Alkaline Pretreatment and Pre-Hydrolysis Using Acidic Biowastes to Increase Methane Production from Sugarcane Bagasse
Abstract
:1. Introduction
2. Material and Methods
2.1. Feedstock and Inoculum
2.2. Pretreatment Procedure
2.3. Biochemical Methane Potential (BMP) Assay
2.4. Analytical Methods
2.5. Data Analysis
2.6. Kinetic Study
3. Results and Discussion
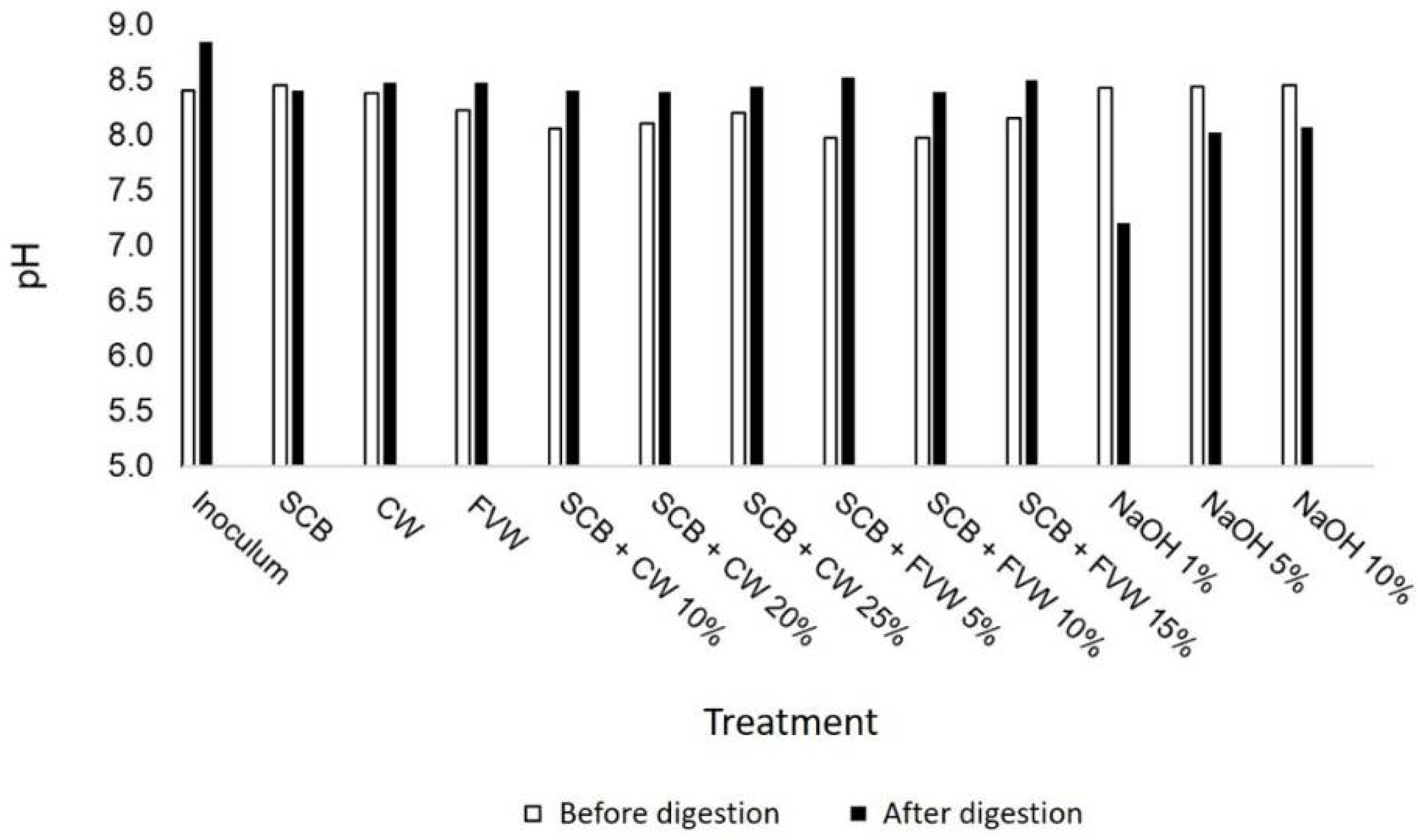
3.1. Characterization of Substrates and Inoculum
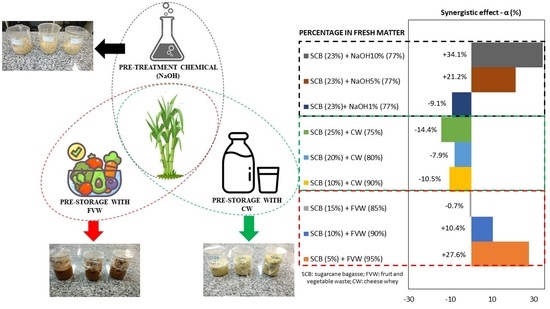
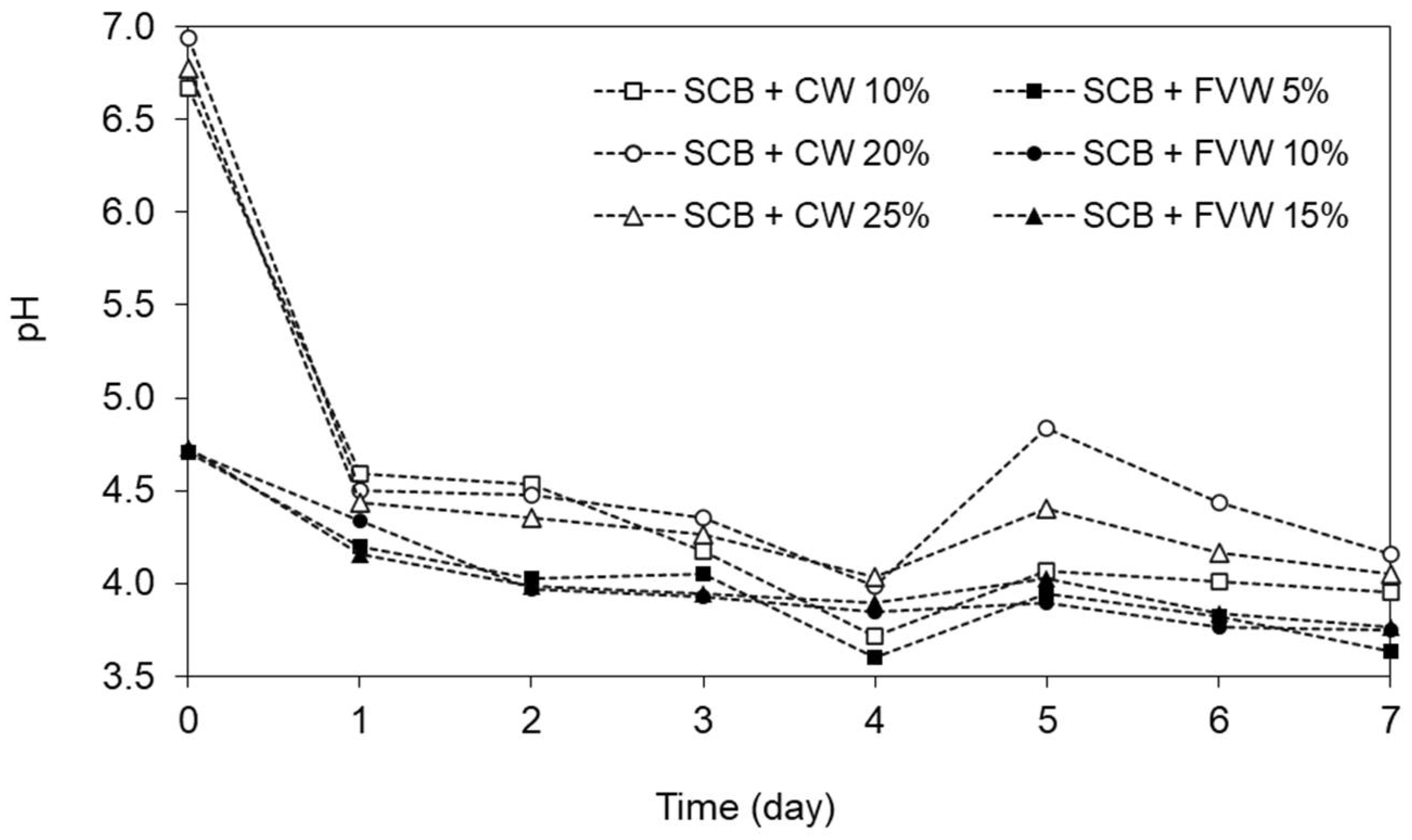
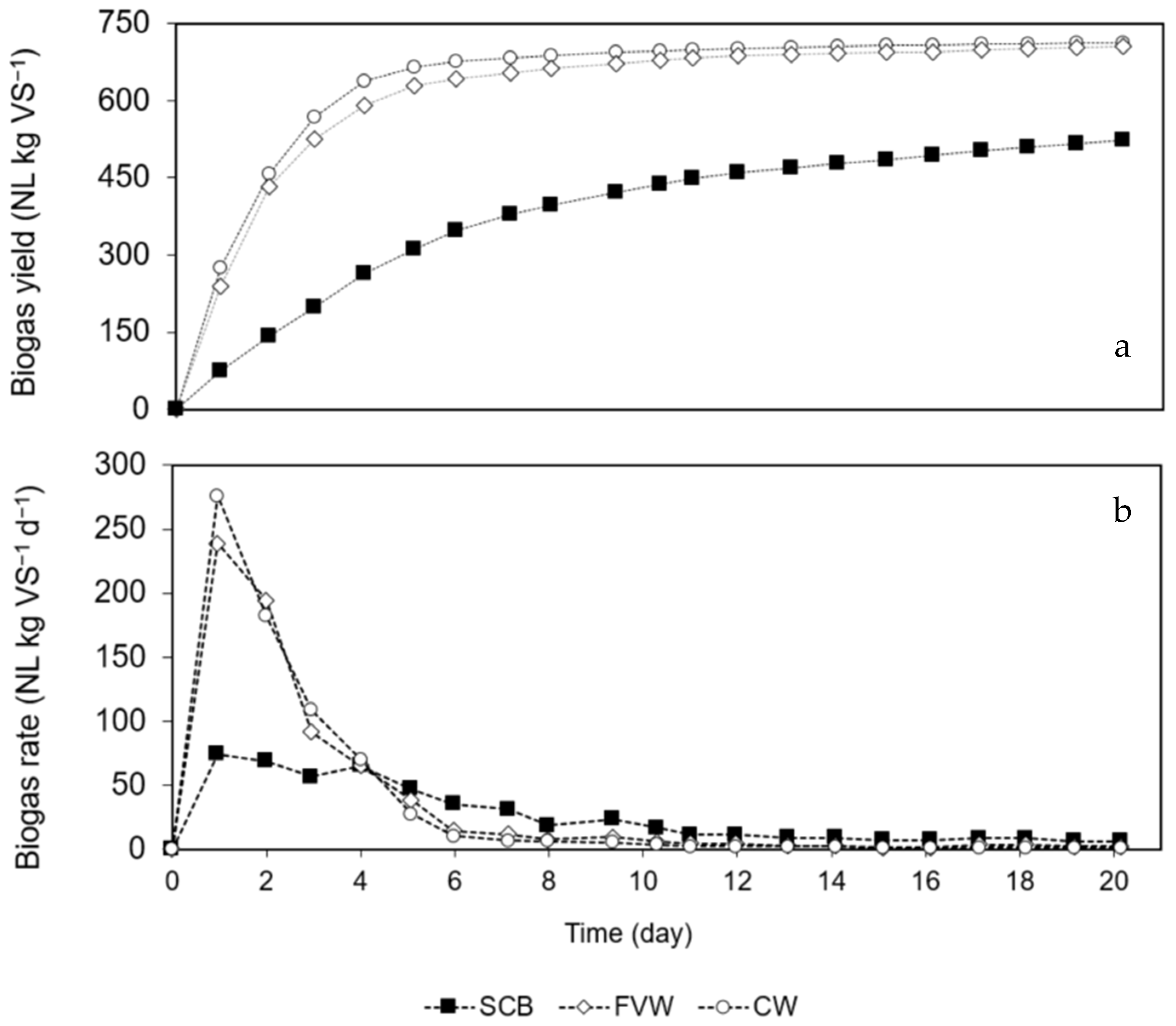
3.2. Effect of Pre-Hydrolysis between SCB + FVW and SCB + CW on Methane Potential (Experiment I)
3.3. Effect of Alkaline Pretreatment on Methane Potential of SCB (Experiment II)
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- REN21. Renewables 2020—Global Status Report; 2020 Update; REN21: Paris, France, 2020; ISBN 9783948393007. [Google Scholar]
- Anu, K.A.; Rapoport, A.; Kunze, G.; Kumar, S.; Singh, D.; Singh, B. Multifarious pretreatment strategies for the lignocellulosic substrates for the generation of renewable and sustainable biofuels: A review. Renew. Energy 2020, 160, 1228–1252. [Google Scholar] [CrossRef]
- Rivera, E.C.; Yamakawa, C.K.; Saad, M.B.W.; Atala, D.I.P.; Ambrosio, W.B.; Bonomi, A.; Junior, J.N.; Rossell, C.E.V. Effect of temperature on sugarcane ethanol fermentation: Kinetic modeling and validation under very-high-gravity fermentation conditions. Biochem. Eng. J. 2017, 119, 42–51. [Google Scholar] [CrossRef]
- Nosratpour, M.J.; Karimi, K.; Sadeghi, M. Improvement of ethanol and biogas production from sugarcane bagasse using sodium alkaline pretreatments. J. Environ. Manag. 2018, 226, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Daniyanto, S.; Deendarlianto, B.A. Torrefaction of Indonesian sugar-cane bagasse to improve bio-syngas quality for gasification process. Energy Procedia 2015, 68, 157–166. [Google Scholar] [CrossRef]
