Bariatric–Metabolic Surgery: The State of the Art and the Management of Complications
Abstract
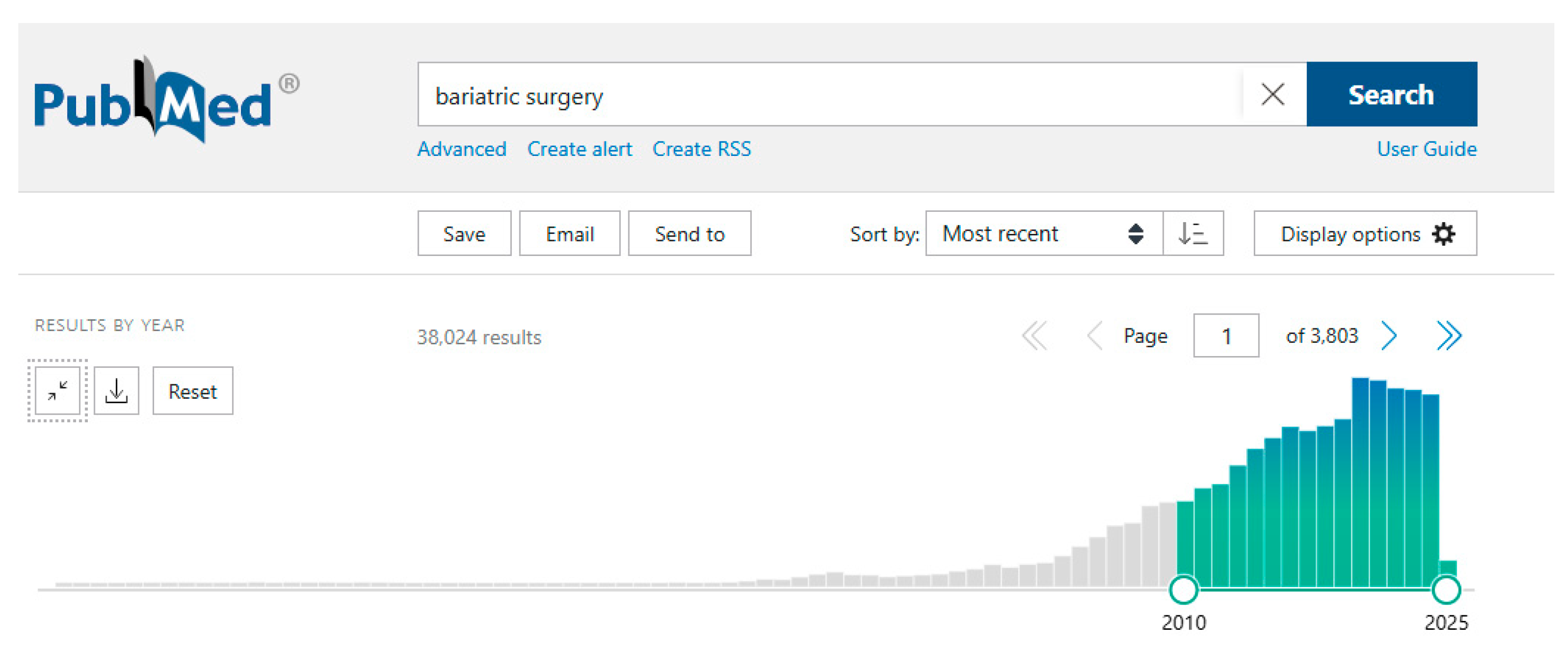
1. Introduction
- -
- Changes in lifestyle;
- -
- Increased consumption of energy-dense foods;
- -
- Reduced physical activity;
- -
- Socioeconomic and cultural factors;
- -
- Psychological factors.
- Nutritional interventions based on nutritional re-education with a balanced low-calorie or ketogenic diet depending on the patient’s metabolic condition;
- Increased personalized physical activity;
- Behavioral changes (with possible psychotherapeutic support).
2. Overview of Procedures
3. Surgical Treatments
3.1. Restrictive Procedures (Figure 2)
3.2. Malabsorptive Procedures
3.3. Restrictive–Malabsorptive Procedures
4. Patient Selection
4.1. Preliminary Assessments
- Accurate medical and dietary history;
- Complete blood chemistry tests, hormone assays, and endocrinological tests;
- Psychological assessment—MMPI, SF 36, and BITE tests;
- BMI;
- Bioimpedance analysis (BIA) and calorimetry to assess body composition and basal metabolic rate;
- Abdominal ultrasound;
- Thyroid ultrasound;
- Cardio-respiratory sleep monitoring;
- Internal medicine, gastroenterology, cardiology, endocrinology, surgery, and anesthesiology assessments;
- Esophagogastroduodenoscopy (EGDS) and digestive tract X-ray;
- Ergospirometry and PFT (pulmonary function tests).
