In Situ Tracking of Nanoparticles During Electrophoresis in Hydrogels Using a Fiber-Based UV-Vis System
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Modification of Au NPs
2.2. Synthesis and Modification of Fe3O4 NPs
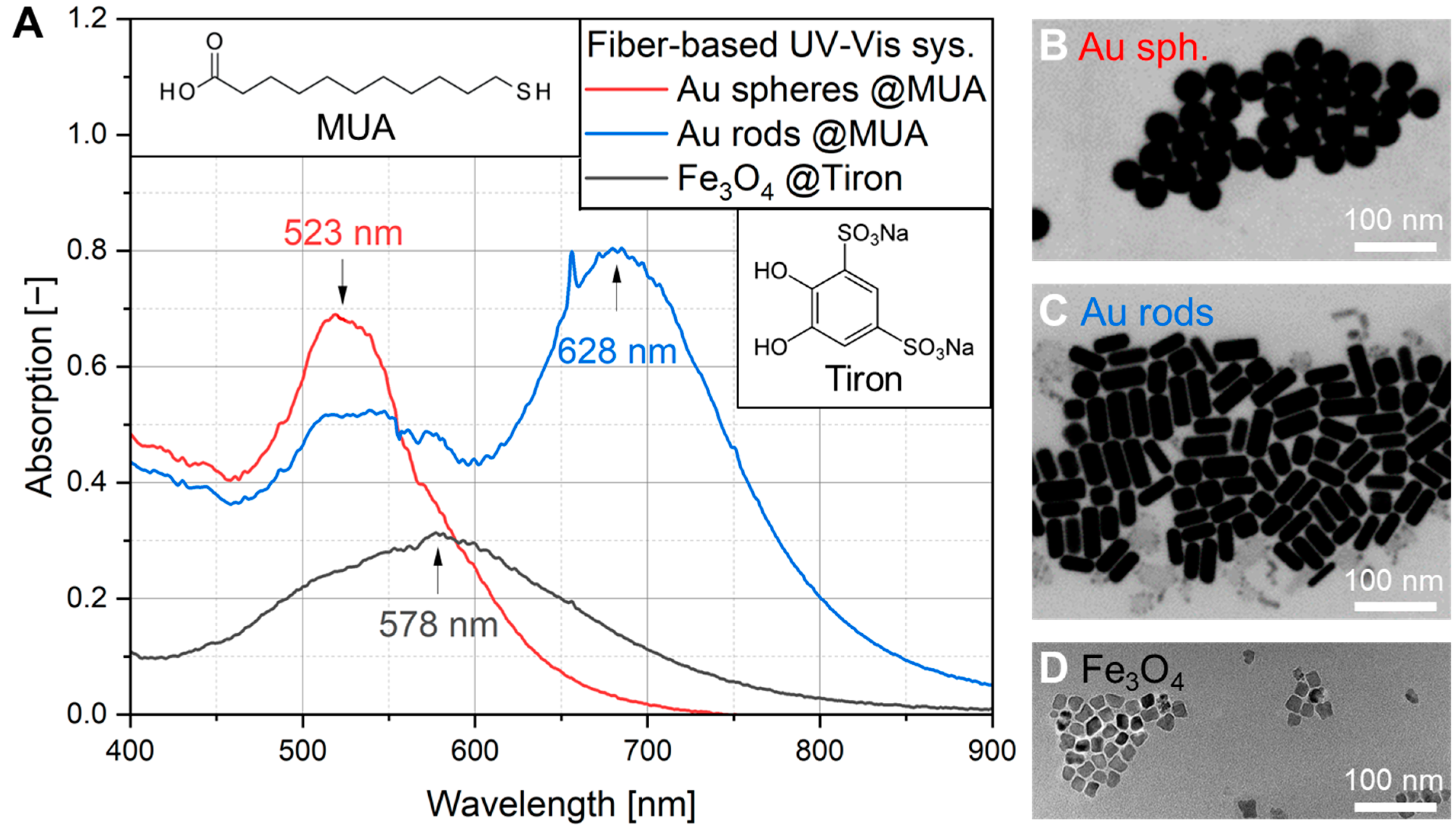
2.3. Characterization of Au and Fe3O4 NPs by UV-Vis, SEM and TEM
2.4. Preparation of Agarose Gels Followed by Electrophoretic Experiment
3. Results
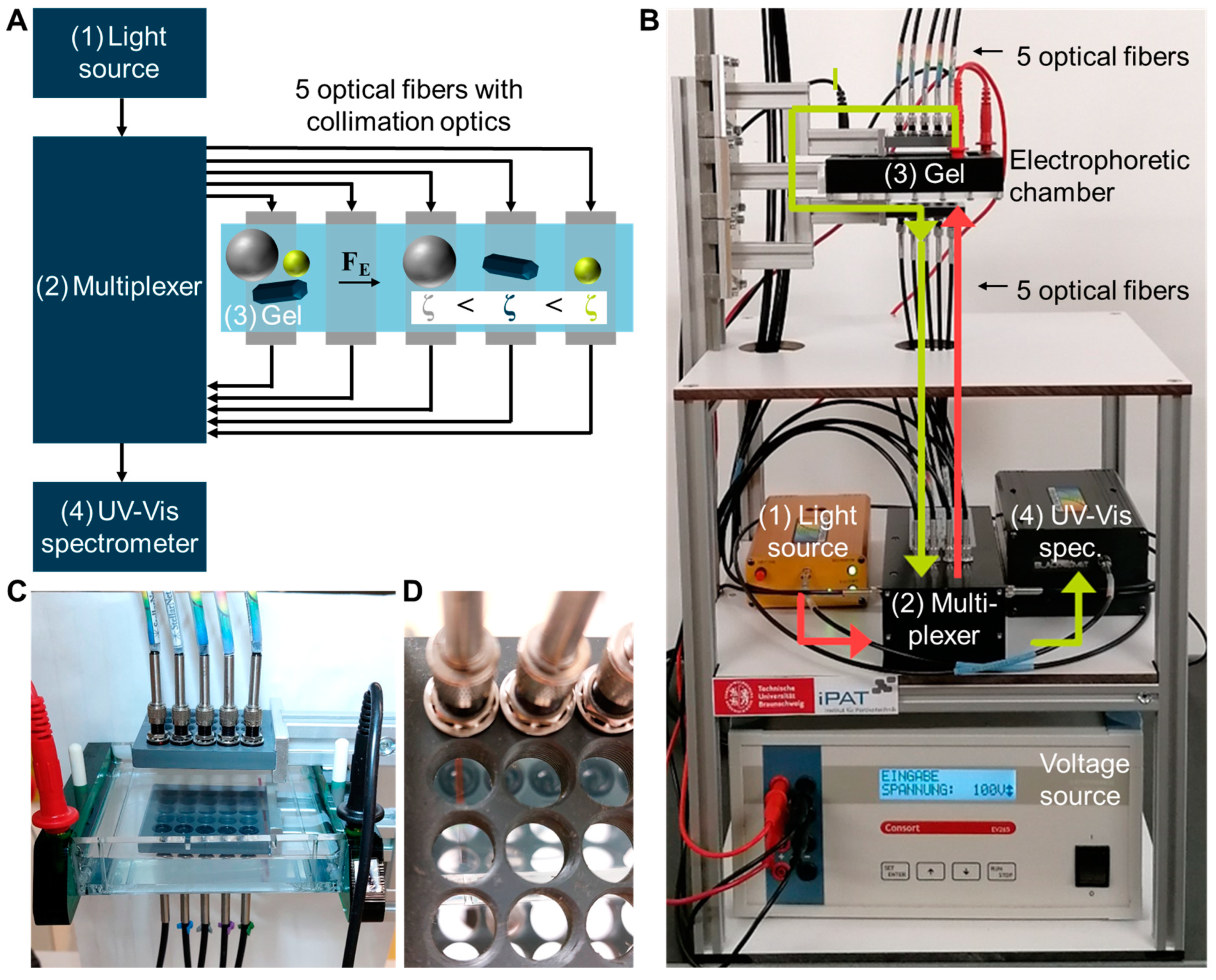
3.1. Set-Up of the Fiber-Based UV-Vis Measurement System
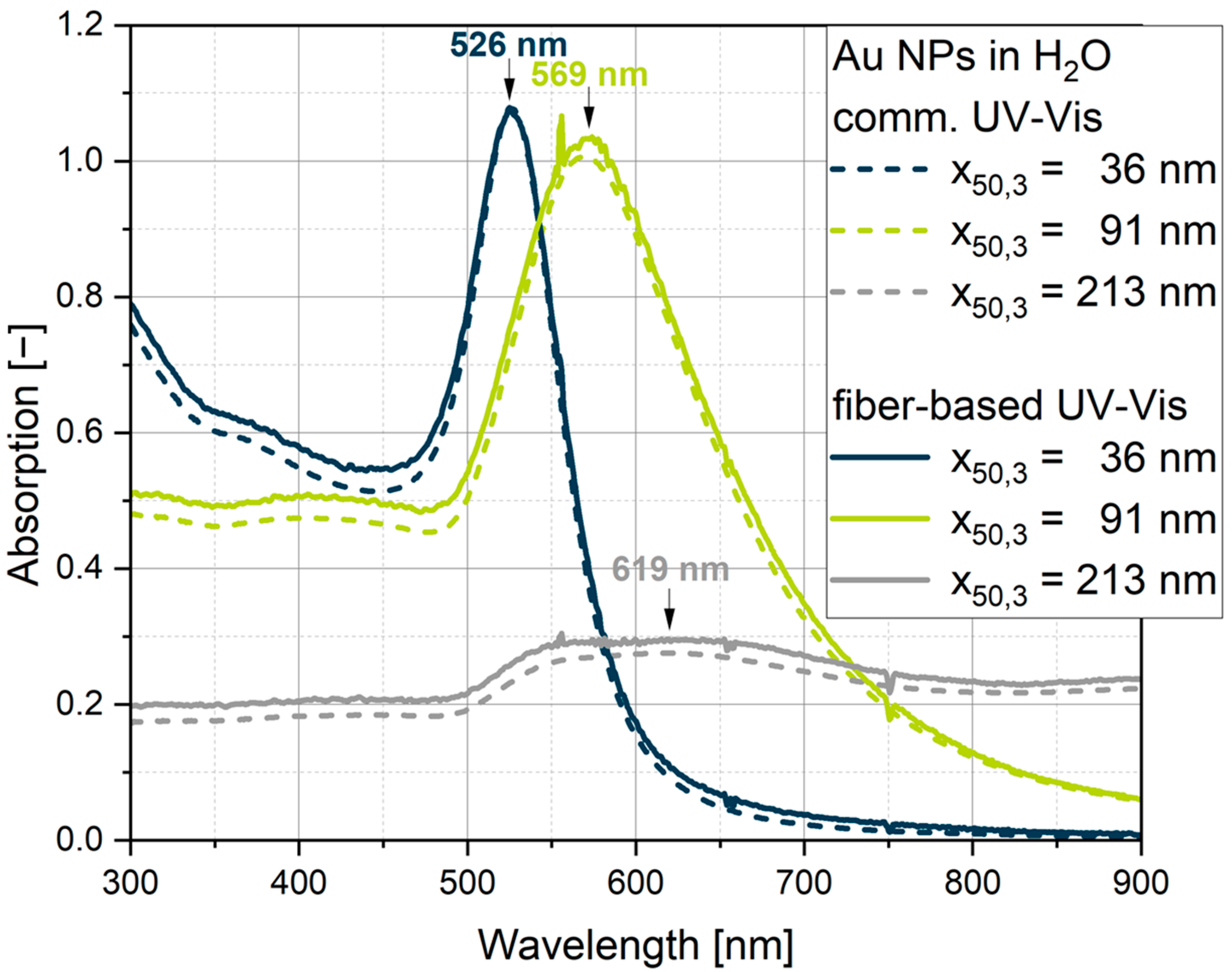
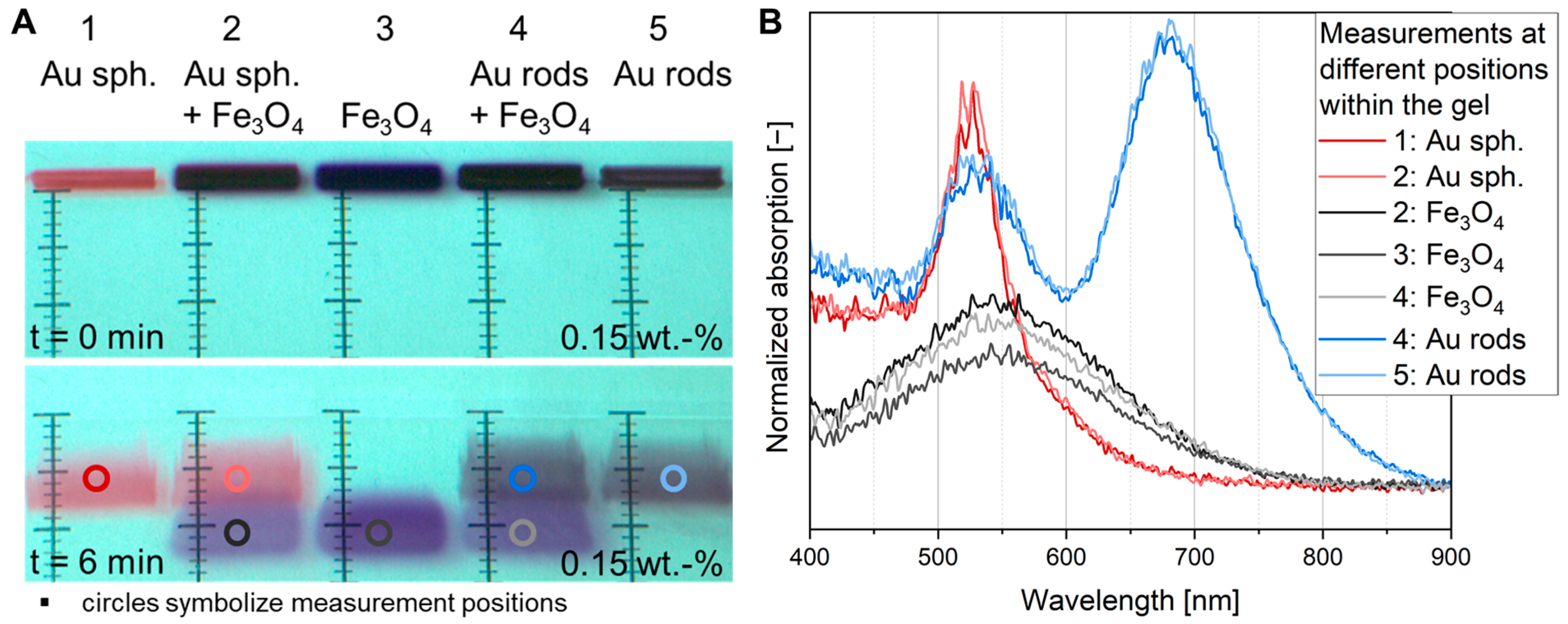
3.2. Comparison Between Commercial and Fiber-Based UV-Vis Measurement Systems
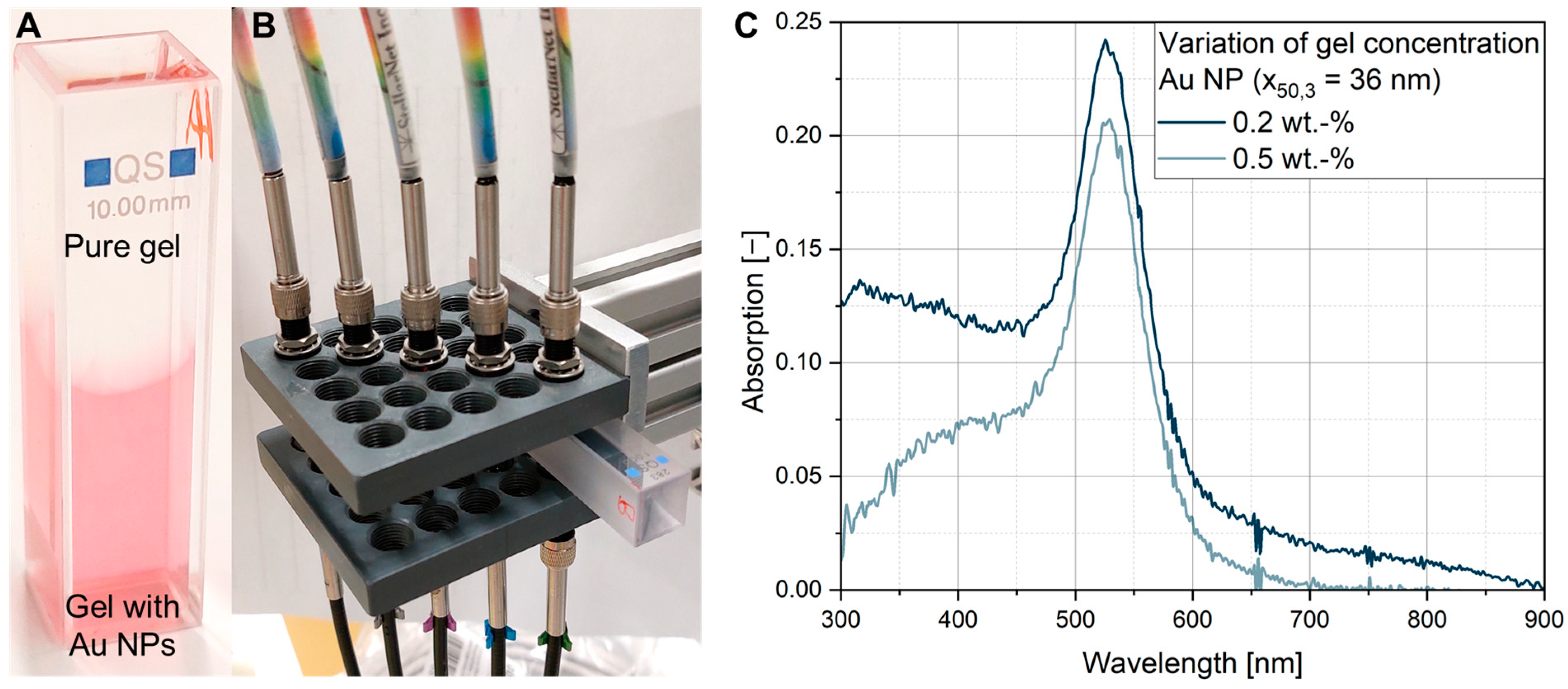
3.3. UV-Vis Measurements with Varied Gel and Particle Concentrations in Quartz Cuvettes
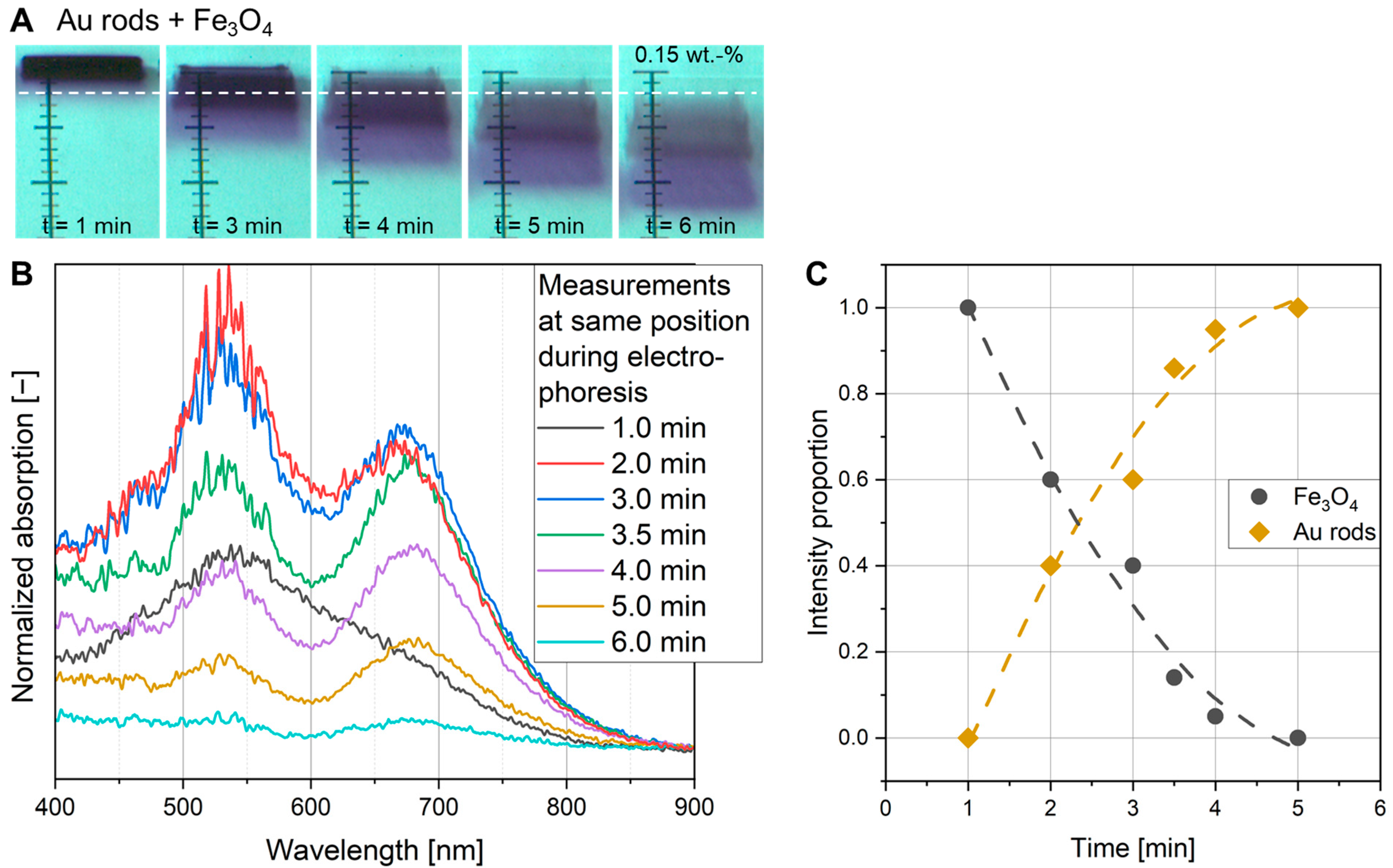
3.4. In Situ UV-Vis Measurements
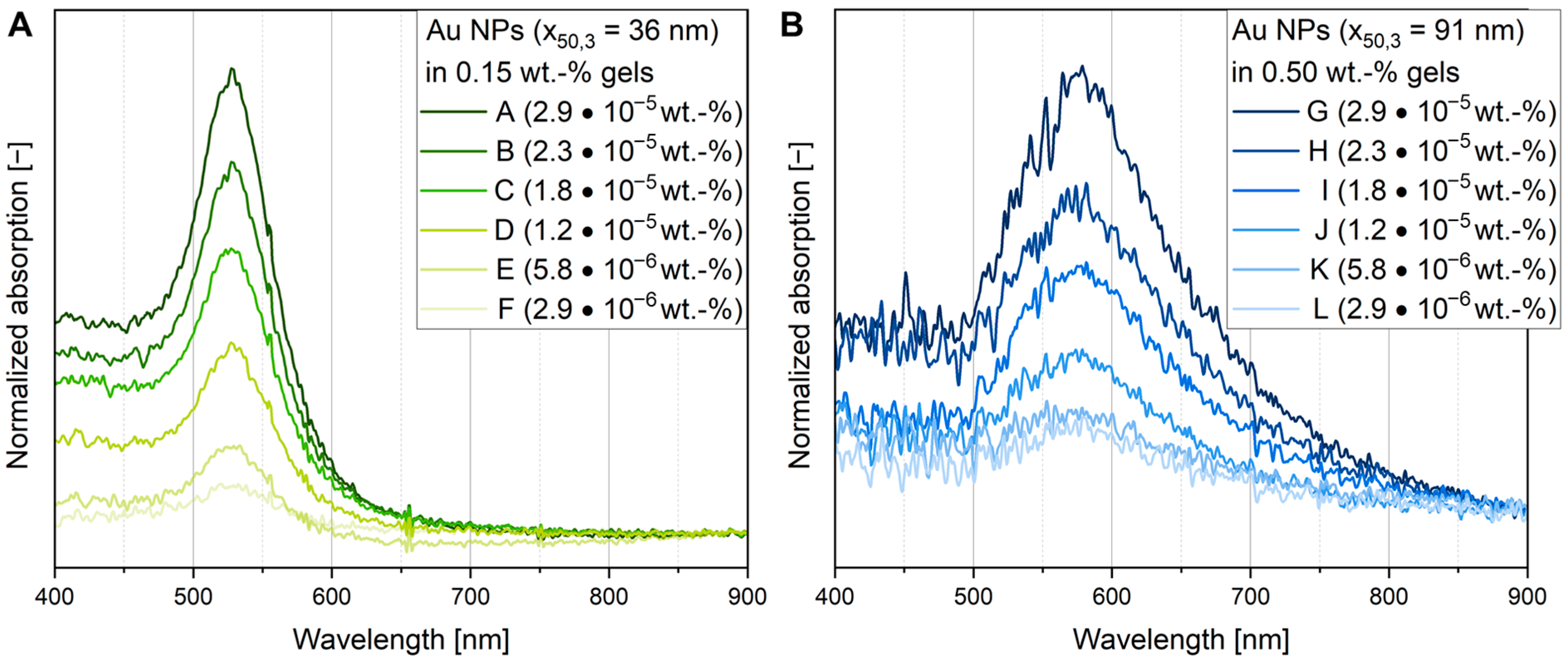
3.5. Determination of Particle Concentration Within the Gel by Calibrated UV-Vis Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNO3 | Silver nitrate |
| BE | Dibenzyl ether |
| CTAB | Cetyltrimethylammonium bromide |
| CTAC | Cetyltrimethylammonium chloride |
| EDTA | Ethylenediaminetetraacetic acid |
| Fe(acac)3 | Iron(III)acetylacetonate |
| HAuCl4 | Gold(III)chloride |
| HDD | 1,2-Hexadecanediol |
| LSPR | Localized surface plasmon resonance |
| MUA | 11-Mercaptoundecanoic acid |
| NP | Nanoparticle |
| PSD | Particle size distribution |
| NaBH4 | Sodium borohydride |
| NaOL | Sodium oleate |
| OAc | Oleic acid |