- Adarme, O.F.H.; Baêta, B.E.L.; Lima, D.R.S.; Gurgel, L.V.A.; Aquino, S.F. Methane and hydrogen production from anaerobic digestion of soluble fraction obtained by sugarcane bagasse ozonation. Ind. Crop. Prod. 2017, 109, 288–299. [Google Scholar] [CrossRef]
- Mari, A.G.; Secco, D.; Kunz, A.; Edwiges, T.; Mari Junior, A.; Frigo, E.P.; Alves, H.J. Performance of up-flow anaerobic digester in solids removal and biogas production. J. Food Agric. Environ. 2014, 12, 1135–1139. [Google Scholar]
- Martínez, E.J.; Rosas, J.G.; Sotres, A.; Moran, A.; Cara, J.; Sánchez, M.E.; Gómez, X. Codigestion of sludge and citrus peel wastes: Evaluating the effect of biochar addition on microbial communities. Biochem. Eng. J. 2018, 137, 314–325. [Google Scholar] [CrossRef]
- Wu, D.; Li, L.; Zhao, X.; Peng, Y.; Yang, P.; Peng, X. Anaerobic digestion: A review on process monitoring. Renew. Sustain. Energy Rev. 2019, 103, 1–12. [Google Scholar] [CrossRef]
- Baêta, B.E.L.; Lima, D.R.S.; Adarme, O.F.H.; Gurgel, L.V.A.; Aquino, S.F. Optimization of sugarcane bagasse autohydrolysis for methane production from hemicellulose hydrolyzates in a biorefinery concept. Bioresour. Technol. 2016, 200, 137–146. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. A review on enhanced biogas production from anaerobic digestion of lignocellulosic biomass by different enhancement techniques. Process Biochem. 2019, 84, 81–90. [Google Scholar] [CrossRef]
- Guan, R.; Xiujin, L.; Wachemo, A.C.; Hairong, Y.; Yanping, L.; Dexun, Z.; Xiaoyu, Z.; Junyu, G. Enhancing anaerobic digestion performance and degradation of lignocellulosic components of rice straw by combined biological and chemical pretreatment. Sci. Total Environ. 2018, 637, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Liu, R.; Li, K.; Ma, R. A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China. Renew. Sustain. Energy Rev. 2019, 107, 51–58. [Google Scholar] [CrossRef]
