Improving the Powder Properties of an Active Pharmaceutical Ingredient (Ethenzamide) with a Silica Nanoparticle Coating for Direct Compaction into Tablets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ETZ with Silica Nanoparticles Dry Coated by the Mechanomill
2.3. ETZ with Silica Nanoparticles Dry Coated by the Comil
2.4. Particle Characterization
2.5. Surface Energy Measurements by Inverse Gas Chromatography
2.6. Powder Flowability
2.7. Preparation of ETZ Tablets from Formulated Powder and Evaluation of Tablet Properties
3. Result and Discussion
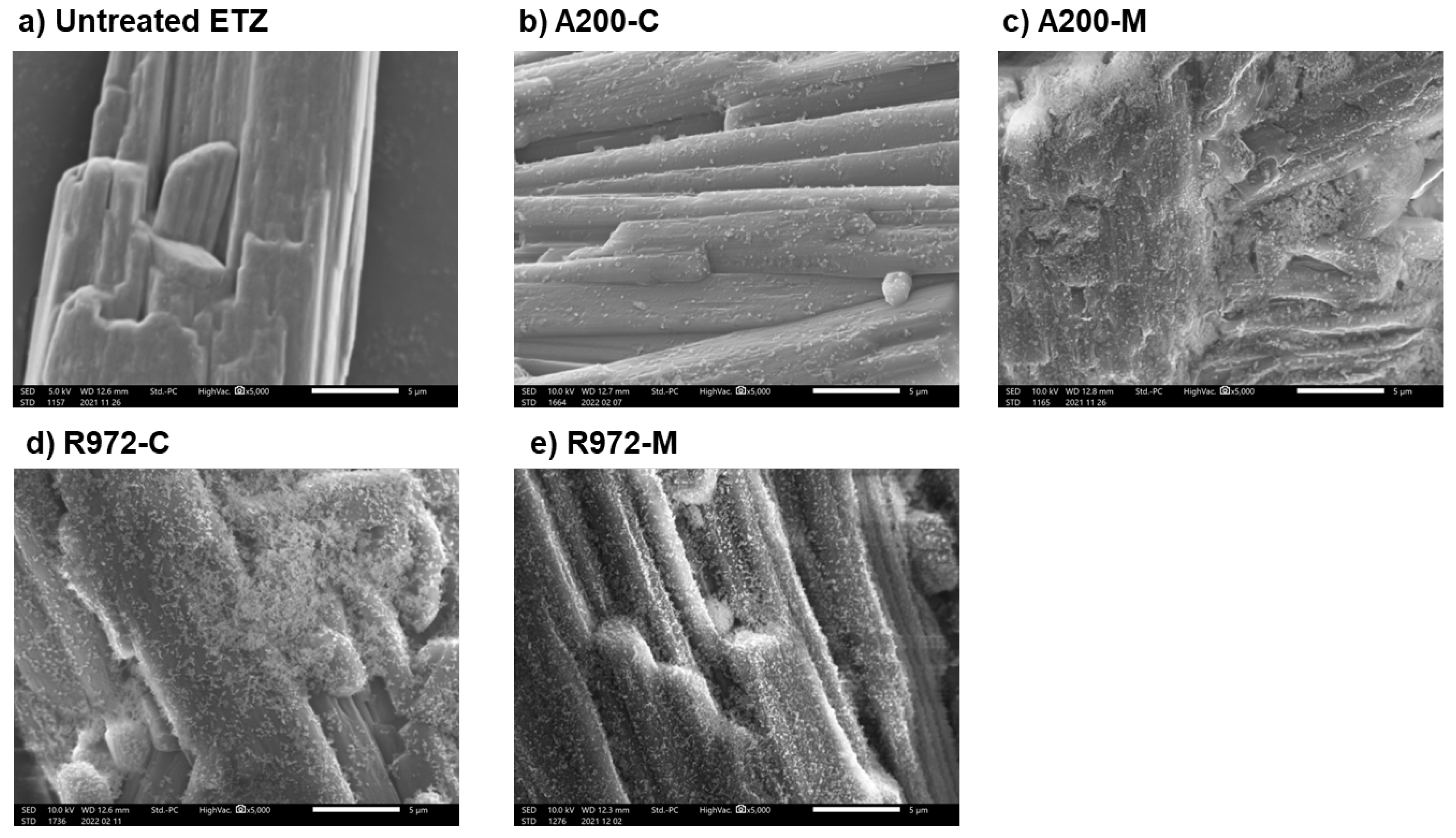
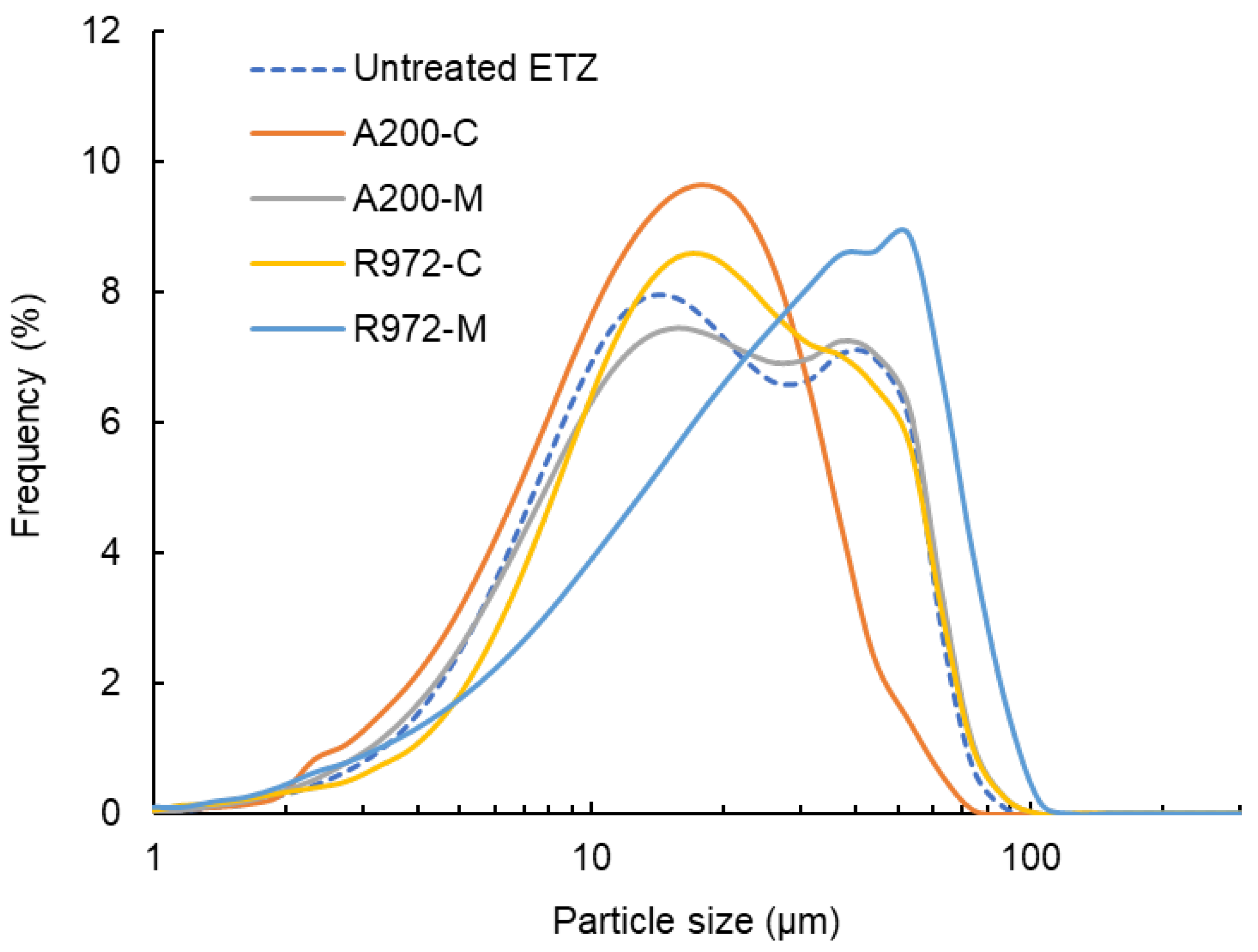
3.1. Particle Morphology and Size Distribution of Ethenzamide Dry Coated with Silica Nanoparticles
3.2. Static Flowability Evaluation of the Ethenzamide Powder Dry Coated with Silica Nanoparticles
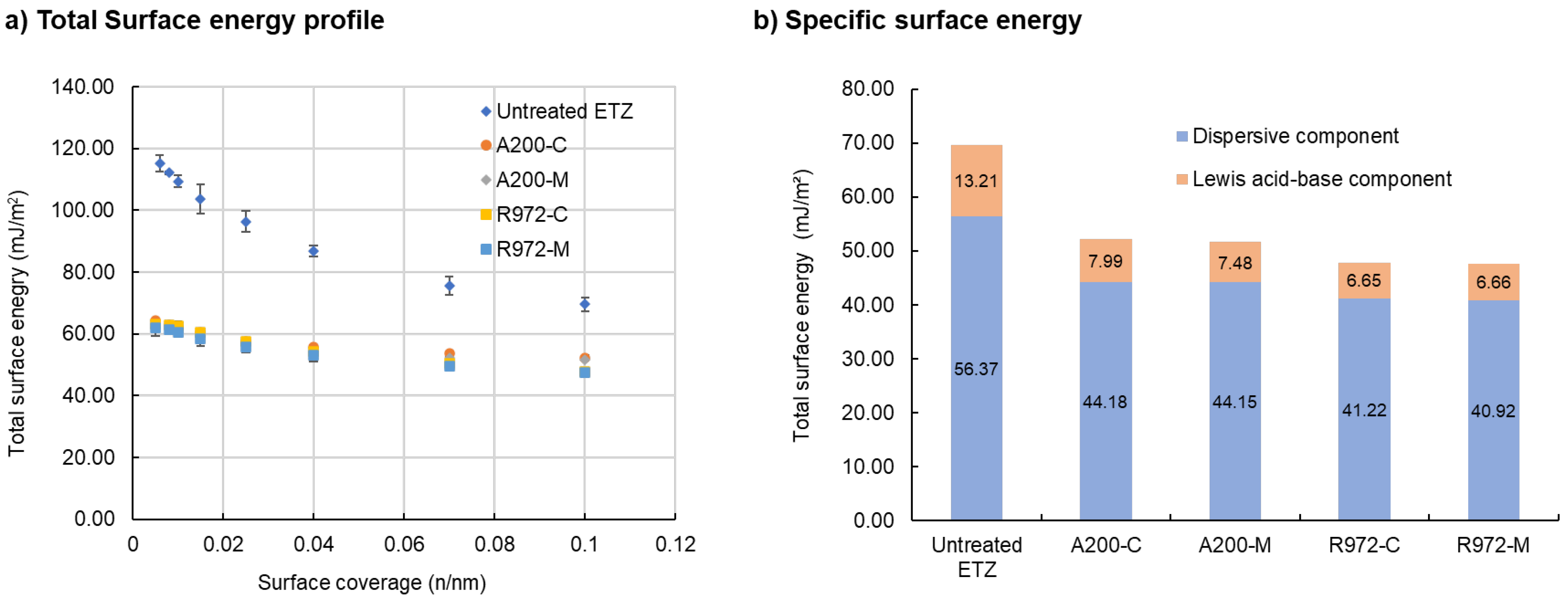
3.3. Surface Free Energy of Silica-Nanoparticle-Coated Ethenzamide Measured by IGC
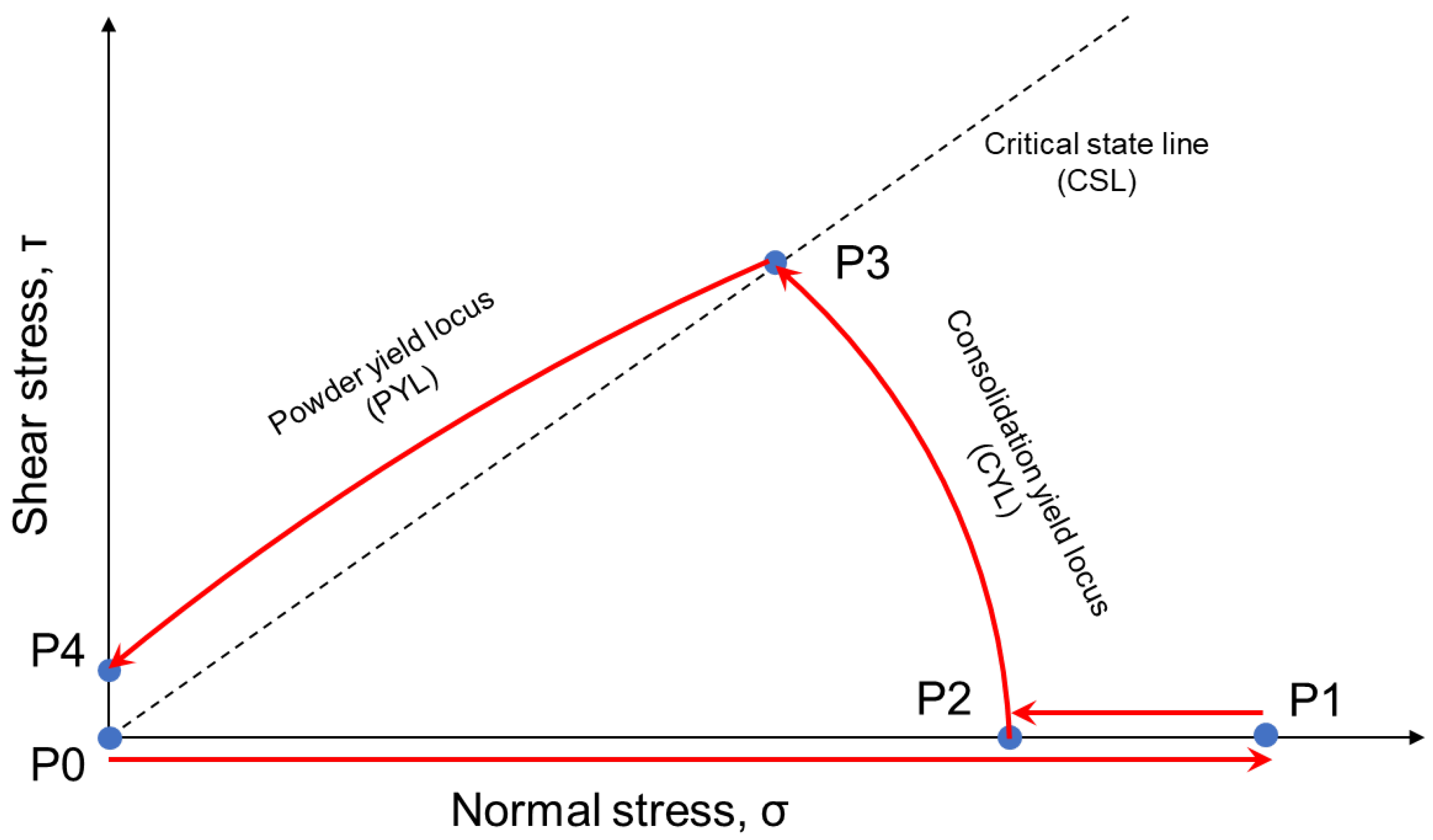
3.4. Powder Flowability of Ethenzamide Particles Coated with Silica Nanoparticles by the Shear Cell Method
3.5. Effect of Dry Coating of Silica Nanoparticles on ETZ Tablet Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leane, M.; Pitt, K.; Reynolds, G.; Manufacturing Classification System Working Group. A proposal for a drug product Manufacturing Classification System (MCS) for oral solid dosage forms. Pharm. Dev. Technol. 2015, 20, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Kleinebudde, P. Enabling the direct compression of metformin hydrochloride through QESD crystallization. Int. J. Pharm. 