4.1.1. Body Mass Index (BMI): Indications for Surgery
- -
- BMI ≥ 40 kg/m2;
- -
- BMI ≥ 35 kg/m2 and comorbidities: high blood pressure, type 2 diabetes mellitus (T2DM), dyslipidemia, and obstructive sleep apnea syndrome (OSAS);
- -
- BMI ≥ 30 kg/m2 in selected cases after collegial evaluation (osteoarticular pathologies of the lower limbs; T2DM not controlled with medical therapy; and at least one uncontrolled comorbidity among T2DM, arterial hypertension, dyslipidemia, and OSAS).
4.1.2. Body Mass Index (BMI): Indications for the Choice of Bariatric Procedure
- -
- BMI ≥ 40 kg/m2:
- ▪
- Roux-en-Y Gastric Bypass (RYGB);
- ▪
- Biliopancreatic diversion with duodenal switch;
- ▪
- One-Anastomosis Gastric Bypass (OAGB);
- ▪
- Sleeve gastrectomy;
- ▪
- Laparoscopic gastric banding.
- -
- BMI ≥ 35 kg/m2 and ≥ 1 comorbidity or uncontrolled T2DM:
- ▪
- RYGB;
- ▪
- Biliopancreatic diversion with duodenal switch;
- ▪
- OAGB.
- -
- BMI ≥ 30 kg/m2 and uncontrolled T2DM:
- ▪
- RYGB;
- ▪
- Laparoscopic gastric banding;
- ▪
- Sleeve gastrectomy.
4.1.3. Personalization in the Choice Based on
- -
- BMI;
- -
- Surgical experience, facility potential, and follow-up;
- -
- Compliance;
- -
- Comorbidities;
- -
- Type of obesity;
- -
- Eating habits.
4.1.4. Psychological Evaluation
- Pathological addictions;
- Psychopathologies;
- Eating disorders.
- (a)
- Rule out untreated psychiatric disorders (such as severe depression, eating disorders, and substance abuse), which could compromise the long-term success of the surgery.
- (b)
- Assess the patient’s attitude toward change and their ability to adopt new eating and behavioral habits. For example, the ability to manage emotions without resorting to food is crucial. Post-operative psychological support is also essential to facilitate adaptation to the new lifestyle and body image and to maintain the results achieved.
4.2. Failure of Conservative Treatments
4.3. Age
4.4. Conclusions
5. Comparing Surgical Procedures
6. Post-Operative Follow-Up
- -
- Weight loss;
- -
- The patient’s psychological state;
- -
- The patient’s well-being in terms of quality of life and control of any associated diseases;
- -
- Prevention and early diagnosis of surgical and non-surgical complications.
- -
- After 15 and 30 days from surgery for the first three months;
- -
- Subsequently every 3 months in the first year after surgery;
- -
- Every 6 months in the second year after surgery;
- -
- Annually from the third year after surgery.
6.1. Follow-Up
6.1.1. Post-Operative Dietary Visits
- -
- A liquid diet in the first two weeks after discharge, consisting of liquid foods and supplemented with normal-calorie and high-protein oral nutritional supplements, which are necessary to meet the patient’s needs;
- -
- A creamy diet for one month with the aim of increasing the consistency of foods and increasing nutrient intake until specific requirements are met, reducing the risk of anastomotic dehiscence as much as possible;
- -
- A soft diet for about one month; the duration may be increased in the case of intolerance to solid foods;
- -
- A personalized solid diet in the long term.
- -
- Behavioral advice to help reduce the onset of complications like eat slowly, chew well, drink between meals, and stop eating as soon as you feel full.
- -
- Nutritional education sessions, which facilitate the long-term maintenance of achieved results.
- -
- Assessment of patient’s clinical and nutritional status;
- -
- Monitoring of body weight and weight loss;
- -
- Monitoring of blood chemistry tests;
- -
- Verification of adherence to the diet and provided behavioral advices;
- -
- Verification of compliance with the recommended physical activity plan;
- -
- Verification of adherence to the prescription of multivitamin supplements.