- Liu, X.; Zicari, S.M.; Liu, G.; Li, Y.; Zhang, R. Improving the bioenergy production from wheat straw with alkaline pretreatment. Biosyst. Eng. 2015, 140, 59–66. [Google Scholar] [CrossRef]
- Edwiges, T.; Bastos, J.A.; Alino, J.H.L.; D’Avila, L.; Frare, L.M.; Somer, J.G. Comparison of various pretreatment techniques to enhance biodegradability of lignocellulosic biomass for methane production. J. Environ. Chem. Eng. 2019, 7, 103–195. [Google Scholar] [CrossRef]
- Vervaeren, H.; Hostyn, K.; Ghekiere, G.; Willems, B. Biological ensilage additives as pretreatment for maize to increase the biogas production. Renew. Energy 2010, 35, 2089–2093. [Google Scholar] [CrossRef]
- Bochmann, G.; Montgomery, L.F.R. Storage and pre-treatment of substrates for biogas production. In The Biogas Handbook; Elsevier: Amsterdam, The Netherlands, 2013; pp. 85–103. [Google Scholar]
- Chen, Y.; Sharma-Shivappa, R.R.; Chen, C. Ensiling agricultural residues for bioethanol production. Appl. Biochem. Biotechnol. 2007, 143, 80–92. [Google Scholar] [CrossRef]
- FAO. FAO Statistical Yearbook 2014: Latin America and the Caribbean Food and Agriculture; FAO: Santiago, Chile, 2014. [Google Scholar]
- Edwiges, T.; Frare, L.; Mayer, B.; Lins, L.; Triolo, J.L.; Flotats, X.; Costa, M.S.S.M. Influence of chemical composition on biochemical methane potential of fruit and vegetable waste. Waste Manag. 2018, 71, 618–625. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Brazilian Agricultural Research Company. Yearbook of Milk; EMBRAPA: Brasilia, Brazil, 2018; p. 103. [Google Scholar]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffiere, P.; Carballa, M.; de Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Steinmetz, R.L.R.; Mezzari, M.P.; Da Silva, M.L.B.; Kunz, A.; Do Amaral, A.C.; Tápparo, D.C.; Soares, H.M. Enrichment and acclimation of an anaerobic mesophilic microorganism’s inoculum for standardization of BMP assays. Bioresour. Technol. 2016, 219, 21–28. [Google Scholar] [CrossRef] [PubMed]