2021, 605, 120796. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ghoroi, C.; To, D.; Chen, Y.; Dave, R. Simultaneous micronization and surface modification for improvement of flow and dissolution of drug particles. Int. J. Pharm. 2011, 415, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Leane, M.; Pitt, K.; Reynolds, G.K.; Dawson, N.; Ziegler, I.; Szepes, A.; Crean, A.M.; Dall Agnol, R.; The Manufacturing Classification System McS Working Group. Manufacturing classification system in the real world: Factors influencing manufacturing process choices for filed commercial oral solid dosage formulations, case studies from industry and considerations for continuous processing. Pharm. Dev. Technol. 2018, 23, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Morin, G.; Briens, L. The effect of lubricants on powder flowability for pharmaceutical application. AAPS PharmSciTech 2013, 14, 1158–1168. [Google Scholar] [CrossRef]
- Liu, L.X.; Marziano, I.; Bentham, A.C.; Litster, J.D.; White, E.T.; Howes, T. Effect of particle properties on the flowability of ibuprofen powders. Int. J. Pharm. 2008, 362, 109–117. [Google Scholar] [CrossRef]
- Sharma, R.; Setia, G. Mechanical dry particle coating on cohesive pharmaceutical powders for improving flowability—A review. Powder Technol. 2019, 356, 458–479. [Google Scholar] [CrossRef]
- Huang, Z.; Scicolone, J.V.; Han, X.; Dave, R.N. Improved blend and tablet properties of fine pharmaceutical powders via dry particle coating. Int. J. Pharm. 2015, 478, 447–455. [Google Scholar] [CrossRef]
