Effect of Pressure on Hydrogen Isotope Fractionation in Methane during Methane Hydrate Formation at Temperatures Below the Freezing Point of Water
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Makogon, Y.F.; Holditch, S.A.; Makogon, T.Y. Natural gas-hydrates—A potential energy source for the 21st century. J. Pet. Sci. Eng. 2007, 56, 14–31. [Google Scholar] [CrossRef]
- Boswell, R.; Collett, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206–1215. [Google Scholar] [CrossRef]
- Kennedy, M.; Mrofka, D.; Von Der Borch, C. Snowball Earth termination by destabilization of equatorial permafrost methane clathrate. Nature 2008, 453, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Whiticar, M.J.; Faber, E.; Schoell, M. Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation—Isotope evidence. Geochim. Cosmochim. Acta 1986, 50, 693–709. [Google Scholar] [CrossRef]
- Schoell, M. Multiple origins of methane in the earth. Chem. Geol. 1988, 71, 1–10. [Google Scholar] [CrossRef]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Milkov, A.V.; Etiope, G. Revised genetic diagrams for natural gases based on a global dataset of >20,000 samples. Org. Geochem. 2018, 125, 109–120. [Google Scholar] [CrossRef]
- Hachikubo, A.; Kosaka, T.; Kida, M.; Krylov, A.; Sakagami, H.; Minami, H.; Takahashi, N.; Shoji, H. Isotopic fractionation of methane and ethane hydrates between gas and hydrate phases. Geophys. Res. Lett. 2007, 34, L21502. [Google Scholar] [CrossRef]