- VDI Verein Deutscher Ingenieure. Fermentation of Organic Materials: Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; Verein Deutscher Ingenieure: Berlin, Germany, 2016; p. 132. [Google Scholar]
- Bastos, J.A.; Remor, V.; Alino, J.H.L.; Frare, L.M.; Lofhagen, J.C.P.; Edwiges, T. Hydrolysate recycling improves economic feasibility of alkaline pretreatment for bioenergy production. J. Environ. Chem. Eng. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Remor, V.; Bastos, J.A.; Alino, J.H.L.; Frare, L.M.; Kaparaju, P.; Edwiges, T. Optimization of Chemical Solution Concentration and Exposure time in the Alkaline Pretreatment Applied to Sugarcane Bagasse for Methane Production. Environ. Technol. 2022, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Ceotto, E.; Galetti, S. Potassium hydroxide pre-treatment enhances methane yield from giant reed (Arundo donax L. ). Energies 2021, 14, 630. [Google Scholar] [CrossRef]
- Apha. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Salo-Vaananen, P.; Koivistoinen, E. Determination of protein in foods: Comparison of net protein and crude protein (Nx6.25) values. Food Chem. 1996, 57, 27–31. [Google Scholar] [CrossRef]
- Van Soest, J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Nielfa, A.; Cano, R.; Fdz-Polanco, M. Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol. Rep. 2015, 5, 14–21. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef]
- Leite, A.F.; Janke, L.; Harms, H.; Zang, J.W.; Fonseca-Zang, W.A.; Stinner, W.; Nikolausz, M. Assessment of the Variations in Characteristics and Methane Potential of Major Waste Products from the Brazilian Bioethanol Industry along an Operating Season. Energy Fuels 2015, 29, 4022–4029. [Google Scholar] [CrossRef]
