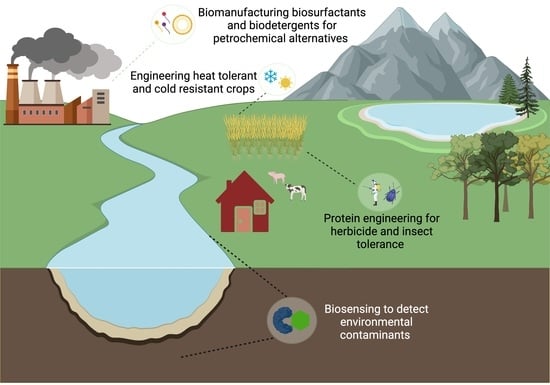
Proteins in Synthetic Biology with Agricultural and Environmental Applications
Abstract
1. Introduction
2. Food Safety and Security
2.1. Herbicide Tolerance
2.2. Insecticidal Activity
2.3. Environmental Change Tolerance
3. Environmental Sensing
3.1. Enzyme Based Sensing
3.2. Binding Affinity-Based Sensing
4. Biomanufacturing
4.1. Naturally Occurring Proteins for Biomanufacturing
4.2. Optimizing Protein Activity within a Pathway
4.3. Strategically Modulating Structural Moieties within a Protein
Author Contributions
Funding
Conflicts of Interest
References
- Synthetic Biology Market Size, Share, Trends, by Technology, by Tools, by Application, by End-Use, and by Region Forecast to 2030; Synthetic Biology Market; Research and Markets. 2022. Available online: https://www.researchandmarkets.com/reports/5648701/synthetic-biology-market-size-share-trends-by (accessed on 1 October 2022).
- USDA ERS-Key Statistics & Graphics. Available online: https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-u-s/key-statistics-graphics/ (accessed on 23 September 2022).
- Daramola, O.S.; Adigun, J.A.; Olorunmaiye, P.M. Challenges of weed management in rice for food security in Africa: A review. Agric. Trop. Subtrop. 2020, 53, 107–115. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Heng, Y.; Li, J.; Pei, J.; Cao, Y.; Deng, X.W.; Ma, L. Generation of a series of mutant lines resistant to imidazolinone by screening an EMS-based mutant library in common wheat. Crop J. 2020, 9, 1030–1038. [Google Scholar] [CrossRef]
- Sarkozi, A. New Standards to Curb the Global Spread of Plant Pests and Diseases; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Murphy, B.P.; Tranel, P.J. Target-Site Mutations Conferring Herbicide Resistance. Plants 2019, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Acetohydroxyacid Synthase Inhibitors (AHAS/ALS). Modern Crop Protection Compounds; Jeschke, P., Witschel, M., Krämer, W., Schirmer, U., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 33–171. ISBN 978-3-527-69926-1. [Google Scholar]
- Fang, J.; Wan, C.; Wang, W.; Ma, L.; Wang, X.; Cheng, C.; Zhou, J.; Qiao, Y.; Wang, X. Engineering Herbicide-Tolerance Rice Expressing an Acetohydroxyacid Synthase with a Single Amino Acid Deletion. Int. J. Mol. Sci. 2020, 21, 1265. [Google Scholar] [CrossRef]
- Fonseca, E.C.M.; da Costa, K.S.; Lameira, J.; Alves, C.N.; Lima, A.H. Investigation of the target-site resistance of EPSP synthase mutants P106T and T102I/P106S against glyphosate. RSC Adv. 2020, 10, 44352–44360. [Google Scholar] [CrossRef]
- Achary, V.M.M.; Sheri, V.; Manna, M.; Panditi, V.; Borphukan, B.; Ram, B.; Agarwal, A.; Fartyal, D.; Teotia, D.; Masakapalli, S.K.; et al. Overexpression of improvedEPSPSgene results in field level glyphosate tolerance and higher grain yield in rice. Plant Biotechnol. J. 2020, 18, 2504–2519. [Google Scholar] [CrossRef]
- Ortega, J.L.; Rajapakse, W.; Bagga, S.; Apodaca, K.; Lucero, Y.; Sengupta-Gopalan, C. An intragenic approach to confer glyphosate resistance in chile (Capsicum annuum) by introducing an in vitro mutagenized chile EPSPS gene encoding for a glyphosate resistant EPSPS protein. PLoS ONE 2018, 13, e0194666. [Google Scholar] [CrossRef]