- Lapham, L.L.; Wilson, R.M.; Chanton, J.P. Pressurized laboratory experiments show no stable carbon isotope fractionation of methane during gas hydrate dissolution and dissociation. Rapid Commun. Mass Spectrom. 2012, 26, 32–36. [Google Scholar] [CrossRef]
- Luzi, M.; Schicks, J.M.; Erzinger, J. Carbon isotopic fractionation of synthetic methane and carbon dioxide hydrates. In Proceedings of the 7th International Conference on Gas Hydrates (ICGH2011), Edinburgh, Scotland, UK, 17–21 July 2011. [Google Scholar]
- Kimura, H.; Fuseya, G.; Takeya, S.; Hachikubo, A. Carbon isotope fractionation during the formation of CO2 hydrate and equilibrium pressures of 12CO2 and 13CO2 hydrates. Molecules 2021, 26, 4215. [Google Scholar] [CrossRef]
- Carvajal-Ortiz, H.; Pratt, L.M. Influences of salinity and temperature on the stable isotopic composition of methane and hydrogen sulfide trapped in pressure-vessel hydrates. Geochim. Cosmochim. Acta 2013, 118, 72–84. [Google Scholar] [CrossRef]
- Hachikubo, A.; Takizawa, K.; Takeya, S. Stable isotope fractionation of nitrogen between gas and hydrate phases. J. Chem. Eng. Data 2023, 68, 694–697. [Google Scholar] [CrossRef]
- Deaton, W.M.; Frost, E.M. Gas Hydrates and Their Relation to the Operation of Natural-Gas Pipe Lines; American Gas Association: Washington, DC, USA, 1946; Volume 8. [Google Scholar]
- Adisasmito, S.; Frank, R.J.; Sloan, E.D. Hydrates of carbon dioxide and methane mixtures. J. Chem. Eng. Data 1991, 36, 68–71. [Google Scholar] [CrossRef]
- Ozeki, T.; Kikuchi, Y.; Takeya, S.; Hachikubo, A. Phase equilibrium of isotopologue methane hydrates enclathrated CH3D and CD4. J. Chem. Eng. Data 2018, 63, 2266–2270. [Google Scholar] [CrossRef]