| OAm | Oleylamine |
| SEM | Scanning electron microscopy |
| STEM | Scanning transmission electron microscopy |
| TBE | Tris/borate/EDTA |
| TEM | Transmission electron microscopy |
| Tris | Tris(hydroxymethyl)aminomethane |
| UV-Vis | Ultraviolet–visible |
| x50,3 | Mean volumetric diameter [nm] |
References
- Tariq, M.; Koch, M.D.; Andrews, J.W.; Knowles, K.E. Correlation between surface chemistry and optical properties in colloidal Cu2O nanoparticles. J. Phys. Chem. C 2020, 124, 4810–4819. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Nemati, Z.; Alonso, J.; Rodrigo, I.; Das, R.; Garaio, E.; García, J.Á.; Orue, I.; Phan, M.-H.; Srikanth, H. Improving the heating efficiency of iron oxide nanoparticles by tuning their shape and size. J. Phys. Chem. C 2018, 122, 2367–2381. [Google Scholar] [CrossRef]
- Golden, M.S.; Bjonnes, A.C.; Georgiadis, R.M. Distance- and wavelength-dependent dielectric function of Au nanoparticles by angle-resolved surface plasmon resonance imaging. J. Phys. Chem. C 2010, 114, 8837–8843. [Google Scholar] [CrossRef]
- Frecker, T.; Bailey, D.; Arzeta-Ferrer, X.; McBride, J.; Rosenthal, S.J. Quantum dots and their application in lighting, displays, and biology. ECS J. Solid State Sci. Technol. 2016, 5, R3019–R3031. [Google Scholar] [CrossRef]
- Segets, D.; Gradl, J.; Taylor, R.K.; Vassilev, V.; Peukert, W. Analysis of optical absorbance spectra for the determination of ZnO nanoparticle size distribution, solubility, and surface energy. ACS Nano 2009, 3, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Singh, V.; Jurney, P.; Shi, L.; Sreenivasan, S.V.; Roy, K. Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. Proc. Natl. Acad. Sci. USA 2013, 110, 17247–17252. [Google Scholar] [CrossRef]