- Liu, Y.; Han, S.; Yang, S.; Chen, Z.; Yin, Y.; Xi, J.; Liu, Q.; Yan, W.; Song, X.; Zhao, F.; et al. Engineered chimeric insecticidal crystalline protein improves resistance to lepidopteran insects in rice (Oryza sativa L.) and maize (Zea mays L.). Sci. Rep. 2022, 12, 12529. [Google Scholar] [CrossRef]
- Hou, J.; Cong, R.; Izumi-Willcoxon, M.; Ali, H.; Zheng, Y.; Bermudez, E.; McDonald, M.; Nelson, M.; Yamamoto, T. Engineering of Bacillus thuringiensis Cry Proteins to Enhance the Activity against Western Corn Rootworm. Toxins 2019, 11, 162. [Google Scholar] [CrossRef]
- Gomis-Cebolla, J.; dos Santos, R.F.; Wang, Y.; Caballero, J.; Caballero, P.; He, K.; Jurat-Fuentes, J.; Ferré, J. Domain Shuffling between Vip3Aa and Vip3Ca: Chimera Stability and Insecticidal Activity against European, American, African, and Asian Pests. Toxins 2020, 12, 99. [Google Scholar] [CrossRef]
- Duman, J.G.; Wisniewski, M.J. The use of antifreeze proteins for frost protection in sensitive crop plants. Environ. Exp. Bot. 2014, 106, 60–69. [Google Scholar] [CrossRef]
- Juurakko, C.L.; Dicenzo, G.C.; Walker, V.K. Cold acclimation and prospects for cold-resilient crops. Plant Stress 2021, 2, 100028. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, R.; Wang, Y.; Zhang, L.; Yao, S. A point mutation in LTT1 enhances cold tolerance at the booting stage in rice. Plant Cell Environ. 2020, 43, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Gómez, R.; Carrillo, N.; Morelli, M.P.; Tula, S.; Shahinnia, F.; Hajirezaei, M.-R.; Lodeyro, A.F. Faster photosynthetic induction in tobacco by expressing cyanobacterial flavodiiron proteins in chloroplasts. Photosynth. Res. 2017, 136, 129–138. [Google Scholar] [CrossRef]
- Wani, U.M.; Majeed, S.T.; Raja, V.; Wani, Z.A.; Jan, N.; Andrabi, K.I.; John, R. Ectopic expression of a novel cold-resistance protein 1 from Brassica oleracea promotes tolerance to chilling stress in transgenic tomato. Sci. Rep. 2021, 11, 16574. [Google Scholar] [CrossRef]
- Selahle, S.K.; Mpupa, A.; Nomngongo, P.N. A review of extraction, analytical, and advanced methods for the determination of neonicotinoid insecticides in environmental water matrices. Rev. Anal. Chem. 2021, 40, 187–203. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Shi, X.-C.; Liu, F.-Q.; Laborda, P. Chromatographic Methods for Detection and Quantification of Carbendazim in Food. J. Agric. Food Chem. 2020, 68, 11880–11894. [Google Scholar] [CrossRef]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Nigam, V.K.; Shukla, P. Enzyme Based Biosensors for Detection of Environmental Pollutants-A Review. J. Microbiol. Biotechnol. 2015, 25, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and their applications–A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Roy, R.; Anand, R. Harnessing the Potential of Biological Recognition Elements for Water Pollution Monitoring. ACS Sensors 2022, 7, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, L.S.B.; Verma, N. Enzyme Inhibition Based Biosensors: A Review. Anal. Lett. 2013, 46, 225–241. [Google Scholar] [CrossRef]
- Chadha, U.; Bhardwaj, P.; Agarwal, R.; Rawat, P.; Agarwal, R.; Gupta, I.; Panjwani, M.; Singh, S.; Ahuja, C.; Selvaraj, S.K.; et al. Recent progress and growth in biosensors technology: A critical review. J. Ind. Eng. Chem. 2022, 109, 21–51. [Google Scholar] [CrossRef]