- Hachikubo, A.; Fuseya, G.; Kikuchi, Y.; Takeya, S. Phase equilibrium of methane hydrate encapsulated isotopologue 13CH4 and singly deuterated methane. J. Chem. Eng. Data 2021, 66, 4663–4669. [Google Scholar] [CrossRef]
- Pellenbarg, R.E.; Max, M.D.; Clifford, S.M. Methane and carbon dioxide hydrates on Mars: Potential origins, distribution, detection, and implications for future in situ resource utilization. J. Geophys. Res. 2003, 108, 8042. [Google Scholar] [CrossRef]
- Kamata, S.; Nimmo, F.; Sekine, Y.; Kuramoto, K.; Noguchi, N.; Kimura, J.; Tani, A. Pluto’s ocean is capped and insulated by gas hydrates. Nat. Geosci. 2019, 12, 407–410. [Google Scholar] [CrossRef]
- Loveday, J.S.; Nelmes, R.J.; Guthrie, M.; Belmonte, S.A.; Allan, D.R.; Klug, D.D.; Tse, J.S.; Handa, Y.P. Stable methane hydrate above 2 GPa and the source of Titan’s atmospheric methane. Nature 2001, 410, 661–663. [Google Scholar] [CrossRef]
- Prieto-Ballesteros, O.; Kargel, J.S.; Fernández-Sampedro, M.; Selsis, F.; Martínez, E.S.; Hogenboom, D.L. Evaluation of the possible presence of clathrate hydrates in Europa’s icy shell or seafloor. Icarus 2005, 177, 491–505. [Google Scholar] [CrossRef]
- Bouquet, A.; Mousis, O.; Waite, J.H.; Picaud, S. Possible evidence for a methane source in Enceladus’ ocean. Geophys. Res. Lett. 2015, 42, 1334–1339. [Google Scholar] [CrossRef]