- Zhang, K.; Fang, H.; Chen, Z.; Taylor, J.-S.A.; Wooley, K.L. Shape effects of nanoparticles conjugated with cell-penetrating peptides (HIV Tat PTD) on CHO cell uptake. Bioconjug. Chem. 2008, 19, 1880–1887. [Google Scholar] [CrossRef]
- Lu, F.; Wu, S.-H.; Hung, Y.; Mou, C.-Y. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef]
- Kowalczyk, B.; Lagzi, I.; Grzybowski, B.A. Nanoseparations: Strategies for size and/or shape-selective purification of nanoparticles. Curr. Opin. Colloid Interface Sci. 2011, 16, 135–148. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, J.; An, H.-Q.; Li, J.-J.; Zhao, J.-W. A two-step approach to realize size- and shape-selective separation of crude gold nanotriangles with high purification. J. Mater. Chem. C 2016, 4, 568–580. [Google Scholar] [CrossRef]
- Barasinski, M.; Pesch, G.R.; Garnweitner, G. Particle Separation Techniques-Fundamentals, Instrumentation, and Selected Applications, 1st ed.; Elsevier Science: Philadelphia, PA, USA, 2022. [Google Scholar]
- Winkler, M.; Sonner, H.; Gleiss, M.; Nirschl, H. Fractionation of ultrafine particles: Evaluation of separation efficiency by UV–vis spectroscopy. Chem. Eng. Sci. 2020, 213, 115374. [Google Scholar] [CrossRef]