- Karadurmus, L.; Kaya, S.I.; Ozkan, S.A. Recent advances of enzyme biosensors for pesticide detection in foods. J. Food Meas. Charact. 2021, 15, 4582–4595. [Google Scholar] [CrossRef]
- Vigneshvar, S.; Sudhakumari, C.C.; Senthilkumaran, B.; Prakash, H. Recent Advances in Biosensor Technology for Potential Applications—An Overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Zhu, A.; Shi, H. Recent Advances in Optical Biosensors for Environmental Monitoring and Early Warning. Sensors 2013, 13, 13928–13948. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, I.S.; Soldatkin, A.; Kucherenko, D.Y.; Soldatkina, O.V.; Dzyadevych, S.V. Advances in nanomaterial application in enzyme-based electrochemical biosensors: A review. Nanoscale Adv. 2019, 1, 4560–4577. [Google Scholar] [CrossRef]
- Cao, J.C.; Wang, M.; Yu, H.; She, Y.; Ye, J.; El-Aty, A.M.A.; Hacimuftuoglu, A.; Wang, J.; Lao, S. An Overview on the Mechanisms and Applications of Enzyme Inhibition-Based Methods for Determination of Organophosphate and Carbamate Pesticides. J. Agric. Food Chem. 2020, 68, 7298–7315. [Google Scholar] [CrossRef]
- Hara, T.O.; Singh, B. Electrochemical Biosensors for Detection of Pesticides and Heavy Metal Toxicants in Water: Recent Trends and Progress. ACS EST Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Khanmohammadi, A.; Ghazizadeh, A.J.; Hashemi, P.; Afkhami, A.; Arduini, F.; Bagheri, H. An overview to electrochemical biosensors and sensors for the detection of environmental contaminants. J. Iran. Chem. Soc. 2020, 17, 2429–2447. [Google Scholar] [CrossRef]
- Wee, Y.; Park, S.; Kwon, Y.H.; Ju, Y.; Yeon, K.-M.; Kim, J. Tyrosinase-immobilized CNT based biosensor for highly-sensitive detection of phenolic compounds. Biosens. Bioelectron. 2019, 132, 279–285. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, Y.; Liu, S.; Wu, D.; Su, Z.; Chen, G.; Liu, J.; Li, G. Recent advances in enzyme immobilization based on novel porous framework materials and its applications in biosensing. Coord. Chem. Rev. 2022, 459, 214414. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, Y.; Zhang, Y.; Zhang, M.; Zhang, Y.; Qiao, L.; Yin, N.; Song, K.; Liu, M.; Wang, D. Recent advances in carbon nanomaterials-based electrochemical sensors for phenolic compounds detection. Microchem. J. 2021, 171, 106776. [Google Scholar] [CrossRef]
- Mathew, M.; Rout, C.S. Electrochemical biosensors based on Ti3C2Tx MXene: Future perspectives for on-site analysis. Curr. Opin. Electrochem. 2021, 30, 100782. [Google Scholar] [CrossRef]
- Soltaninejad, K.; Shadnia, S. History of the Use and Epidemiology of Organophosphorus Poisoning. In Basic and Clinical Toxicology of Organophosphorus Compounds; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-1-4471-5625-3. [Google Scholar]
- Hemingway, J.; Hawkes, N.J.; McCarroll, L.; Ranson, H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem. Mol. Biol. 2004, 34, 653–665. [Google Scholar] [CrossRef]
- Hsieh, B.H.; Deng, J.F.; Ger, J.; Tsai, W.J. Acetylcholinesterase Inhibition and the Extrapyramidal Syndrome: A Review of the Neurotoxicity of Organophosphate. Neurotoxicology 2001, 22, 423–427. [Google Scholar] [CrossRef]
- Hassall, K.A. Biochemistry and Uses of Pesticides; Macmillan Press Ltd.: Basingstoke, UK, 1990. [Google Scholar]