- Pape, T.; Geprägs, P.; Hammerschmidt, S.; Wintersteller, P.; Wei, J.; Fleischmann, T.; Bohrmann, G.; Kopf, A.J. Hydrocarbon seepage and its sources at mud volcanoes of the Kumano forearc basin, Nankai Trough subduction zone. Geochem. Geophys. Geosyst. 2014, 15, 2180–2194. [Google Scholar] [CrossRef]
- Meister, P.; Liu, B.; Khalili, A.; Böttcher, M.E.; Jørgensen, B.B. Factors controlling the carbon isotope composition of dissolved inorganic carbon and methane in marine porewater: An evaluation by reaction-transport modelling. J. Mar. Syst. 2019, 200, 103227. [Google Scholar] [CrossRef]
- Pape, T.; Haeckel, M.; Riedel, M.; Kölling, M.; Schmidt, M.; Wallmann, K.; Bohrmann, G. Formation pathways of light hydrocarbons in deep sediments of the Danube deep-sea fan, western Black Sea. Mar. Pet. Geol. 2020, 122, 104627. [Google Scholar] [CrossRef]
- Snyder, G.T.; Sano, Y.; Takahata, N.; Matsumoto, R.; Kakizaki, Y.; Tomaru, H. Magmatic fluids play a role in the development of active gas chimneys and massive gas hydrates in the Japan Sea. Chem. Geol. 2020, 535, 119462. [Google Scholar] [CrossRef]
- Lalk, E.; Pape, T.; Gruen, D.S.; Kaul, N.; Karolewski, J.S.; Bohrmann, G.; Ono, S. Clumped methane isotopologue-based temperature estimates for sources of methane in marine gas hydrates and associated vent gases. Geochim. Cosmochim. Acta 2022, 327, 276–297. [Google Scholar] [CrossRef]
- Hachikubo, A.; Minami, H.; Sakagami, H.; Yamashita, S.; Krylov, A.; Kalmychkov, G.; Poort, J.; De Batist, M.; Manakov, A.; Khlystov, O. Characteristics and varieties of gases enclathrated in natural gas hydrates retrieved at Lake Baikal. Sci. Rep. 2023, 13, 4440. [Google Scholar] [CrossRef] [PubMed]
