Mechanisms, Growth Rates, and Morphologies of Gas Hydrates of Carbon Dioxide, Methane, and Their Mixtures
Abstract
:1. Introduction
- It is not clear whether morphology is dependent on the guest molecule, the driving force, or other variables. Some authors report morphological differences between CO and CH hydrates and their mixtures, but for other groups, those differences are less evident or even negligible.
- Although there is a clear correlation between and morphology, CO and CH hydrates have not been formed at the exact same temperatures, subcoolings, and precursor phases, which makes it difficult to compare morphology and growth mechanisms between methane, carbon dioxide, and their mixtures.
- It has not been established whether the formation of these hydrates is a mass transfer or heat transfer limited process. Solubilities, heat of formation, and intrinsic rates of formation for CO and CH differ considerably between each other and may have a role on the hydrate growth velocities and morphologies.
2. Materials and Methods
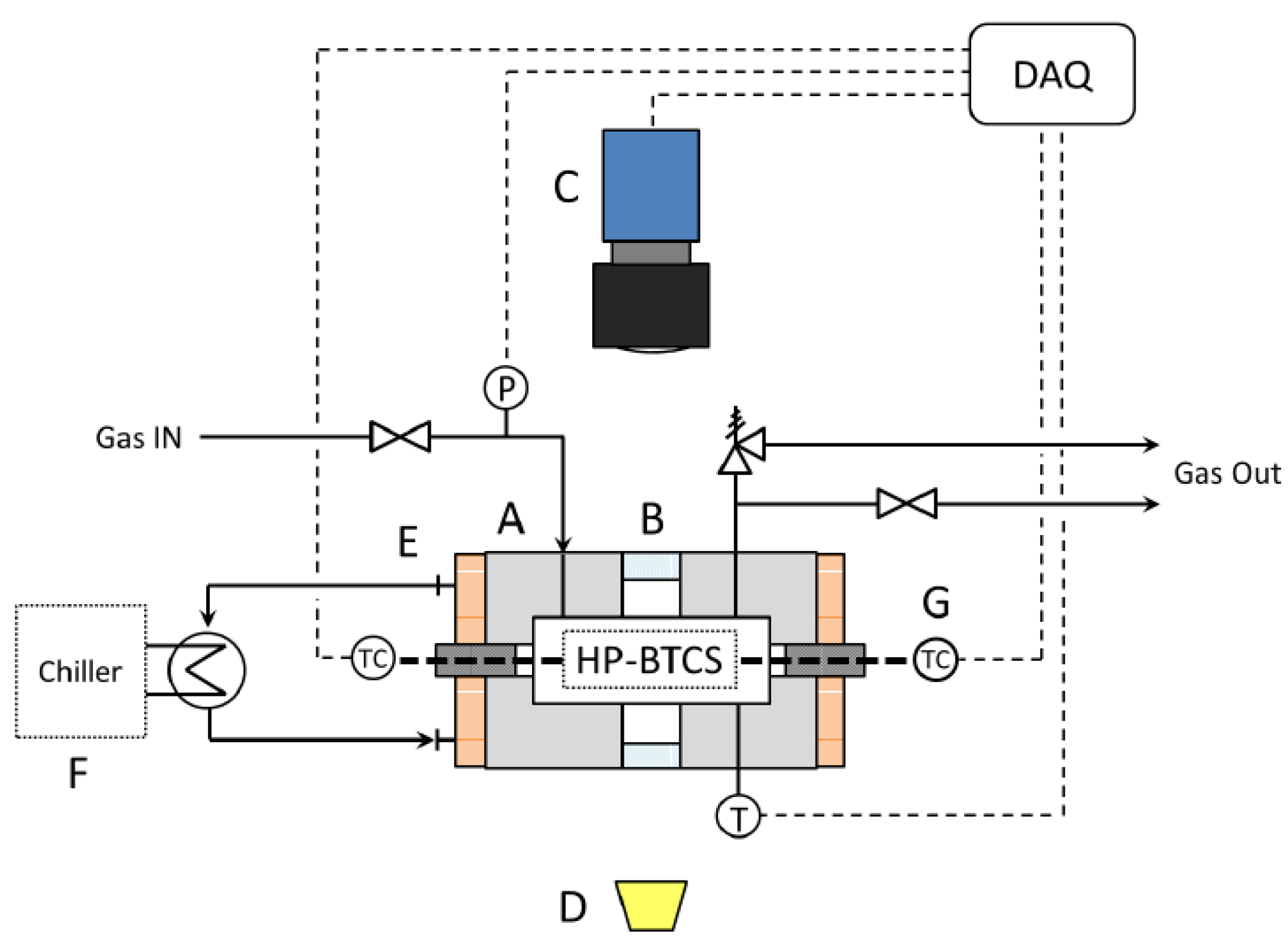
2.1. Apparatus
2.2. Methods
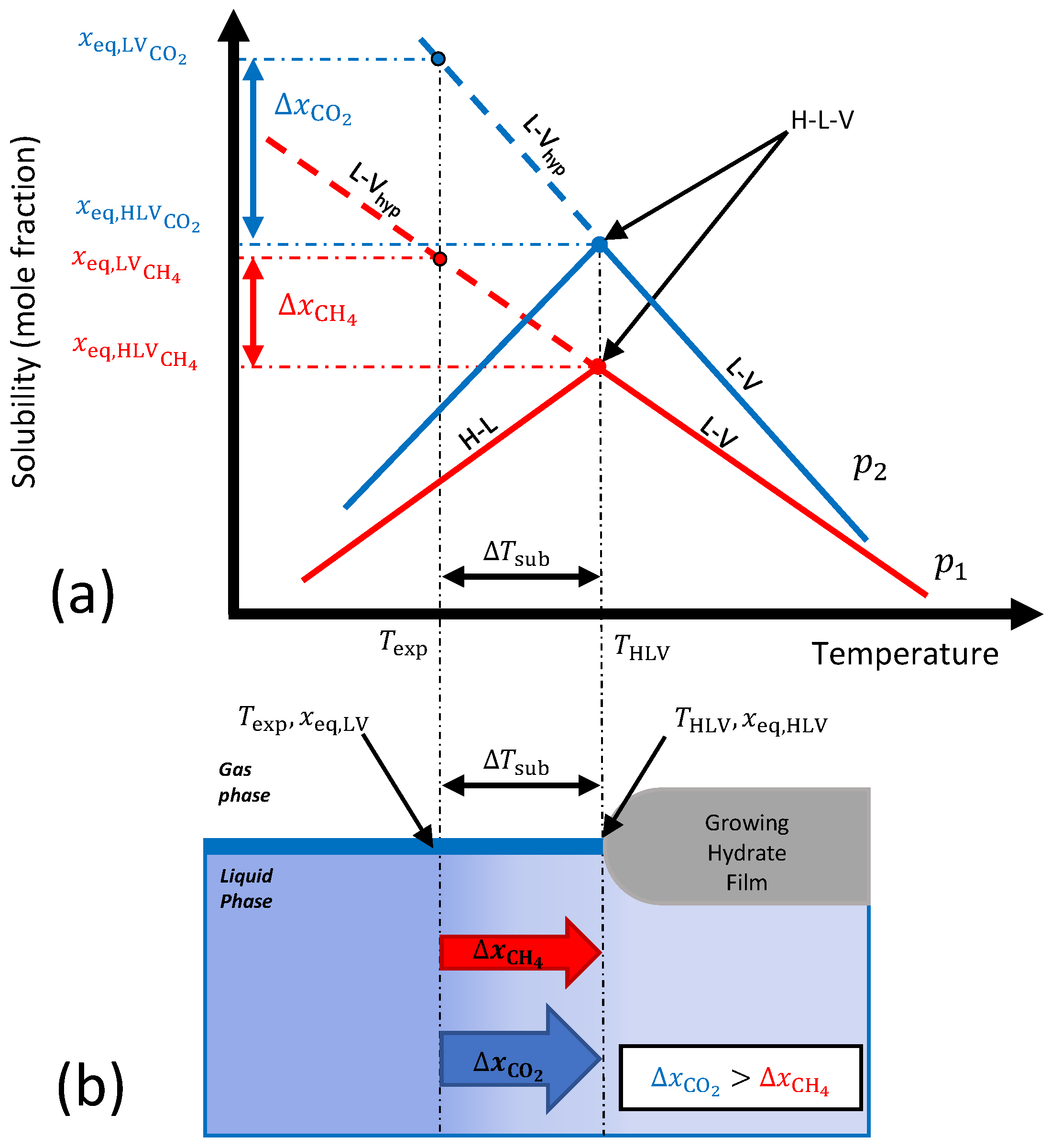
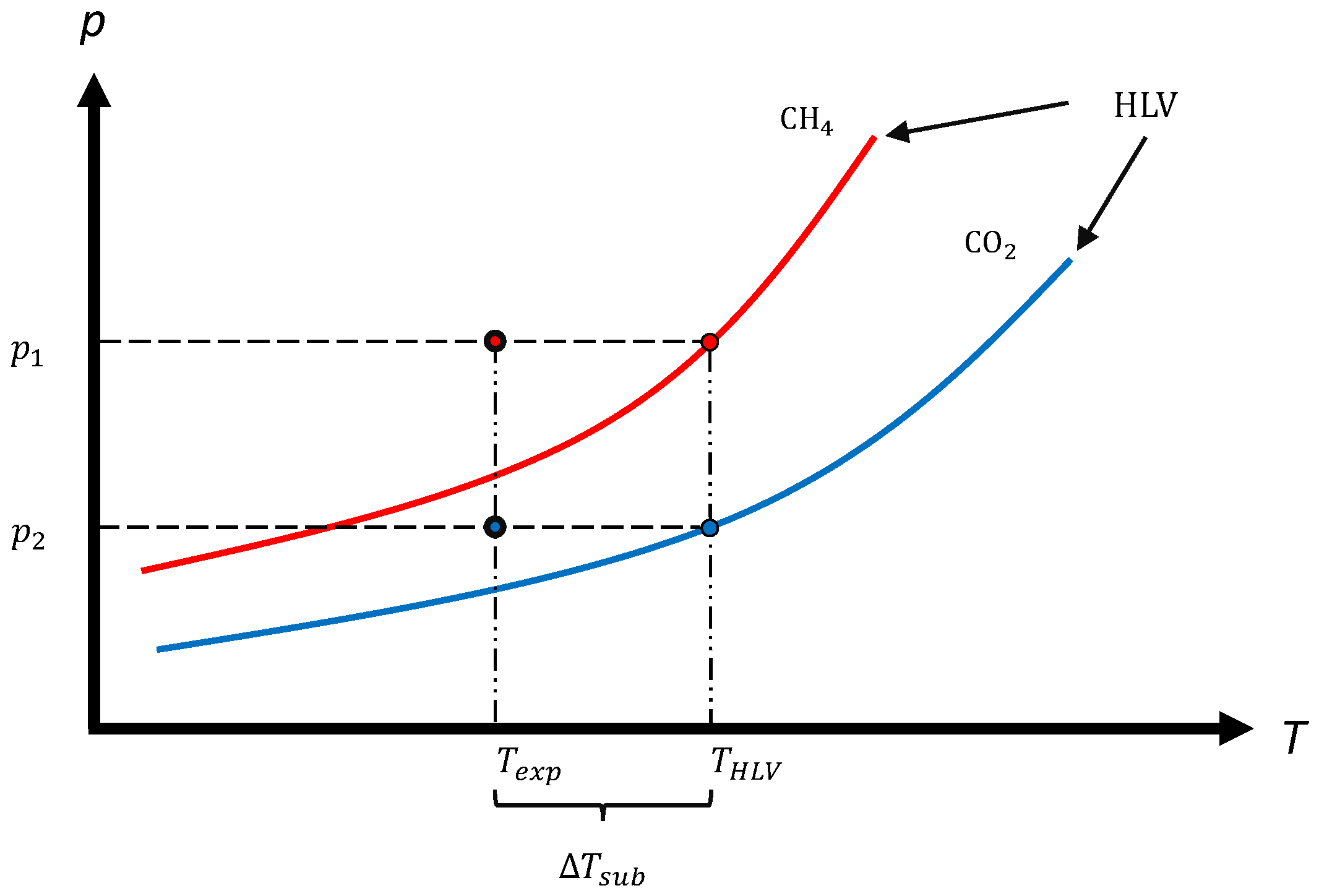
2.2.1. Mass Transfer-Based Driving Force
2.2.2. Extension for Mixtures
2.3. Experimental Conditions
3. Results and Discussion
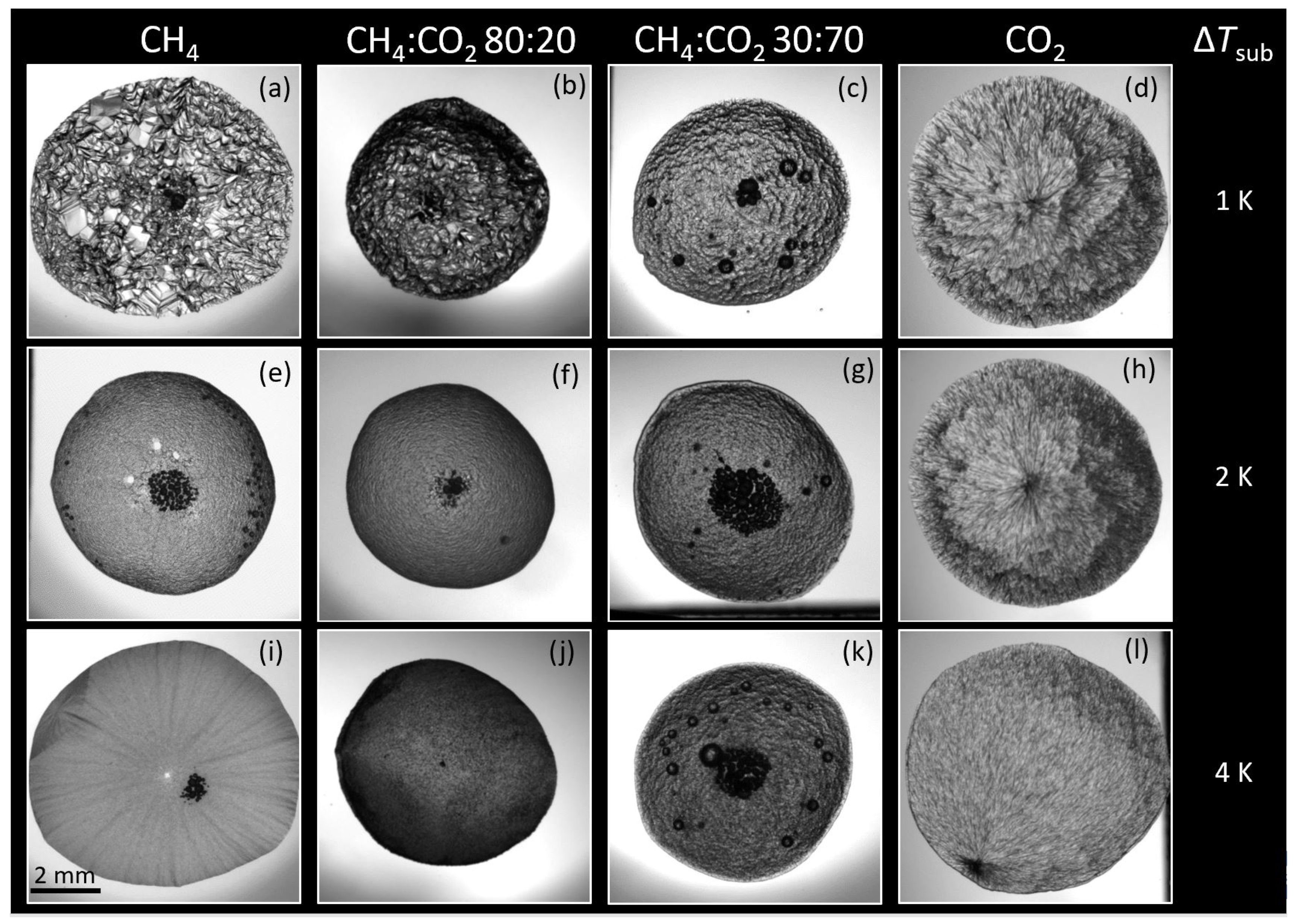
3.1. Hydrate Morphology
3.1.1. Subcooling
3.1.2. Mass Transfer-Based Driving Force
3.1.3. Gradient
3.1.4. Growth Velocity
3.2. Growth and Dissociation
3.2.1. Growth Mechanism
3.2.2. Partial Dissociation during Growth
3.2.3. Dissociation Mechanism
3.3. Growth Rates
Morphology and Growth Velocity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| difference | |
| stoichiometric coefficient | |
| Greek Letters | |
| f | fugacity |
| H | Henry’s law constant |
| v | hydrate film growth velocity |
| n | hydration number |
| x | mole fraction |
| P | pressure |
| g | temperature gradient |
| T | temperature |
| volumetric growth rate | |
| Subscripts and Abbreviations | |
| L | coldest side of the stage |
| j | dissociation step |
