal Degeneration. ACS Chem. Neurosci. 2022, 13, 3188–3197. [Google Scholar] [CrossRef]
- Dupuy, O.; Flocard, F.; Vial, C.; Rode, G.; Charles, N.; Boisson, D.; Flechaire, A. Disulfiram (Esperal) toxicity. Apropos of 3 original cases. Rev. Med. Int. 1995, 16, 67–72. [Google Scholar] [CrossRef]
- Barrow, P.; Houston, P.; Wong, D. Overdose of sustained-release verapamil. BJA Br. J. Anaesth. 1994, 72, 361–365. [Google Scholar] [CrossRef]
- Nastri, L.; De Rosa, A.; De Gregorio, V.; Grassia, V.; Donnarumma, G. A new controlled-release material containing metronidazole and doxycycline for the treatment of periodontal and peri-implant diseases: Formulation and in vitro testing. Int. J. Dent. 2019, 2019, 9374607. [Google Scholar] [CrossRef]
- Sgolastra, F.; Gatto, R.; Petrucci, A.; Monaco, A. Effectiveness of systemic amoxicillin/metronidazole as adjunctive therapy to scaling and root planing in the treatment of chronic periodontitis: A systematic review and meta-analysis. J. Periodontol. 2012, 83, 1257–1269. [Google Scholar] [CrossRef]
- Zhanel, G.; Critchley, I.; Lin, L.-Y.; Alvandi, N. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob. Agents Chemother. 2019, 63, e01297-18. [Google Scholar] [CrossRef]
- Caton, J.; Ryan, M.E. Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD). Pharmacol. Res. 2011, 63, 114–120. [Google Scholar] [CrossRef]
- English, B.K.; Gaur, A.H. The use and abuse of antibiotics and the development of antibiotic resistance. In Hot Topics in Infection and Immunity in Children VI; Springer: New York, NY, USA, 2010; pp. 73–82. [Google Scholar]
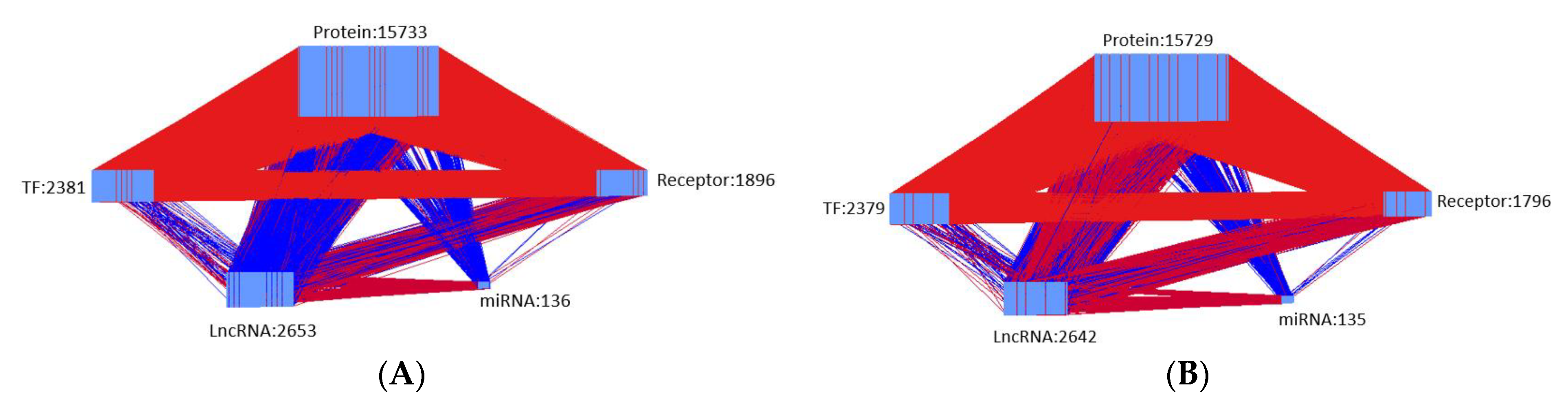

| Nodes | Candidate GWGEN | Real GWGEN of Periodontitis | Real GWGEN of Healthy Control |
| Protein | 20,040 | 15,733 | 15,729 |
| Receptor | 2215 | 1896 | 1796 |
| TF | 2395 | 2381 | 2379 |
| miRNA | 153 | 136 | 135 |
| LncRNA | 3315 | 2653 | 2642 |
| Total nodes | 28,118 | 22,799 | 22,681 |
| Rank | Periodontitis KEGG Pathway | Non-Periodontitis KEGG Pathway | |
| 1 | Cell Cycle (p-Value = 1.9 × 10−12) | Cell Cycle (p-Value = 1.9 × 10−16) | |
| 2 | FOXO Signaling Pathway (p-Value = 2.4 × 10−7) | Insulin Signaling Pathway (p-Value = 1.8 × 10−7) | |
| 3 | Autophagy-Animal (p-Value = 2.3 × 10−6) | mRNA Surveillance Pathway (p-Value = 7.7 × 10−7) | |
| 4 | Apoptosis (p-Value = 2.9 × 10−6) | FOXO Signaling Pathway (p-Value = 2.9 × 10−5) | |
| Candidate Drugs | Regulation Ability (L1000) | Sensitivity (PRISM) | Toxicity (LC50, mol/kg) |