- Arlt, C.-R.; Brekel, D.; Franzreb, M. Continuous fractionation of nanoparticles based on their magnetic properties applying simulated moving bed chromatography. Sep. Purif. Technol. 2021, 259, 118123. [Google Scholar] [CrossRef]
- Hanauer, M.; Pierrat, S.; Zins, I.; Lotz, A.; Sönnichsen, C. Separation of nanoparticles by gel electrophoresis according to size and shape. Nano Lett. 2007, 7, 2881–2885. [Google Scholar] [CrossRef]
- Zhu, X.; Mason, T.G. Separating nanoparticles by surface charge group using pH-controlled passivated gel electrophoresis. Soft Mater. 2016, 14, 204–209. [Google Scholar] [CrossRef]
- Barasinski, M.; Garnweitner, G. Restricted and unrestricted migration mechanisms of silica nanoparticles in agarose gels and their utilization for the separation of binary mixtures. J. Phys. Chem. C 2020, 124, 5157–5166. [Google Scholar] [CrossRef]
- Xu, X.; Caswell, K.K.; Tucker, E.; Kabisatpathy, S.; Brodhacker, K.L.; Scrivens, W.A. Size and shape separation of gold nanoparticles with preparative gel electrophoresis. J. Chromatogr. A 2007, 1167, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Maaloum, M.; Pernodet, N.; Tinland, B. Agarose gel structure using atomic force microscopy: Gel concentration and ionic strength effects. Electrophoresis 1998, 19, 1606–1610. [Google Scholar] [CrossRef]
- Ben Ammar, N.E.; Saied, T.; Barbouche, M.; Hosni, F.; Hamzaoui, A.H.; Şen, M. A comparative study between three different methods of hydrogel network characterization: Effect of composition on the crosslinking properties using sol–gel, rheological and mechanical analyses. Polym. Bull. 2018, 75, 3825–3841. [Google Scholar] [CrossRef]