- Upadhyay, S.; Rao, G.R.; Sharma, M.K.; Bhattacharya, B.K.; Rao, V.K.; Vijayaraghavan, R. Immobilization of acetylcholineesterase–choline oxidase on a gold–platinum bimetallic nanoparticles modified glassy carbon electrode for the sensitive detection of organophosphate pesticides, carbamates and nerve agents. Biosens. Bioelectron. 2009, 25, 832–838. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Shojaosadati, S.; Ranaie, S.; Mousavi, S. Optimization and evaluation of acetylcholine esterase immobilization on ceramic packing using response surface methodology. Process Biochem. 2010, 45, 81–87. [Google Scholar] [CrossRef]
- Anzai, J. Use of biosensors for detecting organophosphorus agents. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2006, 126, 1301–1308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tran-Minh, C.; Pandey, P.C.; Kumaran, S. Studies on acetylcholine sensor and its analytical application based on the inhibition of cholinesterase. Biosens. Bioelectron. 1990, 5, 461–471. [Google Scholar] [CrossRef]
- Tang, W.; Yang, J.; Wang, F.; Wang, J.; Li, Z. Thiocholine-triggered reaction in personal glucose meters for portable quantitative detection of organophosphorus pesticide. Anal. Chim. Acta 2019, 1060, 97–102. [Google Scholar] [CrossRef]
- Regulation (EC) No 396/2005 of the European Parliament and of the Council 2005. Available online: http://data.europa.eu/eli/reg/2005/396/2022-09-19 (accessed on 1 October 2022).
- Su, L.; Jia, W.; Hou, C.; Lei, Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef]
- Shahar, H.; Tan, L.L.; Ta, G.C.; Heng, L.Y. Detection of halogenated hydrocarbon pollutants using enzymatic reflectance biosensor. Sens. Actuators B Chem. 2019, 281, 80–89. [Google Scholar] [CrossRef]
- Behera, B.K.; Das, A.; Sarkar, D.J.; Weerathunge, P.; Parida, P.K.; Das, B.K.; Thavamani, P.; Ramanathan, R.; Bansal, V. Polycyclic Aromatic Hydrocarbons (PAHs) in inland aquatic ecosystems: Perils and remedies through biosensors and bioremediation. Environ. Pollut. 2018, 241, 212–233. [Google Scholar] [CrossRef] [PubMed]
- Plotnikova, E.G.; Shumkova, E.S.; Shumkov, M.S. Whole-cell bacterial biosensors for the detection of aromatic hydrocarbons and their chlorinated derivatives (Review). Appl. Biochem. Microbiol. 2016, 52, 347–357. [Google Scholar] [CrossRef]
- O’Neill, E.; Ng, L.C.; Sze, C.C.; Shingler, V. Aromatic ligand binding and intramolecular signalling of the phenol-responsive σ54-dependent regulator DmpR: Ligand Binding and Intramolecular Signalling of DmpR. Mol. Microbiol. 2002, 28, 131–141. [Google Scholar] [CrossRef]
- Ray, S.; Gunzburg, M.J.; Wilce, M.; Panjikar, S.; Anand, R. Structural Basis of Selective Aromatic Pollutant Sensing by the Effector Binding Domain of MopR, an NtrC Family Transcriptional Regulator. ACS Chem. Biol. 2016, 11, 2357–2365. [Google Scholar] [CrossRef]
- Ng, L.C.; O’Neill, E.; Shingler, V. Genetic Evidence for Interdomain Regulation of the Phenol-responsive 54-dependent Activator DmpR. J. Biol. Chem. 1996, 271, 17281–17286. [Google Scholar] [CrossRef]