| experimental value | |
| gradient experiments | |
| h | halo |
| H | hottest side of the stage |
| hydrate front | |
| hydrate–Liquid water–vapor | |
| interface | |
| subcooling | |
| uniform experiments | |
References
- Englezos, P. Clathrate Hydrates. Ind. Eng. Chem. Res. 1993, 32, 1251–1274. [Google Scholar] [CrossRef]
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; Chemical Industries; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Englezos, P.; Kalogerakis, N.; Dholabhai, P.D.; Bishnoi, P.R. Kinetics of formation of methane and ethane gas hydrates. Chem. Eng. Sci. 1987, 42, 2647–2658. [Google Scholar] [CrossRef]
- Natarajan, V.; Bishnoi, P.R.; Kalogerakis, N. Induction phenomena in gas hydrate nucleation. Chem. Eng. Sci. 1994, 49, 2075–2087. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Maeda, N.; Wells, D.; Becker, N.C.; Hartley, P.G.; Wilson, P.W.; Haymet, A.D.J.; Kozielski, K.A. Development of a high pressure automated lag time apparatus for experimental studies and statistical analyses of nucleation and growth of gas hydrates. Rev. Sci. Instruments 2011, 82, 65109. [Google Scholar] [CrossRef]
- Metaxas, P.J.; Lim, V.W.; Booth, C.; Zhen, J.; Stanwix, P.L.; Johns, M.L.; Aman, Z.M.; Haandrikman, G.; Crosby, D.; May, E.F. Gas hydrate formation probability distributions: Induction times, rates of nucleation and growth. Fuel 2019, 252, 448–457. [Google Scholar] [CrossRef]
- Maeda, N. Nucleation of Gas Hydrates; Springer International Publishing: New York, NY, USA, 2020. [Google Scholar]
- Buchanan, P.; Soper, A.; Thompson, H.; Westacott, R.; Creek, J.; Hobson, G.; Koh, C. Search for memory effects in methane hydrate: Structure of water before hydrate formation and after hydrate decomposition. J. Chem. Phys. 2005, 123, 164507. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.; Soper, A.; Buchanan, P.; Aldiwan, N.; Creek, J.; Koh, C. Methane hydrate formation and decomposition: Structural studies via neutron diffraction and empirical potential structure refinement. J. Chem. Phys. 2006, 124, 164508. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Svartaas, T.M.; Chen, D. A review of gas hydrate nucleation theories and growth models. J. Nat. Gas Sci. Eng. 2019, 61, 169–196. [Google Scholar] [CrossRef]