- Jallo, L.J.; Ghoroi, C.; Gurumurthy, L.; Patel, U.; Dave, R.N. Improvement of flow and bulk density of pharmaceutical powders using surface modification. Int. J. Pharm. 2012, 423, 213–225. [Google Scholar] [CrossRef]
- Pfeffer, R.; Dave, R.N.; Wei, D.; Ramlakhan, M. Synthesis of engineered particulates with tailored properties using dry particle coating. Powder Technol. 2001, 117, 40–67. [Google Scholar] [CrossRef]
- Chattoraj, S.; Shi, L.; Sun, C.C. Profoundly improving flow properties of a cohesive cellulose powder by surface coating with nano-silica through comilling. J. Pharm. Sci. 2011, 100, 4943–4952. [Google Scholar] [CrossRef]
- Mullarney, M.P.; Beach, L.E.; Davé, R.N.; Langdon, B.A.; Polizzi, M.; Blackwood, D.O. Applying dry powder coatings to pharmaceutical powders using a comil for improving powder flow and bulk density. Powder Technol. 2011, 212, 397–402. [Google Scholar] [CrossRef]
- Capece, M.; Larson, J. Improving the Effectiveness of the Conical Screen Mill as a Dry-Coating Process at Lab and Manufacturing Scale. Pharm. Res. 2022. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, L.; Marinaro, W.; Lu, Q.; Sun, C.C. Improving manufacturability of an ibuprofen powder blend by surface coating with silica nanoparticles. Powder Technol. 2013, 249, 290–296. [Google Scholar] [CrossRef]
- Tahara, K. Pharmaceutical formulation and manufacturing using particle/powder technology for personalized medicines. Adv. Powder Technol. 2020, 31, 387–392. [Google Scholar] [CrossRef]