- Bush, M.; Dixon, R. The Role of Bacterial Enhancer Binding Proteins as Specialized Activators of σ54-Dependent Transcription. Microbiol. Mol. Biol. Rev. 2012, 76, 497–529. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Panjikar, S.; Anand, R. Structure Guided Design of Protein Biosensors for Phenolic Pollutants. ACS Sens. 2017, 2, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Senapati, T.; Sahu, S.; Bandyopadhyaya, R.; Anand, R. Design of Ultrasensitive Protein Biosensor Strips for Selective Detection of Aromatic Contaminants in Environmental Wastewater. Anal. Chem. 2018, 90, 8960–8968. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Ray, S.; Chowdhury, A.; Anand, R. Tunable Multiplexed Whole-Cell Biosensors as Environmental Diagnostics for ppb-Level Detection of Aromatic Pollutants. ACS Sens. 2021, 6, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Soleja, N.; Jairajpuri, M.A.; Queen, A.; Mohsin, M. Genetically encoded FRET-based optical sensor for Hg2+ detection and intracellular imaging in living cells. J. Ind. Microbiol. Biotechnol. 2019, 46, 1669–1683. [Google Scholar] [CrossRef]
- Ji, Y.; Guan, F.; Zhou, X.; Liu, X.; Wu, N.; Liu, D.; Tian, J. Construction of a mApple-D6A3-mediated biosensor for detection of heavy metal ions. AMB Express 2020, 10, 213. [Google Scholar] [CrossRef]
- Mattocks, J.A.; Ho, J.V.; Cotruvo, J.A. A Selective, Protein-Based Fluorescent Sensor with Picomolar Affinity for Rare Earth Elements. J. Am. Chem. Soc. 2019, 141, 2857–2861. [Google Scholar] [CrossRef]
- Lee, W.; Kim, H.; Kang, Y.; Lee, Y.; Yoon, Y. A Biosensor Platform for Metal Detection Based on Enhanced Green Fluorescent Protein. Sensors 2019, 19, 1846. [Google Scholar] [CrossRef]
- Yoon, Y.; Kang, Y.; Lee, W.; Oh, K.-C.; Jang, G.; Kim, B.-G. Modulating the Properties of Metal-Sensing Whole-Cell Bioreporters by Interfering with Escherichia coli Metal Homeostasis. J. Microbiol. Biotechnol. 2018, 28, 323–329. [Google Scholar] [CrossRef]
- Mann, M.M.; Tang, J.D.; Berger, B.W. Engineering human liver fatty acid binding protein for detection of poly- and perfluoroalkyl substances. Biotechnol. Bioeng. 2022, 119, 513–522. [Google Scholar] [CrossRef]
- N’Guetta, P.-E.Y.; Fink, M.M.; Rizk, S.S. Engineering a fluorescence biosensor for the herbicide glyphosate. Protein Eng. Des. Sel. 2020, 33, gzaa021. [Google Scholar] [CrossRef] [PubMed]
- Sahay, S.; Chouhan, D. Study on the potential of cold-active lipases from psychrotrophic fungi for detergent formulation. J. Genet. Eng. Biotechnol. 2018, 16, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; He, L.; Wang, Q.; Ye, H.; Jiang, C. Effects of inoculation of biosurfactant-producing Bacillus sp. J119 on plant growth and cadmium uptake in a cadmium-amended soil. J. Hazard. Mater. 2008, 155, 17–22. [Google Scholar] [CrossRef]
- Kavitha, M.; Shanthi, C. Alkaline Thermostable Cold Active Lipase from Halotolerant Pseudomonas sp. VITCLP4 as Detergent Additive. Indian J. Biotechnol. 2017, 16, 446–455. [Google Scholar]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Genet. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Lai, C.-C.; Huang, Y.C.; Wei, Y.-H.; Chang, J.-S. Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J. Hazard. Mater. 2009, 167, 609–614. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Heavy metal removal from sediments by biosurfactants. J. Hazard. Mater. 2001, 85, 111–125. [Google Scholar] [CrossRef]
- Singh, A.K.; Cameotra, S.S. Efficiency of lipopeptide biosurfactants in removal of petroleum hydrocarbons and heavy metals from contaminated soil. Environ. Sci. Pollut. Res. 2013, 20, 7367–7376. [Google Scholar] [CrossRef]