- Esmail, S.; Beltran, J. Methane hydrate propagation on surfaces of varying wettability. J. Nat. Gas Sci. Eng. 2016, 35, 1535–1543. [Google Scholar] [CrossRef]
- Chatti, I.; Delahaye, A.; Fournaison, L.; Petitet, J.P. Benefits and drawbacks of clathrate hydrates: A review of their areas of interest. Energy Convers. Manag. 2005, 46, 1333–1343. [Google Scholar] [CrossRef]
- Dashti, H.; Yew, L.; Lou, X. Recent advances in gas hydrate-based CO2 capture. J. Nat. Gas Sci. Eng. 2015, 23, 195–207. [Google Scholar] [CrossRef]
- Uchida, T.; Ebinuma, T.; Kawabata, J.; Narita, H. Microscopic observations of formation processes of clathrate-hydrate films at an interface between water and carbon dioxide. J. Cryst. Growth 1999, 204, 348–356. [Google Scholar] [CrossRef]
- Circone, S.; Stern, L.; Kirby, S.; Durham, W.; Chakoumakos, B.; Rawn, C.; Rondinone, A.; Ishii, Y. CO2 Hydrate: Synthesis, Composition, Structure, Dissociation Behavior, and a Comparisonto Structure I CH4 Hydrate. J. Phys. Chem. B 2003, 107, 5529–5539. [Google Scholar] [CrossRef]
- Ohmura, R.; Shimada, W.; Uchida, T.; Mori, Y.; Takeya, S.; Nagao, J.; Minagawa, H.; Ebinuma, T.; Narita, H. Clathrate hydrate crystal growth in liquid water saturated with a hydrate-forming subtance: Variations in crystal morphology. Philos. Mag. 2004, 84, 1–16. [Google Scholar] [CrossRef]
- Servio, P.; Englezos, P. Morphology of Methane and Carbon Dioxide Hydrates Formed from Water Droplets. AIChE J. 2003, 49, 269–276. [Google Scholar] [CrossRef]
- Ohmura, R.; Matsuda, S.; Uchida, T.; Ebinuma, T.; Narita, H. Clathrate Hydrate Crystal Growth in Liquid Water Saturated with a Guest Substance: Observations in a Methane + Water System. Cryst. Growth Des. 2005, 5, 953–957. [Google Scholar] [CrossRef]
- Peng, B.; Dandekar, A.; Sun, C.; Luo, H.; Ma, Q.; Pang, W.; Chen, G. Hydrate Film Growth on the Surface of a Gas Bubble Suspended in Water. J. Phys. Chem. B 2007, 111, 12485–12493. [Google Scholar] [CrossRef]