- Shiino, K.; Iwao, Y.; Fujinami, Y.; Itai, S. Preparation and evaluation of granules with pH-dependent release by melt granulation. Int. J. Pharm. 2012, 431, 70–77. [Google Scholar] [CrossRef]
- Takeuchi, H.; Nagira, S.; Yamamoto, H.; Kawashima, Y. Solid dispersion particles of tolbutamide prepared with fine silica particles by the spray-drying method. Powder Technol. 2004, 141, 187–195. [Google Scholar] [CrossRef]
- Jonat, S.; Hasenzahl, S.; Drechsler, M.; Albers, P.; Wagner, K.G.; Schmidt, P.C. Investigation of compacted hydrophilic and hydrophobic colloidal silicon dioxides as glidants for pharmaceutical excipients. Powder Technol. 2004, 141, 31–43. [Google Scholar] [CrossRef]
- Ho, R.; Heng, J.Y.Y. A Review of Inverse Gas Chromatography and its Development as a Tool to Characterize Anisotropic Surface Properties of Pharmaceutical Solids. KONA Powder Part. J. 2013, 30, 164–180. [Google Scholar] [CrossRef]
- Karde, V.; Ghoroi, C. Influence of surface modification on wettability and surface energy characteristics of pharmaceutical excipient powders. Int. J. Pharm. 2014, 475, 351–363. [Google Scholar] [CrossRef]
- Shimada, Y.; Hatano, S.; Matsusaka, S. A new method for evaluating powder flowability using constant-volume shear tester. Adv. Powder Technol. 2018, 29, 3577–3583. [Google Scholar] [CrossRef]
- Shimada, Y.; Kawata, T.; Matsusaka, S. Analysis of constant-volume shear tests based on precise measurement of stresses in powder beds. Adv. Powder Technol. 2018, 29, 1372–1378. [Google Scholar] [CrossRef]