- Park, H.J.; Han, S.J.; Yim, J.H.; Kim, D. Characterization of an Antarctic Alkaline Protease, a Cold-Active Enzyme for Laundry Detergents. Korean J. Microbiol. 2018, 54, 60–68. [Google Scholar] [CrossRef]
- Wang, X.; Kan, G.; Ren, X.; Yu, G.; Shi, C.; Xie, Q.; Wen, H.; Betenbaugh, M. Molecular Cloning and Characterization of a Novel α-Amylase from Antarctic Sea Ice Bacterium Pseudoalteromonas sp. M175 and Its Primary Application in Detergent. BioMed Res. Int. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Li, C.; Zhang, R.; Wang, J.; Wilson, L.M.; Yan, Y. Protein Engineering for Improving and Diversifying Natural Product Biosynthesis. Trends Biotechnol. 2020, 38, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Ma, T.; Liu, M.; Qu, J.; Liu, Z.; Zhang, H.; Shi, B.; Fu, S.; Ma, J.; Lai, L.T.F.; et al. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux. Nat. Commun. 2019, 10, 4248. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Zhang, C.; Du, G.; Chen, J. Metabolic Engineering of Escherichia coli for Production of 2-phenylethanol from Renewable Glucose. Appl. Biochem. Biotechnol. 2013, 172, 2012–2021. [Google Scholar] [CrossRef]
- Lee, H.-J.; Ravn, M.M.; Coates, R.M. Synthesis and characterization of abietadiene, levopimaradiene, palustradiene, and neoabietadiene: Hydrocarbon precursors of the abietane diterpene resin acids. Tetrahedron 2001, 57, 6155–6167. [Google Scholar] [CrossRef]
- Leonard, E.; Ajikumar, P.K.; Thayer, K.; Xiao, W.-H.; Mo, J.D.; Tidor, B.; Stephanopoulos, G.; Prather, K.L.J. Combining metabolic and protein engineering of a terpenoid biosynthetic pathway for overproduction and selectivity control. Proc. Natl. Acad. Sci. USA 2010, 107, 13654–13659. [Google Scholar] [CrossRef]
- Wu, X.; Tovilla-Coutiño, D.B.; Eiteman, M.A. Engineered citrate synthase improves citramalic acid generation in Escherichia coli. Biotechnol. Bioeng. 2020, 117, 2781–2790. [Google Scholar] [CrossRef]
- Bibik, J.D.; Weraduwage, S.M.; Banerjee, A.; Robertson, K.; Espinoza-Corral, R.; Sharkey, T.D.; Lundquist, P.K.; Hamberger, B.R. Pathway Engineering, Re-targeting, and Synthetic Scaffolding Improve the Production of Squalene in Plants. ACS Synth. Biol. 2022, 11, 2121–2133. [Google Scholar] [CrossRef]
- Kwan, D.H.; Schulz, F. The Stereochemistry of Complex Polyketide Biosynthesis by Modular Polyketide Synthases. Molecules 2011, 16, 6092–6115. [Google Scholar] [CrossRef]
- Pan, G.; Xu, Z.; Guo, Z.; Hindra; Ma, M.; Yang, D.; Zhou, H.; Gansemans, Y.; Zhu, X.; Huang, Y.; et al. Discovery of the leinamycin family of natural products by mining actinobacterial genomes. Proc. Natl. Acad. Sci. USA 2017, 114, E11131–E11140. [Google Scholar] [CrossRef]
- McDaniel, R.; Thamchaipenet, A.; Gustafsson, C.; Fu, H.; Betlach, M.; Betlach, M.; Ashley, G. Multiple genetic modifications of the erythromycin polyketide synthase to produce a library of novel “unnatural” natural products. Proc. Natl. Acad. Sci. USA 1999, 96, 1846–1851. [Google Scholar] [CrossRef]
- Jacobsen, J.R.; Hutchinson, C.R.; Cane, D.E.; Khosla, C. Precursor-Directed Biosynthesis of Erythromycin Analogs by an Engineered Polyketide Synthase. Science 1997, 277, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Type I Polyketide Synthase Requiring a Discrete Acyltransferase for Polyketide Biosynthesis. Available online: https://www.pnas.org/doi/10.1073/pnas.0537286100 (accessed on 5 September 2022).