- Sikora, A.; Bartczak, D.; Geißler, D.; Kestens, V.; Roebben, G.; Ramaye, Y.; Varga, Z.; Palmai, M.; Shard, A.G.; Goenaga-Infante, H.; et al. A systematic comparison of different techniques to determine the zeta potential of silica nanoparticles in biological medium. Anal. Methods 2015, 7, 9835–9843. [Google Scholar] [CrossRef]
- Jansohn, M.; Rothhämel, S. Gentechnische Methoden: Eine Sammlung von Arbeitsanleitungen für das Molekularbiologische Labor, 5th ed.; Spektrum Akademischer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Bikos, D.A.; Mason, T.G. Influence of ionic constituents and electrical conductivity on the propagation of charged nanoscale objects in passivated gel electrophoresis. Electrophoresis 2018, 39, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.J.; Li, F.; Doane, T.L.; Burda, C. Electrophoretic interpretation of PEGylated NP structure with and without peripheral charge. Langmuir 2015, 31, 10246–10253. [Google Scholar] [CrossRef]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Ohshima, H. Gel electrophoresis of a soft particle. Adv. Colloid Interface Sci. 2019, 271, 101977. [Google Scholar] [CrossRef]
- Hlaváček, A.; Mickert, M.J.; Soukka, T.; Lahtinen, S.; Tallgren, T.; Pizurova, N.; Król, A.; Gorris, H.H. Large-scale purification of photon-upconversion nanoparticles by gel electrophoresis for analog and digital bioassays. Anal. Chem. 2019, 91, 1241–1246. [Google Scholar] [CrossRef]