- Tsunakawa, H.; Aoki, R. A direct shear test for powders and granular materials. J. Res. Assoc. Powder Technol. Jpn. 1974, 11, 263–268. [Google Scholar] [CrossRef][Green Version]
- Honda, H.; Kimura, M.; Honda, F.; Matsuno, T.; Koishi, M. Preparation of monolayer particle coated powder by the dry impact blending process utilizing mechanochemical treatment. Colloids Surf. Physicochem. Eng. Asp. 1994, 82, 117–128. [Google Scholar] [CrossRef]
- Jager, P.D.; Bramante, T.; Luner, P.E. Assessment of Pharmaceutical Powder Flowability using Shear Cell-Based Methods and Application of Jenike’s Methodology. J. Pharm. Sci. 2015, 104, 3804–3813. [Google Scholar] [CrossRef]
- Salustio, P.J.; Inacio, C.; Nunes, T.; Sousa, E.S.J.P.; Costa, P.C. Flow characterization of a pharmaceutical excipient using the shear cell method. Pharm. Dev. Technol. 2020, 25, 237–244. [Google Scholar] [CrossRef]


| Untreated ETZ | A200-C | A200-M | R972-C | R972-M | |
|---|---|---|---|---|---|
| Angle of repose (degree) | 57.37 ± 1.86 | 43.70 ± 1.27 | 44.30 ± 0.29 | 44.37 ± 0.34 | 41.30 ± 0.00 |
| Bulk density (g/cm3) | 0.26 ± 0.01 | 0.36 ± 0.01 | 0.45 ± 0.00 | 0.45 ± 0.01 | 0.52 ± 0.00 |
| Tap density (g/cm3) | 0.56 ± 0.01 | 0.61 ± 0.01 | 0.70 ± 0.00 | 0.68 ± 0.01 | 0.75 ± 0.00 |
| Compressibility (%) | 54.2 ± 3.48 | 41.50 ± 0.54 | 36.03 ± 0.09 | 34.30 ± 0.36 | 30.23 ± 0.38 |
| Untreated ETZ | A200-C | A200-M | R972-C | R972-M | |
|---|---|---|---|---|---|
| Internal frictional angle (degree) | 28.97 ± 1.73 | 42.43 ± 1.05 | 41.93 ± 1.74 | 40.00 ± 0.16 | 40.47 ± 0.24 |