- Deng, Z.; Liu, J.; Li, T.; Li, H.; Liu, Z.; Dong, Y.; Li, W. An Unusual Type II Polyketide Synthase System Involved in Cinnamoyl Lipid Biosynthesis. Angew. Chem. 2021, 133, 155–160. [Google Scholar] [CrossRef]
- Dutta, S.; Whicher, J.R.; Hansen, D.A.; Hale, W.A.; Chemler, J.A.; Congdon, G.R.; Narayan, A.R.H.; Håkansson, K.; Sherman, D.H.; Smith, J.L.; et al. Structure of a modular polyketide synthase. Nature 2014, 510, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.A.; Schmidt, J.J.; Lowell, A.N.; Hansen, D.A.; Coburn, K.M.; Chemler, J.A.; Sherman, D.H. Probing Selectivity and Creating Structural Diversity Through Hybrid Polyketide Synthases. Angew. Chem. Int. Ed. 2020, 59, 13575–13580. [Google Scholar] [CrossRef]
- Furuya, T.; Kamlet, A.S.; Ritter, T. Catalysis for fluorination and trifluoromethylation. Nature 2011, 473, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Sirirungruang, S.; Ad, O.; Privalsky, T.M.; Ramesh, S.; Sax, J.L.; Dong, H.; Baidoo, E.E.K.; Amer, B.; Khosla, C.; Chang, M.C.Y. Engineering site-selective incorporation of fluorine into polyketides. Nat. Chem. Biol. 2022, 18, 886–893. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mann, M.M.; Vigil, T.N.; Felton, S.M.; Fahy, W.E.; Kinkeade, M.A.; Kartseva, V.K.; Rowson, M.-J.C.; Frost, A.J.; Berger, B.W. Proteins in Synthetic Biology with Agricultural and Environmental Applications. SynBio 2023, 1, 77-88. https://doi.org/10.3390/synbio1010006
Mann MM, Vigil TN, Felton SM, Fahy WE, Kinkeade MA, Kartseva VK, Rowson M-JC, Frost AJ, Berger BW. Proteins in Synthetic Biology with Agricultural and Environmental Applications. SynBio. 2023; 1(1):77-88. https://doi.org/10.3390/synbio1010006
Chicago/Turabian StyleMann, Madison M., Toriana N. Vigil, Samantha M. Felton, William E. Fahy, Mason A. Kinkeade, Victoria K. Kartseva, Mary-Jean C. Rowson, Abigail J. Frost, and Bryan W. Berger. 2023. "Proteins in Synthetic Biology with Agricultural and Environmental Applications" SynBio 1, no. 1: 77-88. https://doi.org/10.3390/synbio1010006
APA StyleMann, M. M., Vigil, T. N., Felton, S. M., Fahy, W. E., Kinkeade, M. A., Kartseva, V. K., Rowson, M.-J. C., Frost, A. J., & Berger, B. W. (2023). Proteins in Synthetic Biology with Agricultural and Environmental Applications. SynBio, 1(1), 77-88. https://doi.org/10.3390/synbio1010006