- Daniel-David, D.; Guerton, F.; Dicharry, C.; Torre, J.; Broseta, D. Hydrate growth at the interface between water and pure or mixed CO2/CH4 gases: Influence of pressure, temperature, gas composition and water-soluble surfactants. Chem. Eng. Sci. 2015, 132, 118–127. [Google Scholar] [CrossRef]
- Ueno, H.; Akiba, H.; Akatsu, S.; Ohmura, R. Crystal growth of clathrate hydrates formed with methane + carbon dioxide mixed gas at the gas/liquid interface and in liquid water. New J. Chem. 2015, 39, 8254–8262. [Google Scholar] [CrossRef]
- Beltran, J.; Servio, P. Morphological Investigations of Methane-Hydrate Films Formed on a Glass Surface. J. Cryst. Growth Des. 2010, 10, 4339–4347. [Google Scholar] [CrossRef]
- Decarie, G.; Beltran, J. Effect of hydrate guest on clathrate morphology. In Proceedings of the 7th International Conference on Gas Hydrates, Edinburgh, UK, 17–21 July 2011. [Google Scholar]
- Koga, T.; Wong, J.; Endoh, M.; Mahajan, D.; Gutt, C.; Satija, S. Hydrate formation at the methane/water interface on the molecular scale. Langmuir 2010, 26, 4627–4630. [Google Scholar] [CrossRef] [PubMed]
- Lehmkuhler, F.; Paulus, M.; Sternemman, C.; Lietz, D.; Venturini, F.; Gutt, C.; Tolan, M. The carbon dioxide water interface at conditions of gas hydrate formation. J. Am. Chem. Soc. 2009, 131, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Servio, P. Bulk liquid and gas mole fraction measurements during hydrate growth for the CH+ CO2+ H2O system. J. Chem. Thermodyn. 2018, 117, 113–118. [Google Scholar] [CrossRef]
- Schicks, J.M.; Luzi-Helbing, M. Cage occupancy and structural changes during hydrate formation from initial stages to resulting hydrate phase. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Horvat, K.; Kerkar, P.; Jones, K.; Mahajan, D. Kinetics of the Formation and Dissociation of Gas Hydrates from CO2-CH4 Mixtures. Energies 2012, 5, 2248–2262. [Google Scholar] [CrossRef]
- Schicks, J.M.; Luzi-Helbing, M. Kinetic and Thermodynamic aspects of Clathrate Hydrate Nucleation and growth. J. Chem. Eng. Data 2015, 60, 269–277. [Google Scholar] [CrossRef]