- Pasricha, R.; Singh, A.; Sastry, M. Shape and size selective separation of gold nanoclusters by competitive complexation with octadecylamine monolayers at the air-water interface. J. Colloid Interface Sci. 2009, 333, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hong, Y.; Wu, W.; Sun, D.; Wang, Y.; Huang, J.; Li, Q. Separation of different shape biosynthesized gold nanoparticles via agarose gel electrophoresis. Sep. Purif. Technol. 2015, 151, 332–337. [Google Scholar] [CrossRef]
- Barasinski, M.; Hilbig, J.; Neumann, S.; Rafaja, D.; Garnweitner, G. Simple model of the electrophoretic migration of spherical and rod-shaped Au nanoparticles in gels with varied mesh sizes. Colloids Surf. A 2022, 651, 129716. [Google Scholar] [CrossRef]
- Del Caño, R.; Gisbert-González, J.M.; González-Rodríguez, J.; Sánchez-Obrero, G.; Madueño, R.; Blázquez, M.; Pineda, T. Effective replacement of cetyltrimethylammonium bromide (CTAB) by mercaptoalkanoic acids on gold nanorod (AuNR) surfaces in aqueous solutions. Nanoscale 2020, 12, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Dragoman, R.M.; Grogg, M.; Bodnarchuk, M.I.; Tiefenboeck, P.; Hilvert, D.; Dirin, D.N.; Kovalenko, M.V. Surface-engineered cationic nanocrystals stable in biological buffers and high ionic strength solutions. Chem. Mater. 2017, 29, 9416–9428. [Google Scholar] [CrossRef] [PubMed]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Ansar, S.M.; Chakraborty, S.; Kitchens, C.L. pH-responsive mercaptoundecanoic acid functionalized gold nanoparticles and applications in catalysis. Nanomaterials 