- Hachikubo, A.; Khlystov, O.; Manakov, A.; Kida, M.; Krylov, A.; Sakagami, H.; Minami, H.; Takahashi, N.; Shoji, H.; Kalmychkov, G.; et al. Model of formation of double structure gas hydrates in Lake Baikal based on isotopic data. Geophys. Res. Lett. 2009, 36, L18504. [Google Scholar] [CrossRef]
- Hachikubo, A.; Krylov, A.; Sakagami, H.; Minami, H.; Nunokawa, Y.; Shoji, H.; Matveeva, T.; Jin, Y.K.; Obzhirov, A. Isotopic composition of gas hydrates in subsurface sediments from offshore Sakhalin Island, Sea of Okhotsk. Geo-Mar. Lett. 2010, 30, 313–319. [Google Scholar] [CrossRef]
- Hachikubo, A.; Minami, H.; Yamashita, S.; Khabuev, A.; Krylov, A.; Kalmychkov, G.; Poort, J.; De Batist, M.; Chenskiy, A.; Manakov, A.; et al. Characteristics of hydrate-bound gas retrieved at the Kedr mud volcano (southern Lake Baikal). Sci. Rep. 2020, 10, 14747. [Google Scholar] [CrossRef]
- Hachikubo, A.; Fuseya, G.; Sugimori, E.; Takeya, S. Effect of pressure on the hydration number of argon hydrate. J. Chem. Eng. Data 2022, 67, 3757–3762. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
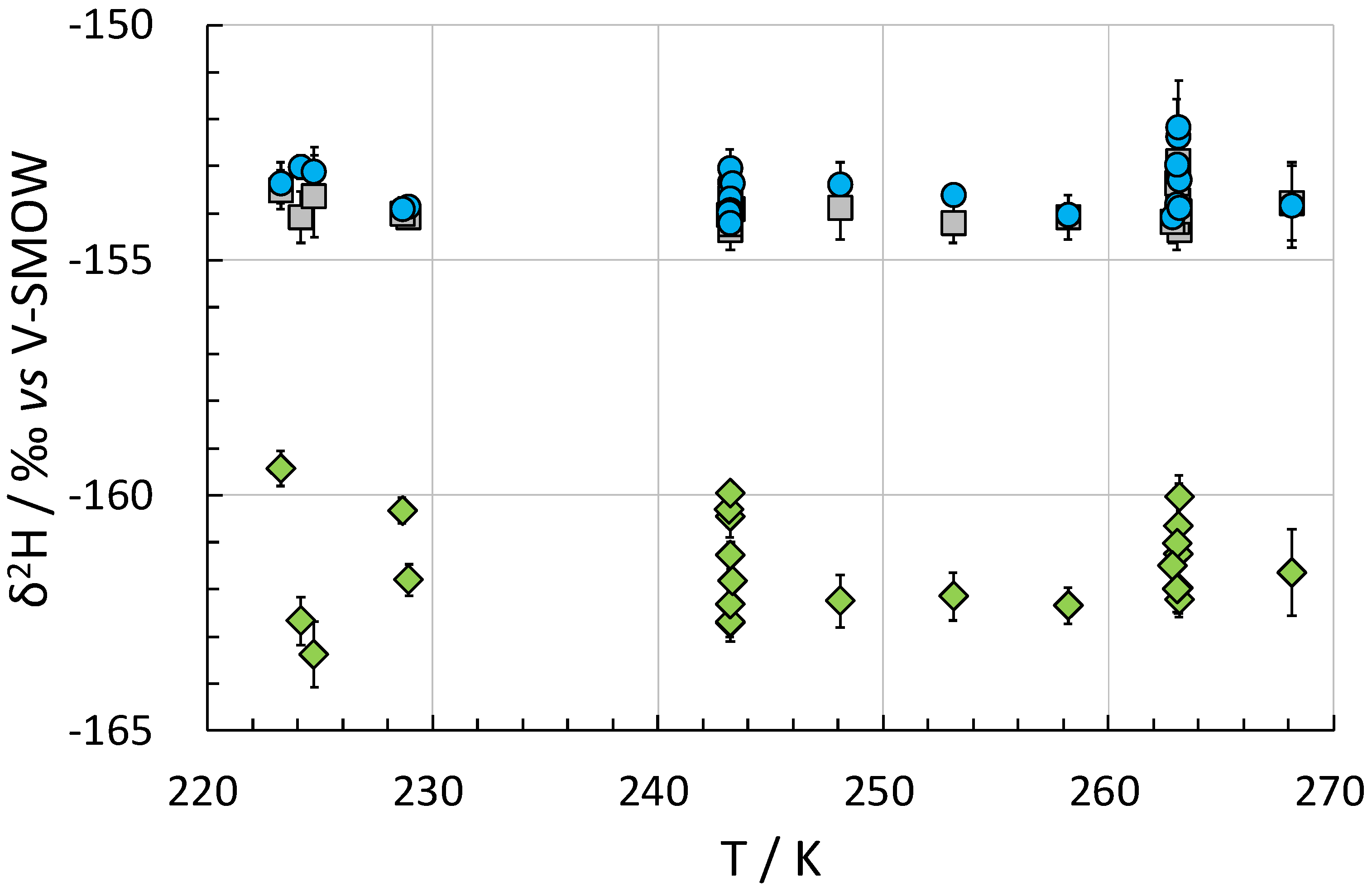
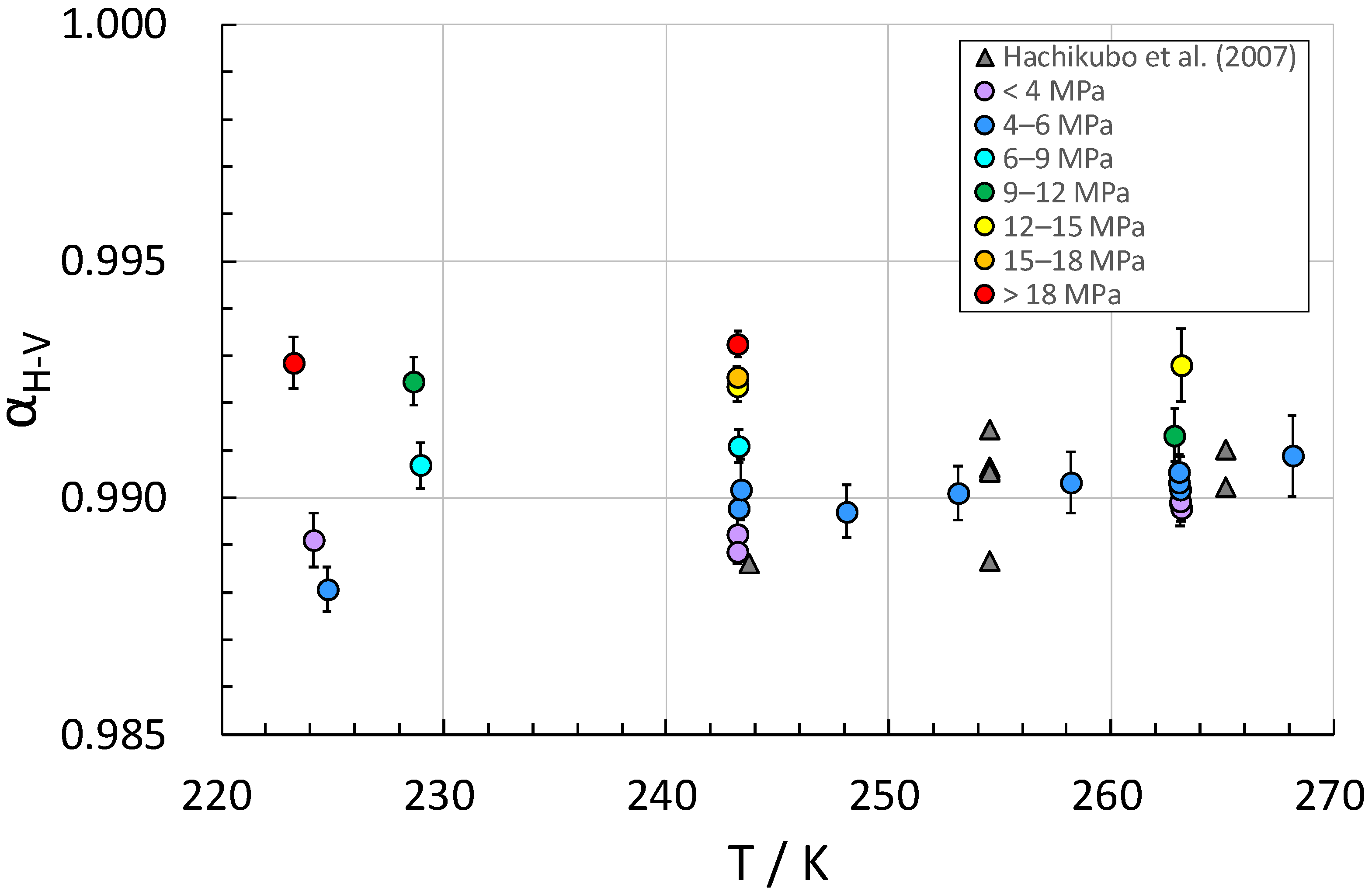
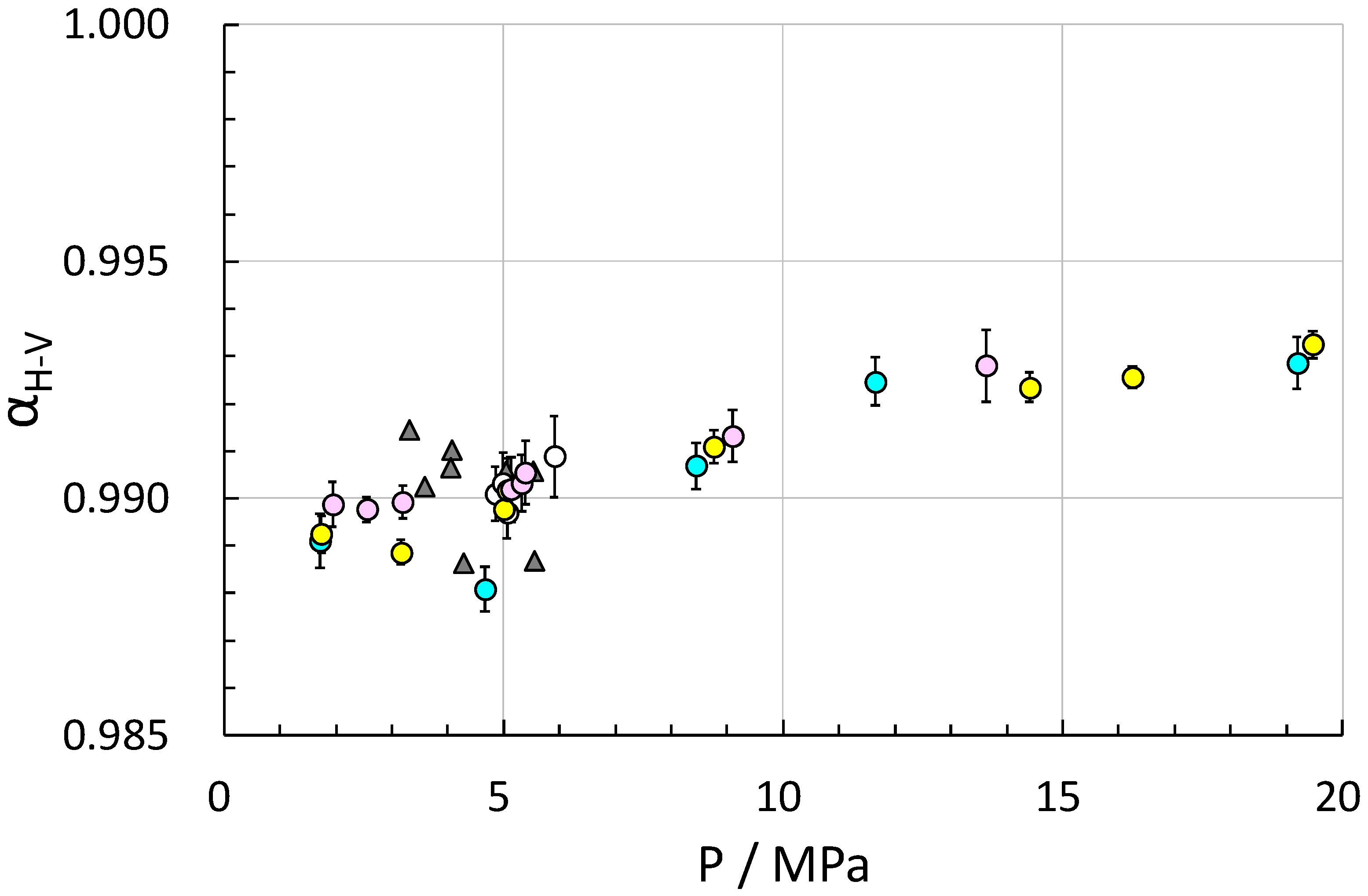

| T/K a | p/MPa a | Original Gas [‰] | Residual Gas [‰] | Hydrate-Bound Gas [‰] | f | αH-V |
|---|---|---|---|---|---|---|
| 224.2 | 1.7 | −154.1 ± 0.5 | −153.0 ± 0.2 | −162.7 ± 0.5 | 0.912 | 0.9891 ± 0.0006 |
| 224.8 | 4.7 | −153.6 ± 0.9 | −153.1 ± 0.5 | −163.4 ± 0.7 | 0.966 | 0.9881 ± 0.0005 |
| 229.0 | 8.4 | −154.1 ± 0.2 | −153.9 ± 0.2 | −161.8 ± 0.3 | 0.981 | 0.9907 ± 0.0005 |