- Uchida, T.; Ikeda, M.; Takeya, S.; Kamata, Y.; Ohmura, R.; Nagao, J.; Zatsepina, O.Y.; Buffet, B.A. Kinetics and Stability of CH4-CO2 Mixed Gas Hydrates during Formation and Long-Term Storage. ChemPhysChem 2005, 6, 646–654. [Google Scholar] [CrossRef]
- Belosludov, R.V.; Zhdanov, R.K.; Gets, K.V.; Bozhko, Y.Y.; Belosludov, V.R.; Kawazoe, Y. Role of Methane as a Second Guest Component in Thermodynamic Stability and Isomer Selectivity of Butane Clathrate Hydrates. J. Phys. Chem. C 2020, 124, 18474–18481. [Google Scholar] [CrossRef]
- Kodera, M.; Matsueda, T.; Belosludov, R.V.; Zhdanov, R.K.; Belosludov, V.R.; Takeya, S.; Alavi, S.; Ohmura, R. Physical Properties and Characterization of the Binary Clathrate Hydrate with Methane + 1,1,1,3,3-Pentafluoropropane (HFC-245fa) + Water. J. Phys. Chem. C 2020, 124, 20736–20745. [Google Scholar] [CrossRef]
- Kwon, T.H.; Kneafsey, T.J.; Rees, E.V.L. Thermal Dissociation Behavior and Dissociation Enthalpies of Methane–Carbon Dioxide Mixed Hydrates. J. Phys. Chem. B 2011, 115, 8169–8175. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Sakemoto, R.; Ohmura, R. Crystal Growth of Clathrate Hydrates Formed at the Interface of Liquid Water and Gaseous Methane, Ethane, or Propane: Variations in Crystal Morphology. Cryst. Growth Des. 2009, 9, 2529–2536. [Google Scholar] [CrossRef]
- Freer, E.M.; Selim, M.S.; Sloan, E.D. Methane hydrate film growth kinetics. Fluid Phase Equilibria 2001, 185, 65–75. [Google Scholar] [CrossRef]
- Magill, J. Spherulites: A personal perspective. J. Mater. Sci. 2001, 36, 3143–3164. [Google Scholar] [CrossRef]
- Kitamura, M.; Mori, Y. Clathrate-hydrate film growth growth along water/methane phase boundaries–and observational study. Cryst. Res. Technol. 2013, 48, 511–519. [Google Scholar] [CrossRef]
- Kishimoto, M.; Ohmura, R. Correlation of the Growth Rate of the Hydrate Layer at a Guest/Liquid-Water Interface to Mass Transfer Resistance. Energies 2012, 5, 92–100. [Google Scholar] [CrossRef]