2018, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Sharifi Dehsari, H.; Harris, R.A.; Ribeiro, A.H.; Tremel, W.; Asadi, K. Optimizing the binding energy of the surfactant to iron oxide yields truly monodisperse nanoparticles. Langmuir 2018, 34, 6582–6590. [Google Scholar] [CrossRef] [PubMed]
- Görke, M.; Okeil, S.; Menzel, D.; Semenenko, B.; Garnweitner, G. Tuning the properties of iron oxide nanoparticles in thermal decomposition synthesis: A comparative study of the influence of temperature, ligand length and ligand concentration. Part. Part. Syst. Charact. 2024, 41, 2400059. [Google Scholar] [CrossRef]
- Akbulut, M.K.; Harreiß, C.; Löffler, M.; Mayrhofer, K.J.J.; Schöbitz, M.; Bachmann, J.; Spiecker, E.; Hock, R.; Kryschi, C. Facile one-pot synthesis of water-soluble fcc FePt3 alloy nanostructures. SN Appl. Sci. 2020, 2, 1744. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; El-Sayed, M.A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Tyagi, H.; Kushwaha, A.; Kumar, A.; Aslam, M. A facile pH controlled citrate-based reduction method for gold nanoparticle synthesis at room temperature. Nanoscale Res. Lett. 2016, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Afrooz, A.R.M.N.; Sivalapalan, S.T.; Murphy, C.J.; Hussain, S.M.; Schlager, J.J.; Saleh, N.B. Spheres vs. rods: The shape of gold nanoparticles influences aggregation and deposition behavior. Chemosphere 2013, 91, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.J.; Sau, T.K.; Gole, A.M.; Orendorff, C.J.; Gao, J.; Gou, L.; Hunyadi, S.E.; Li, T. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J. Phys. Chem. B 2005, 109, 13857–13870. [Google Scholar] [CrossRef]