| 228.7 | 11.7 | −154.0 ± 0.2 | −153.9 ± 0.2 | −160.3 ± 0.3 | 0.986 | 0.9925 ± 0.0005 |
| 223.3 | 19.2 | −153.5 ± 0.4 | −153.4 ± 0.4 | −159.4 ± 0.4 | 0.991 | 0.9929 ± 0.0005 |
| 243.2 | 1.7 | −154.4 ± 0.4 | −153.3 ± 0.3 | −162.7 ± 0.4 | 0.941 | 0.9892 ± 0.0004 |
| 243.2 | 3.2 | −153.8 ± 0.2 | −153.0 ± 0.4 | −162.7 ± 0.3 | 0.955 | 0.9889 ± 0.0003 |
| 243.2 | 5.0 | −153.7 ± 0.4 | −153.5 ± 0.3 | −162.3 ± 0.3 | 0.963 | 0.9898 ± 0.0001 |
| 243.3 | 5.1 | −153.9 ± 0.5 | −153.4 ± 0.4 | −161.8 ± 0.4 | 0.967 | 0.9902 ± 0.0007 |
| 243.2 | 8.8 | −153.7 ± 0.2 | −153.7 ± 0.3 | −161.3 ± 0.3 | 0.988 | 0.9911 ± 0.0003 |
| 243.2 | 14.4 | −154.1 ± 0.2 | −153.9 ± 0.4 | −160.4 ± 0.5 | 0.992 | 0.9923 ± 0.0003 |
| 243.2 | 16.2 | −154.0 ± 0.2 | −154.0 ± 0.3 | −160.3 ± 0.4 | 0.990 | 0.9926 ± 0.0002 |
| 243.2 | 19.5 | −154.2 ± 0.3 | −154.2 ± 0.4 | −160.0 ± 0.2 | 0.990 | 0.9932 ± 0.0003 |
| 248.1 | 5.1 | −153.9 ± 0.7 | −153.4 ± 0.5 | −162.2 ± 0.6 | 0.966 | 0.9897 ± 0.0006 |
| 253.1 | 4.9 | −154.2 ± 0.4 | −153.6 ± 0.1 | −162.2 ± 0.5 | 0.962 | 0.9901 ± 0.0006 |
| 258.2 | 5.0 | −154.1 ± 0.5 | −154.0 ± 0.3 | −162.3 ± 0.4 | 0.968 | 0.9903 ± 0.0006 |
| 263.1 | 1.9 | −153.8 ± 0.6 | −153.1 ± 0.2 | −162.0 ± 0.6 | 0.942 | 0.9899 ± 0.0005 |
| 263.1 | 2.6 | −154.4 ± 0.2 | −153.3 ± 0.3 | −162.2 ± 0.4 | 0.944 | 0.9898 ± 0.0003 |
| 263.1 | 3.2 | −153.7 ± 0.6 | −152.4 ± 0.4 | −161.2 ± 0.2 | 0.925 | 0.9899 ± 0.0003 |
| 263.1 | 5.1 | −152.9 ± 0.8 | −152.2 ± 1.0 | −160.7 ± 0.9 | 0.962 | 0.9902 ± 0.0007 |
| 263.0 | 5.3 | −154.2 ± 0.6 | −153.8 ± 0.6 | −162.0 ± 0.5 | 0.998 b | 0.9903 ± 0.0006 |