|---|---|---|---|
| FOS(−) | |||
| brucine | 0.337173 | −0.37421 | 5.564 |
| terfenadine | 0.360951 | −0.21913 | 5.437 |
| sulfaphenazole | 0.255523 | −0.32539 | 3.044 |
| alfuzosin | 1.314529 | 0.210014 | 3.945 |
| NF-κB(+) | |||
| imipramine | −0.35669 | −0.10246 | 4.588 |
| terfenadine | −0.76653 | −0.74069 | 5.437 |
| SB-218078 | −0.72856 | −0.2355 | 4.24 |
| verapamil | 0.048613 | −0.06036 | 6.13 |
| FOXO1(−) | |||
| disulfiram | 0.073034 | 0.433672 | 8.023 |
| indinavir | 1.023422 | 0.248016 | 4.009 |
| E-4031 | 0.511786 | 0.049582 | 3.265 |
| triclosan | 0.605751 | 0.064844 | 5.892 |
| TSC2(+) | |||
| erastin | −0.04114 | 0.418918 | 5.621 |
| PK-11195 | −0.28626 | −0.49959 | 5.578 |
| phenothiazine | −0.05596 | −0.43309 | 4.718 |
| loperamide | −0.37009 | 0.003406 | 4.076 |
| Potential. Multiple Molecule Drugs | FOS(−) | Regulation Abilities | TSC2(+) | Sensitivity (PRISM) | Toxicity (LC50, mol/kg) | |
|---|---|---|---|---|---|---|
| NF-κB(+) | FOXO1(−) | |||||
| brucine | 0.337173● | −0.11899● | 0.423386● | −0.28488● | −0.2282 | 5.564 |
| disulfiram | 0.250077● | −0.26205● | 0.073034● | −0.40304● | −0.34918 | 8.023 |
| verapamil | 0.211182● | 0.048613 | 0.086545● | −0.08621● | −0.06036 | 6.13 |
| PK-11195 | 0.482221● | −0.29659● | 0.205624● | −0.28626● | −0.19207 | 5.578 |
| brucine | disulfiram | |||||
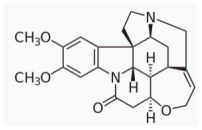 | 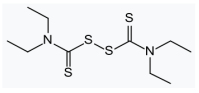 | |||||
| Verapamil | PK-11195 | |||||
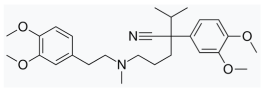 | 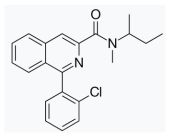 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-T.; Chen, B.-S. Drug Discovery for Periodontitis Treatment Based on Big Data Mining, Systems Biology, and Deep Learning Methods. SynBio 2023, 1, 116-143. https://doi.org/10.3390/synbio1010009
Wang C-T, Chen B-S. Drug Discovery for Periodontitis Treatment Based on Big Data Mining, Systems Biology, and Deep Learning Methods. SynBio. 2023; 1(1):116-143. https://doi.org/10.3390/synbio1010009
Chicago/Turabian StyleWang, Chun-Tse, and Bor-Sen Chen. 2023. "Drug Discovery for Periodontitis Treatment Based on Big Data Mining, Systems Biology, and Deep Learning Methods" SynBio 1, no. 1: 116-143. https://doi.org/10.3390/synbio1010009
APA StyleWang, C.-T., & Chen, B.-S. (2023). Drug Discovery for Periodontitis Treatment Based on Big Data Mining, Systems Biology, and Deep Learning Methods. SynBio, 1(1), 116-143. https://doi.org/10.3390/synbio1010009