- Hashemi, S.; Macchi, A.; Bergeron, S.; Servio, P. Prediction of methane and carbon dioxide solubility in water in the presence of hydrate. Fluid Phase Equilibria 2006, 246, 131–136. [Google Scholar] [CrossRef]
- Servio, P.; Englezos, P. Measurement of dissolved methane in water in equilibrium with its hydrate. J. Chem. Eng. Data 2002, 47, 87–90. [Google Scholar] [CrossRef]
- Saito, K.; Sum, A.; Ohmura, R. Correlation of Hydrate-Film Growth Rate at the Guest/Liquid-Water Interface to Mass Transfer Resistance. Ind. Eng. Chem. Res. 2010, 49, 7102–7103. [Google Scholar] [CrossRef]
- Oya, S.; Aifaa, M.; Ohmura, R. Formation, growth and sintering of CO2 hydrate crystals in liquid water with continuous CO2 supply: Implication for subsurface CO2 sequestration. Int. J. Greenh. Gas Control. 2017, 63, 386–391. [Google Scholar] [CrossRef]
- Li, S.; Sun, C.; Liu, B.; Li, B.; Chen, G.; Sum, A. New Observations and Insights into the Morphology and Growth Kinetics of Hydrate Film. Sci. Rep. 2014, 4, 4129. [Google Scholar] [CrossRef]
- Duquesnay, J.; Diaz Posada, M.; Beltran, J. Novel gas hydrate reactor design: 3-in-1 assessment of phase equilibria, morphology and kinetics. Fluid Phase Equilibria 2016, 413, 148–157. [Google Scholar] [CrossRef]
- Trebble, M.A.; Bishnoi, P.R. Development of a new equation of state. Fluid Phase Equilibria 1987, 35, 1–18. [Google Scholar] [CrossRef]
- Fogg, P.; Gerrard, W. Solubility of Gases in Liquids: A Critical Evaluation of Gas/Liquid Systems in Theory and Practice; Wiley: Hoboken, NJ, USA, 1991. [Google Scholar]
- Sun, C.; Chen, G.; Ma, C.; Huang, Q.; Luo, H.; Li, Q. The growth kinetics of hydrate film on the surface of gas bubble suspended in water or aqueous surfactant solution. J. Cryst. Growth 2007, 306, 491. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Lee, J. Morphology study of methane-propane clathrate hydrates on the bubble surface in the presence of SDS or PVCap. J. Cryst. Growth 2014, 402, 249–259. [Google Scholar] [CrossRef]
- Li, S.L.; Sun, C.Y.; Liu, B.; Feng, X.J.; Li, F.G.; Chen, L.T.; Chen, G.J. Initial thickness measurements and insights into crystal growth of methane hydrate film. AIChE J. 2013, 59, 2145–2154. [Google Scholar] [CrossRef]