- Risner, D.; Tomasino, E.; Hughes, P.; Meunier-Goddik, L. Volatile aroma composition of distillates produced from fermented sweet and acid whey. J. Dairy Sci. 2019, 102, 202–210. [Google Scholar] [CrossRef]
- Janke, L.; Leite, A.; Nikolausz, M.; Schmidt, T.; Liebetrau, J.; Nelles, M.; Stinner, W. Biogas Production from Sugarcane Waste: Assessment on Kinetic Challenges for Process Designing. Int. J. Mol. Sci. 2015, 16, 20685–20703. [Google Scholar] [CrossRef] [PubMed]
- Lopez, V.M.; De La Cruz, F.B.; Barlaz, M.A. Chemical composition and methane potential of commercial food wastes. Waste Manag. 2016, 56, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Escalante, H.; Castro, L.; Amaya, M.P.; Jaimes, L.; Jaimes-Estévez, J. Anaerobic digestion of cheese whey: Energetic and nutritional potential for the dairy sector in developing countries. Waste Manag. 2018, 71, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.K.; Kumar, M.; Ghosh, P.; Kumar, S.S.; Singh, L.; Vijay, V.K.; Kumar, V. Anaerobic digestion of sugarcane bagasse for biogas production and digestate valorization. Chemosphere 2022, 295, 133893. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.D.; Fuess, L.T.; Mañunga, T.; Gomes, C.F.L.; Zaiat, M. Bacteriocins of lactic acid bacteria as a hindering factor for biohydrogen production from cassava flour wastewater in a continuous multiple tube reactor. Int. J. Hydrog. Energy 2016, 41, 8120–8131. [Google Scholar] [CrossRef]
- Kaur, M.; Verma, Y.P.; Chauhan, S. Effect of chemical pretreatment of sugarcane bagasse on biogas production. Mater. Today Proc. 2020, 21, 1937–1942. [Google Scholar] [CrossRef]
- El Achkar, J.H.; Lendormi, T.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.; Hobaika, Z. Influence of pretreatment conditions on lignocellulosic fractions and methane production from grape pomace. Bioresour. Technol. 2018, 247, 881–889. [Google Scholar] [CrossRef]