| 263.0 | 5.4 | −153.4 ± 0.9 | −153.0 ± 1.4 | −161.0 ± 1.2 | 0.989 c | 0.9905 ± 0.0007 |
| 262.9 | 9.1 | −154.2 ± 0.3 | −154.1 ± 0.6 | −161.5 ± 0.2 | 0.982 | 0.9913 ± 0.0006 |
| 263.1 | 13.6 | −154.0 ± 0.3 | −153.9 ± 0.3 | −160.0 ± 0.5 | 0.988 | 0.9928 ± 0.0008 |
| 268.2 | 5.9 | −153.8 ± 0.8 | −153.8 ± 0.9 | −161.6 ± 0.9 | 0.973 | 0.9909 ± 0.0009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hachikubo, A.; Nezu, T.; Takizawa, K.; Takeya, S. Effect of Pressure on Hydrogen Isotope Fractionation in Methane during Methane Hydrate Formation at Temperatures Below the Freezing Point of Water. Methane 2023, 2, 129-136. https://doi.org/10.3390/methane2020010
Hachikubo A, Nezu T, Takizawa K, Takeya S. Effect of Pressure on Hydrogen Isotope Fractionation in Methane during Methane Hydrate Formation at Temperatures Below the Freezing Point of Water. Methane. 2023; 2(2):129-136. https://doi.org/10.3390/methane2020010
Chicago/Turabian StyleHachikubo, Akihiro, Taichi Nezu, Kaede Takizawa, and Satoshi Takeya. 2023. "Effect of Pressure on Hydrogen Isotope Fractionation in Methane during Methane Hydrate Formation at Temperatures Below the Freezing Point of Water" Methane 2, no. 2: 129-136. https://doi.org/10.3390/methane2020010
APA StyleHachikubo, A., Nezu, T., Takizawa, K., & Takeya, S. (2023). Effect of Pressure on Hydrogen Isotope Fractionation in Methane during Methane Hydrate Formation at Temperatures Below the Freezing Point of Water. Methane, 2(2), 129-136. https://doi.org/10.3390/methane2020010