- Granasy, L.; Pusztai, T.; Tegze, G.; Warren, J.; Douglas, J. Growth and form of spherulites. Phys. Rev. E 2005, 72, 011605. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; Otálora, F. Crystal Growth in Geology. In Handbook of Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–43. [Google Scholar] [CrossRef]
- Sloan, E.D. Clathrate Hydrates of Natural Gases, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1998. [Google Scholar]
- Aya, I.; Yamane, K.; Nariai, H. Solubility of CO2 and Density of CO2 Hydrate at 30 MPa. Energy 1997, 22, 263. [Google Scholar] [CrossRef]
- Teng, H.; Yamasaki, A.; Shindo, Y. Stability of the hydrate layer formed on the surface of a CO2 droplet in high-pressure, low-temperature water. Chem. Eng. Sci. 1996, 51, 4979–4986. [Google Scholar] [CrossRef]
- Ovalle, S.; Beltran, J.G. Growth Mechanisms and Phase Equilibria of Propane and Methane + Propane Hydrates. J. Chem. Eng. Data 2021, 66, 4112–4121. [Google Scholar] [CrossRef]
- Bruusgaard, H.; Beltran, J.; Servio, P. Solubility measurements for the CH4+CO2+H2O system under hydrate–liquid–vapor equilibrium. Fluid Phase Equilibria 2010, 296, 106–109. [Google Scholar] [CrossRef]
- Sunagawa, I. Crystals: Growth, Morphology, and Perfection; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]

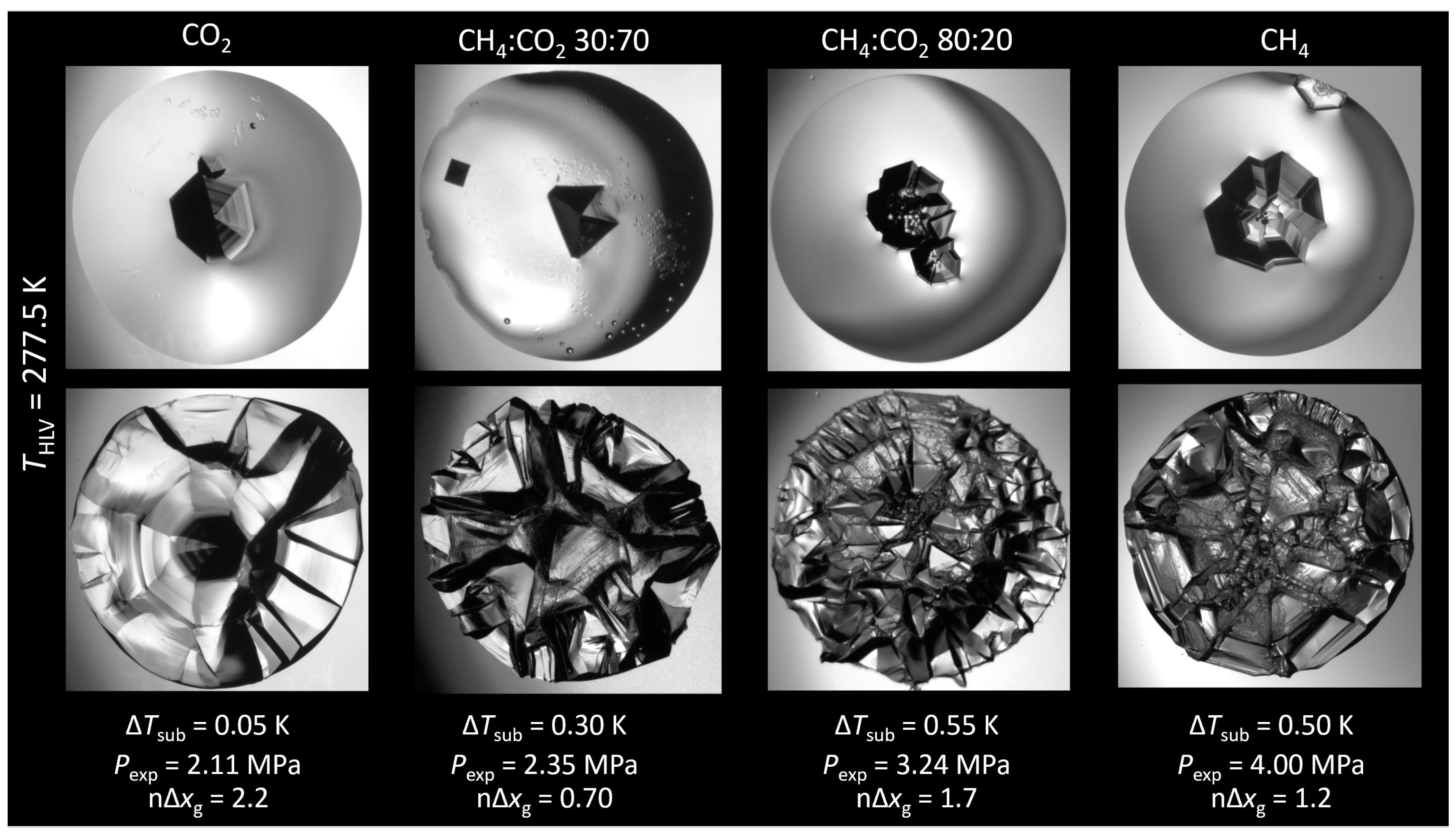
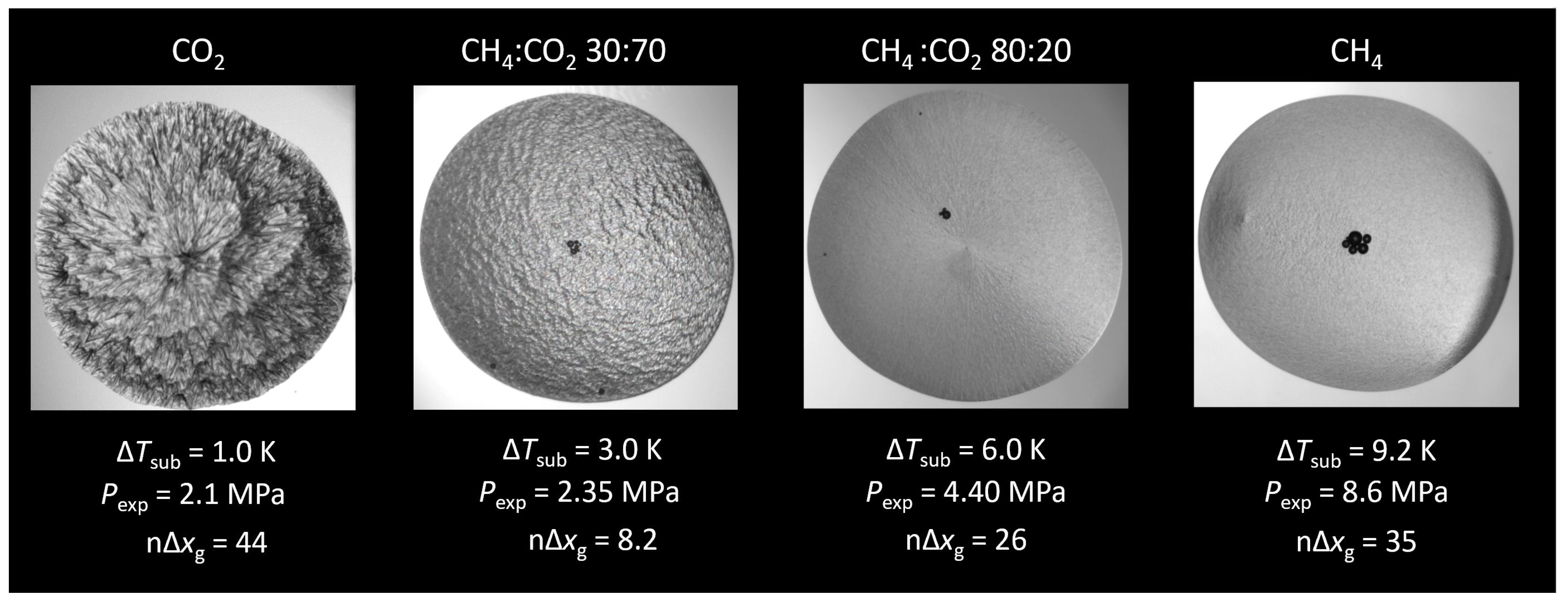
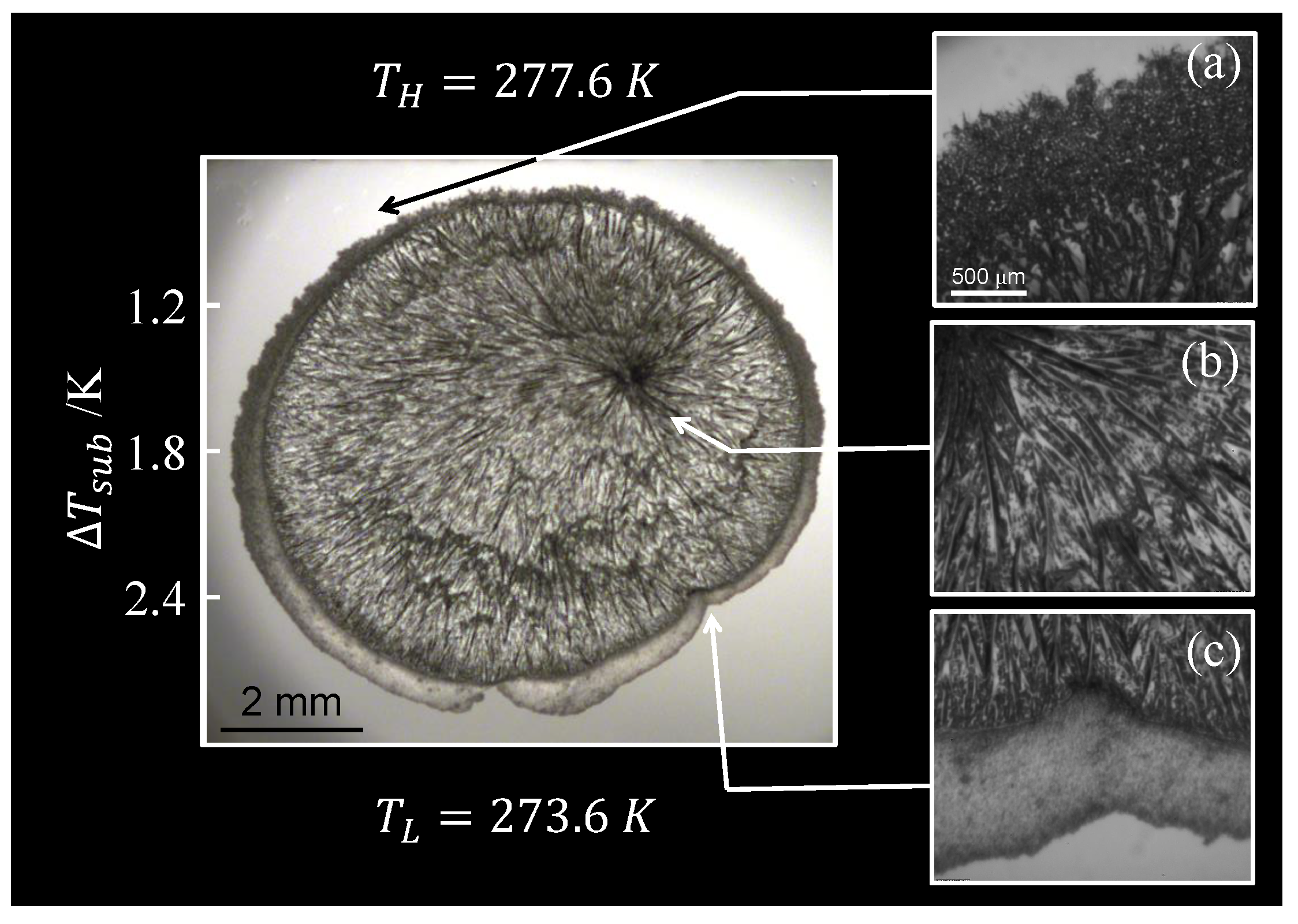


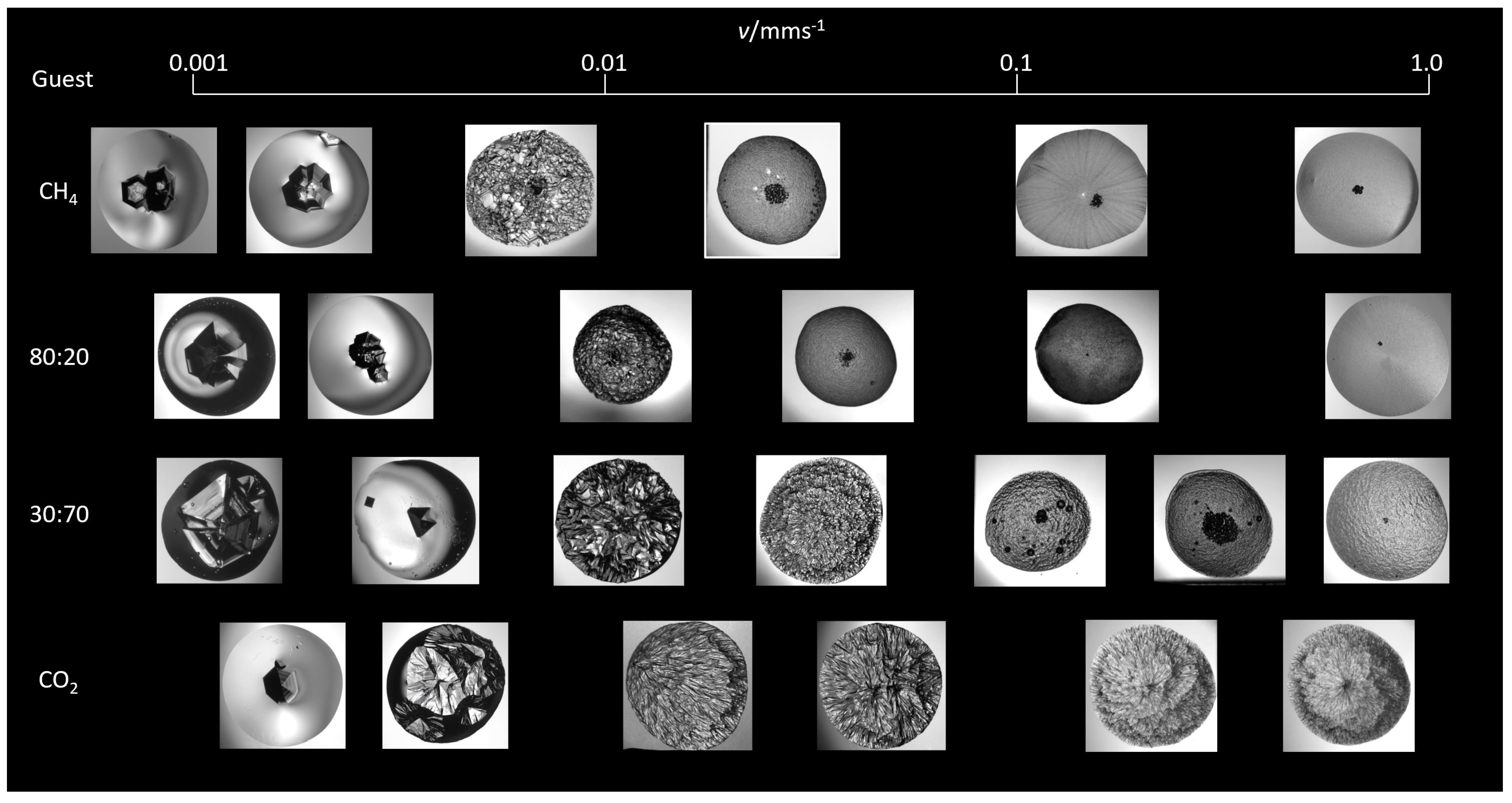


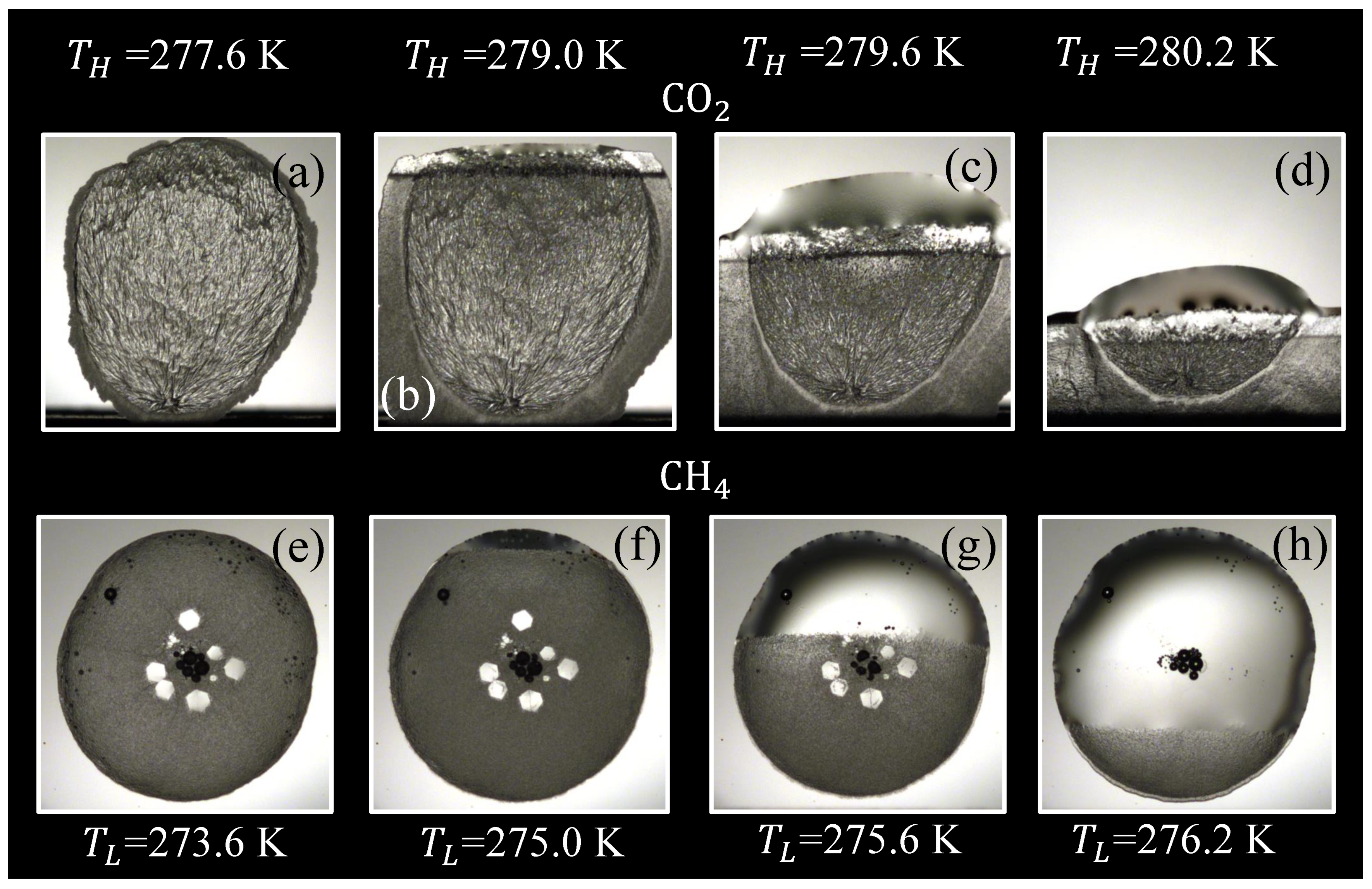
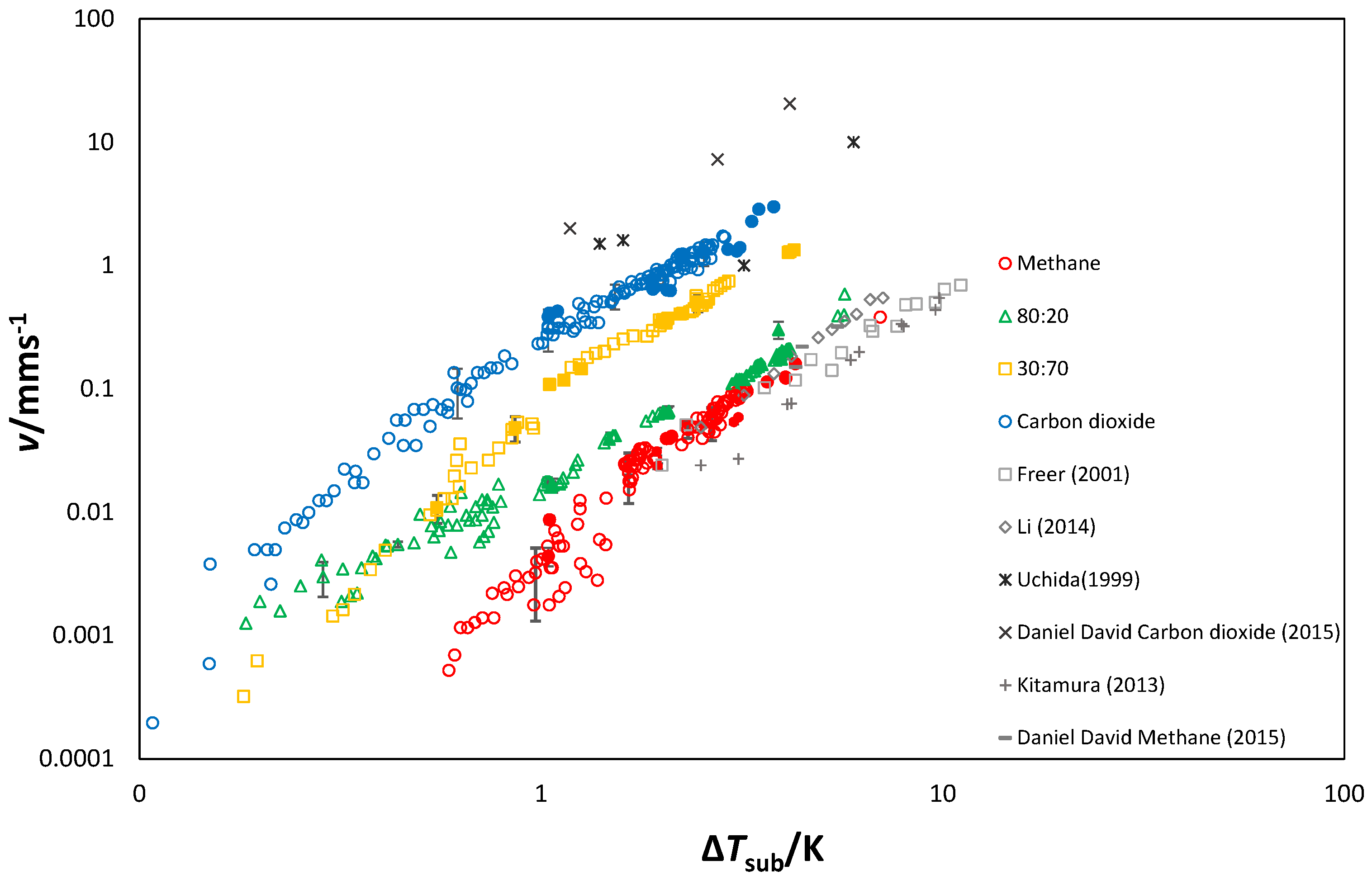

| Composition | 10y | 10y | Source |
|---|---|---|---|
| CH | 99.999 | 0 | Air Liquide, QC, CA |
| 80:20 | 80.02 | 19.98 | |
| 30:70 | 29.94 | 70.06 | |
| CO | 0 | 100 |
| Vapor Phase | P/MPa | /K | /K | /K |
|---|---|---|---|---|
| 4.00 | 277.4 | 279.2 | 275.2 | |
| 4.00 | 277.4 | 279.0 | 275.0 | |
| 4.01 | 277.5 | 279.0 | 275.0 | |
| 4.00 | 277.4 | 276.9 | 276.9 | |
| 4.00 | 277.4 | 277.1 | 277.1 | |
| 6.60 | 282.3 | 274.4 | 274.4 | |
| 8.60 | 284.7 | 275.5 | 275.5 | |
| 8.60 | 284.7 | 276.7 | 276.7 | |