| Treatment | Pretreament Strategy | Mass of the Catalytic Residue or NaOH | Proportions (Fresh Weigh Basis) | Proportions (VS Basis) | TS of the Mixtures (%) |
|---|---|---|---|---|---|
| 1 | SCB pre-stored with FVW | 1 g SCB/19.0 g FVW | 5:95 (SCB:FVW) | 17:83 (SCB:FVW) | 11.9 |
| 2 | 1 g SCB/9.0 g FVW | 10:90 (SCB:FVW) | 30:70 (SCB:FVW) | 13.3 | |
| 3 | 1 g SCB/5.7 g FVW | 15:85 (SCB:FVW) | 40:60 (SCB:FVW) | 14.7 | |
| 4 | SCB pre-stored with CW | 1 g SCB/9.0 g CW | 10:90 (SCB:CW) | 40:60 (SCB:CW) | 10.0 |
| 5 | 1 g SCB/4.0 g CW | 20:80 (SCB:CW) | 60:40 (SCB:CW) | 13.1 | |
| 6 | 1 g SCB/3.0 g CW | 25:75 (SCB:CW) | 66:34 (SCB:CW) | 14.7 | |
| 7 | SCB pretreated with NaOH | 1 g SCB/0.04 g NaOH | NaOH 1% | N.A | 8.5 |
| 8 | 1 g SCB/0.18 g NaOH | NaOH 5% | N.A | 12.3 | |
| 9 | 1 g SCB/0.35 g NaOH | NaOH 10% | N.A | 16.3 |
| Substrate | TS | VS | VS/TS | pH | Crude Protein | Crude Lipid | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (% VS) | (% VS) | (% VS) | (% VS) | (% VS) | |||
| SCB | 38.3 ± 0.1 | 37.4 ± 0.1 | 97.7 | N.D. | 2.3 ± 0.2 | 3.2 ± 0.4 | 30.5 ± 0.3 | 25.6 ± 0.8 | 5.2 ± 0.1 |
| FVW | 10.5 ± 0.2 | 9.8 ± 0.1 | 93.8 | 4.7 ± 0.1 | 9.6 ± 0.1 | 5.0 ± 0.2 | N.D. | N.D. | N.D. |
| CW | 6.8 ± 0.1 | 6.3 ± 0.1 | 92.5 | 6.7 ± 0.1 | 12.1 ± 0.4 | 9.8 ± 0.2 | N.D. | N.D. | N.D. |
| Treatment | Biogas Yield (NL kg VS−1) | BMPE (NL kg VS−1) | BMPC (NL kg VS−1) | T90 (d) | Khyd (d−1) | Tdelay (d) | R2 | α (%) |
|---|---|---|---|---|---|---|---|---|
| SCB | 523 ± 4 | 330 ± 6 | N.A. | 13 | 0.18 | 0.1 | 0.999 | N.A. |
| FVW | 705 ± 10 | 423 ± 6 | N.A. | 6 | 0.48 | 0.1 | 0.998 | N.A. |
| CW | 713 ± 9 | 449 ± 6 | N.A. | 4 | 0.56 | 0.1 | 0.999 | N.A. |
| (1) SCB 5% + FVW 95% | 816 ± 10 | 520 ± 7 | 407 | 7 | 0.42 | 0.4 | 0.999 | 27.6 |
| (2) SCB 10% + FVW 90% | 771 ± 10 | 437 ± 6 | 395 | 8 | 0.37 | 0.3 | 0.996 | 10.4 |