- Porsiel, C.; Temel, B.; Schirmacher, A.; Buhr, E.; Garnweitner, G. Dimensional characterization of cadmium selenide nanocrystals via indirect fourier transform evaluation of small-angle X-ray scattering data. Nano Res. 2019, 12, 2849–2857. [Google Scholar] [CrossRef]
- Barasinski, M.; Garnweitner, G. Aufreinigung von Nano- und Submikronpartikeln durch präparative Gelelektrophorese. Chem. Ing. Tech. 2023, 95, 266–277. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barasinski, M.; Jasper, V.; Görke, M.; Garnweitner, G. In Situ Tracking of Nanoparticles During Electrophoresis in Hydrogels Using a Fiber-Based UV-Vis System. Powders 2025, 4, 3. https://doi.org/10.3390/powders4010003
Barasinski M, Jasper V, Görke M, Garnweitner G. In Situ Tracking of Nanoparticles During Electrophoresis in Hydrogels Using a Fiber-Based UV-Vis System. Powders. 2025; 4(1):3. https://doi.org/10.3390/powders4010003
Chicago/Turabian StyleBarasinski, Matthäus, Valentin Jasper, Marion Görke, and Georg Garnweitner. 2025. "In Situ Tracking of Nanoparticles During Electrophoresis in Hydrogels Using a Fiber-Based UV-Vis System" Powders 4, no. 1: 3. https://doi.org/10.3390/powders4010003
APA StyleBarasinski, M., Jasper, V., Görke, M., & Garnweitner, G. (2025). In Situ Tracking of Nanoparticles During Electrophoresis in Hydrogels Using a Fiber-Based UV-Vis System. Powders, 4(1), 3. https://doi.org/10.3390/powders4010003