| 2.10 | 277.5 | 277.5 | 277.5 | |
| 2.09 | 277.5 | 277.5 | 277.5 | |
| 2.09 | 277.5 | 277.5 | 277.5 | |
| 2.10 | 277.5 | 279.5 | 275.5 | |
| 2.10 | 277.5 | 279.0 | 275.0 | |
| 2.10 | 277.5 | 275.7 | 279.7 | |
| 80:20 | 3.25 | 277.4 | 279.2 | 275.2 |
| 80:20 | 3.25 | 277.4 | 275.0 | 279.0 |
| 80:20 | 3.25 | 277.4 | 273.5 | 277.5 |
| 80:20 | 3.25 | 277.4 | 271.8 | 275.8 |
| 80:20 | 3.25 | 277.4 | 277.1 | 277.1 |
| 80:20 | 3.25 | 277.4 | 277.2 | 277.2 |
| 80:20 | 3.25 | 277.4 | 277.1 | 277.1 |
| 80:20 | 4.40 | 280.2 | 274.2 | 274.2 |
| 80:20 | 4.40 | 280.2 | 273.4 | 273.4 |
| 30:70 | 2.35 | 277.4 | 277.3 | 277.3 |
| 30:70 | 2.35 | 277.4 | 277.2 | 277.2 |
| 30:70 | 2.35 | 277.4 | 277.3 | 277.3 |
| 30:70 | 2.35 | 277.4 | 279.5 | 275.5 |
| 30:70 | 2.35 | 277.4 | 279.2 | 275.2 |
| 30:70 | 2.35 | 277.4 | 277.2 | 277.2 |
| 30:70 | 2.35 | 277.4 | 277.1 | 277.1 |
| 30:70 | 2.35 | 277.4 | 274.2 | 274.2 |
| 30:70 | 2.35 | 277.4 | 276.4 | 276.4 |
| 30:70 | 2.35 | 277.4 | 275.4 | 275.4 |
| 30:70 | 2.35 | 277.4 | 273.4 | 273.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, C.; Sandoval, J.F.; Ortiz, N.; Ovalle, S.; Beltran, J.G. Mechanisms, Growth Rates, and Morphologies of Gas Hydrates of Carbon Dioxide, Methane, and Their Mixtures. Methane 2022, 1, 2-23. https://doi.org/10.3390/methane1010002
Martinez C, Sandoval JF, Ortiz N, Ovalle S, Beltran JG. Mechanisms, Growth Rates, and Morphologies of Gas Hydrates of Carbon Dioxide, Methane, and Their Mixtures. Methane. 2022; 1(1):2-23. https://doi.org/10.3390/methane1010002
Chicago/Turabian StyleMartinez, Camilo, Juan F. Sandoval, Nathalia Ortiz, Sebastian Ovalle, and Juan G. Beltran. 2022. "Mechanisms, Growth Rates, and Morphologies of Gas Hydrates of Carbon Dioxide, Methane, and Their Mixtures" Methane 1, no. 1: 2-23. https://doi.org/10.3390/methane1010002
APA StyleMartinez, C., Sandoval, J. F., Ortiz, N., Ovalle, S., & Beltran, J. G. (2022). Mechanisms, Growth Rates, and Morphologies of Gas Hydrates of Carbon Dioxide, Methane, and Their Mixtures. Methane, 1(1), 2-23. https://doi.org/10.3390/methane1010002