| Shear cohesion (kPa) | 9.93 ± 1.31 | 11.37 ± 1.76 | 5.80 ± 2.67 | 8.43 ± 2.65 | 4.67 ± 0.94 |
| Flow function coefficient (ffc) | 4.07 ± 0.37 | 3.60 ± 0.36 | 11.03 ± 5.31 | 6.50 ± 2.33 | 11.93 ± 4.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadauchi, T.; Yamada, D.; Koide, Y.; Yamada, M.; Shimada, Y.; Yamazoe, E.; Ito, T.; Tahara, K. Improving the Powder Properties of an Active Pharmaceutical Ingredient (Ethenzamide) with a Silica Nanoparticle Coating for Direct Compaction into Tablets. Powders 2022, 1, 231-242. https://doi.org/10.3390/powders1040016
Tadauchi T, Yamada D, Koide Y, Yamada M, Shimada Y, Yamazoe E, Ito T, Tahara K. Improving the Powder Properties of an Active Pharmaceutical Ingredient (Ethenzamide) with a Silica Nanoparticle Coating for Direct Compaction into Tablets. Powders. 2022; 1(4):231-242. https://doi.org/10.3390/powders1040016
Chicago/Turabian StyleTadauchi, Tatsuki, Daiki Yamada, Yoko Koide, Mayumi Yamada, Yasuhiro Shimada, Eriko Yamazoe, Takaaki Ito, and Kohei Tahara. 2022. "Improving the Powder Properties of an Active Pharmaceutical Ingredient (Ethenzamide) with a Silica Nanoparticle Coating for Direct Compaction into Tablets" Powders 1, no. 4: 231-242. https://doi.org/10.3390/powders1040016
APA StyleTadauchi, T., Yamada, D., Koide, Y., Yamada, M., Shimada, Y., Yamazoe, E., Ito, T., & Tahara, K. (2022). Improving the Powder Properties of an Active Pharmaceutical Ingredient (Ethenzamide) with a Silica Nanoparticle Coating for Direct Compaction into Tablets. Powders, 1(4), 231-242. https://doi.org/10.3390/powders1040016