| (3) SCB 15% + FVW 85% | 638 ± 7 | 383 ± 4 | 386 | 9 | 0.36 | 0.4 | 0.997 | −0.7 |
| (4) SCB 10% + CW 90% | 583 ± 10 | 345 ± 6 | 381 | 10 | 0.28 | 0.1 | 0.997 | −10.5 |
| (5) SCB 20% + CW 80% | 587 ± 15 | 338 ± 9 | 365 | 11 | 0.24 | 0.1 | 0.998 | −7.9 |
| (6) SCB 25% + CW 75% | 536 ± 18 | 309 ± 11 | 353 | 11 | 0.36 | 0.1 | 0.998 | −14.4 |
| SCB1 | 449 ± 11 | 246 ± 6 | N.A. | 12 | 0.20 | 0.1 | 0.999 | N.A. |
| (7) SCB + NaOH 1% | 405 ± 13 | 223 ± 7 | N.A. | 12 | 0.13 | 1.9 | 0.997 | −9.1 |
| (8) SCB + NaOH 5% | 567 ± 28 | 298 ± 15 | N.A. | 12 | 0.28 | 1.3 | 0.998 | 21.2 |
| (9) SCB + NaOH 10% | 632 ± 13 | 330 ± 7 | N.A. | 12 | 0.27 | 1.5 | 0.999 | 34.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alino, J.H.L.; Bastos, J.A.; Remor, P.V.; Frare, L.M.; Orssatto, F.; Damaceno, F.M.; Edwiges, T. Alkaline Pretreatment and Pre-Hydrolysis Using Acidic Biowastes to Increase Methane Production from Sugarcane Bagasse. Methane 2022, 1, 189-200. https://doi.org/10.3390/methane1030015
Alino JHL, Bastos JA, Remor PV, Frare LM, Orssatto F, Damaceno FM, Edwiges T. Alkaline Pretreatment and Pre-Hydrolysis Using Acidic Biowastes to Increase Methane Production from Sugarcane Bagasse. Methane. 2022; 1(3):189-200. https://doi.org/10.3390/methane1030015
Chicago/Turabian StyleAlino, João Henrique Lima, Jhenifer Aline Bastos, Paula Verônica Remor, Laercio Mantovani Frare, Fábio Orssatto, Felippe Martins Damaceno, and Thiago Edwiges. 2022. "Alkaline Pretreatment and Pre-Hydrolysis Using Acidic Biowastes to Increase Methane Production from Sugarcane Bagasse" Methane 1, no. 3: 189-200. https://doi.org/10.3390/methane1030015
APA StyleAlino, J. H. L., Bastos, J. A., Remor, P. V., Frare, L. M., Orssatto, F., Damaceno, F. M., & Edwiges, T. (2022). Alkaline Pretreatment and Pre-Hydrolysis Using Acidic Biowastes to Increase Methane Production from Sugarcane Bagasse. Methane, 1(3), 189-200. https://doi.org/10.3390/methane1030015