6.1.2. Post-Operative Psychological Visits
- -
- Loss of motivation;
- -
- The onset of psychological distress;
- -
- The resumption of unhealthy eating habits.
6.1.3. Multidisciplinary Consultations
- -
- Internal medicine: Management of dyspepsia and infectious complications and support in monitoring other obesity-related conditions, such as diabetes, hypertension, and heart disorders;
- -
- Cardiology consultation: Monitoring of drug therapy for heart conditions associated with obesity;
- -
- Endocrinology consultation: Monitoring of type 2 diabetes and any endocrine disorders associated with obesity (e.g., thyroid dysfunction, polycystic ovary syndrome or PCOS, and osteoporosis);
- -
- Surgical consultation: Monitoring and management of any post-bariatric complications such as dehiscence, anastomotic leaks, or adhesions.
7. Benefits on Major Comorbidities
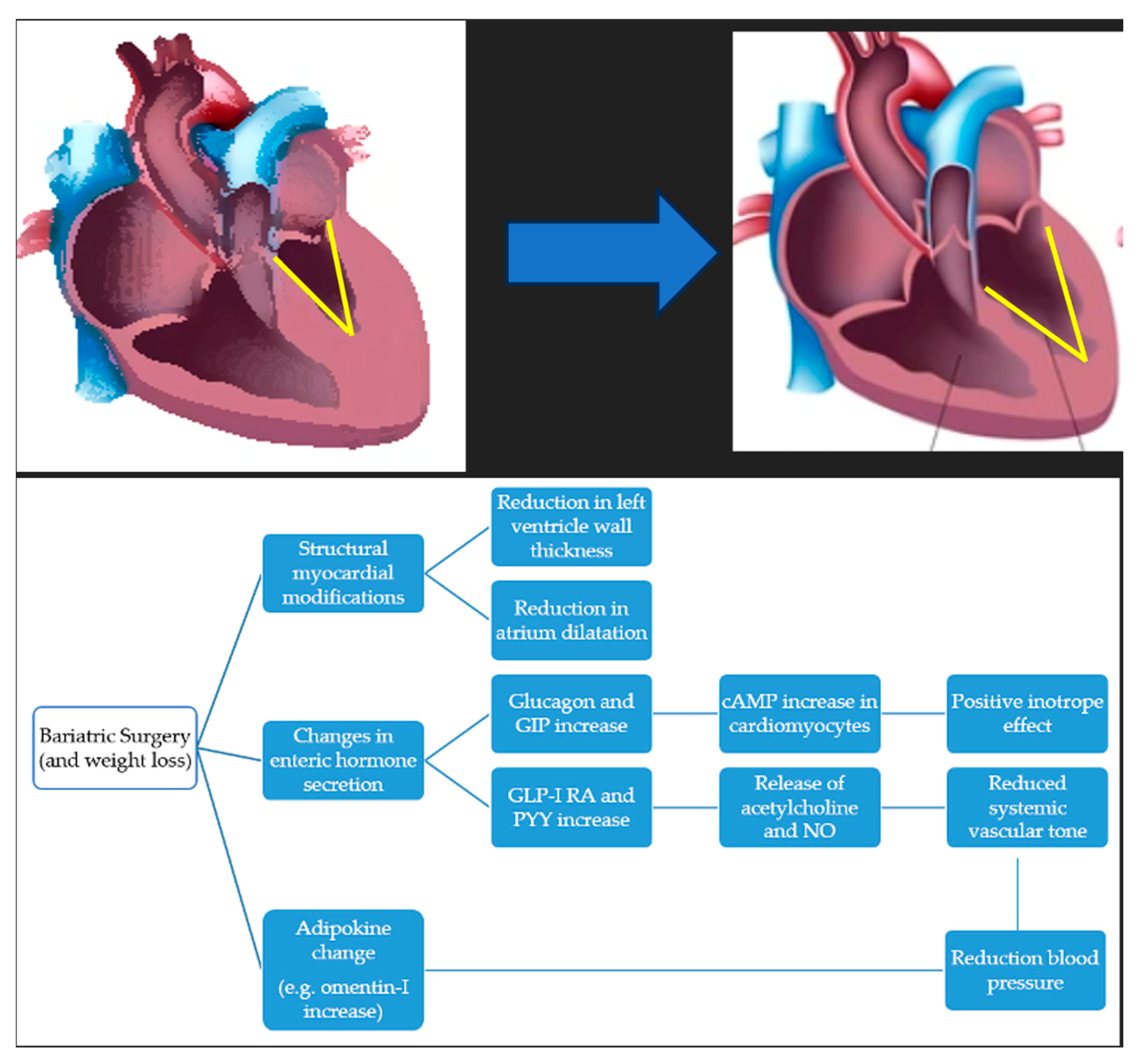
7.1. Cardiovascular Risk and Hypertension
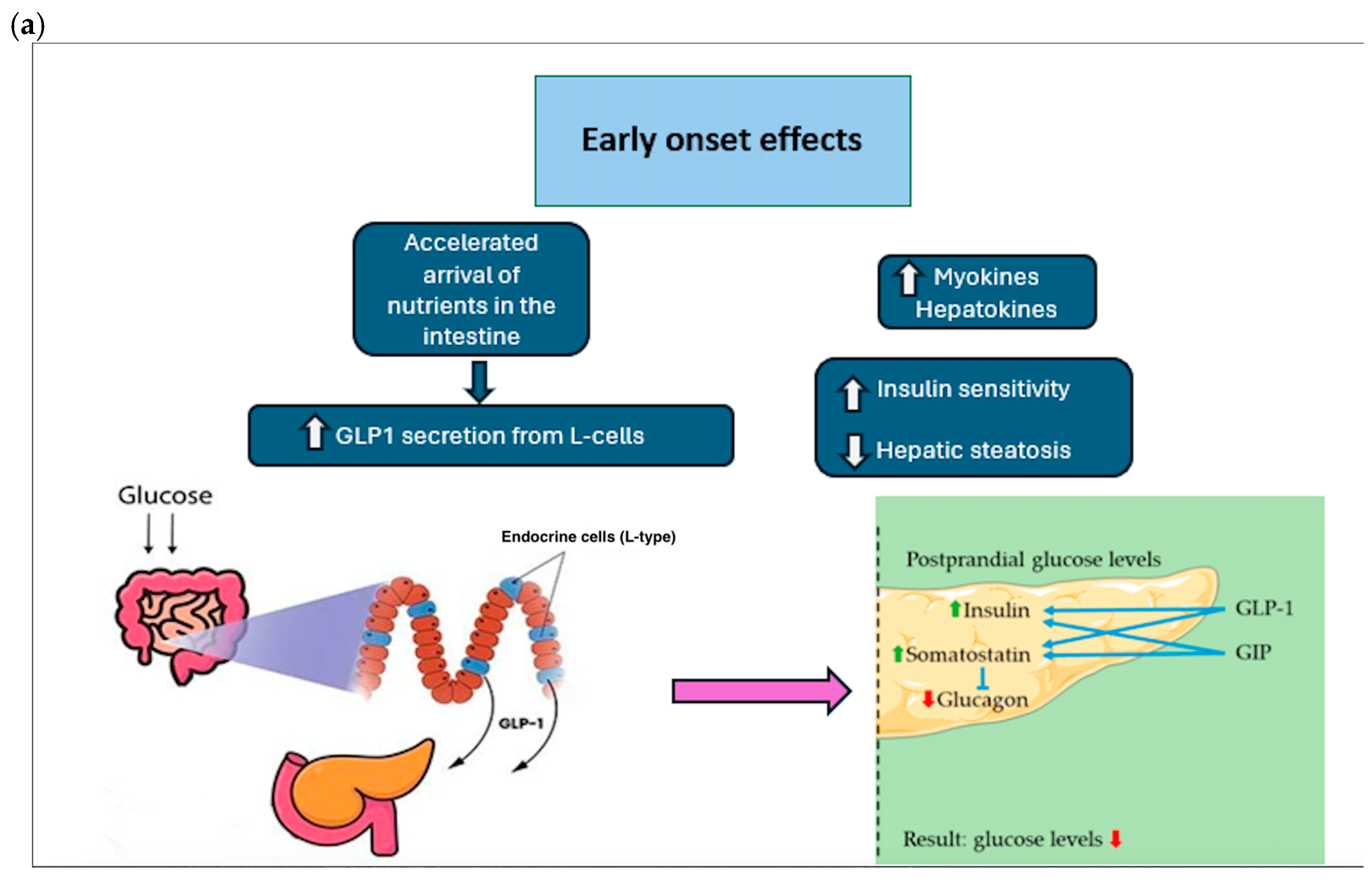
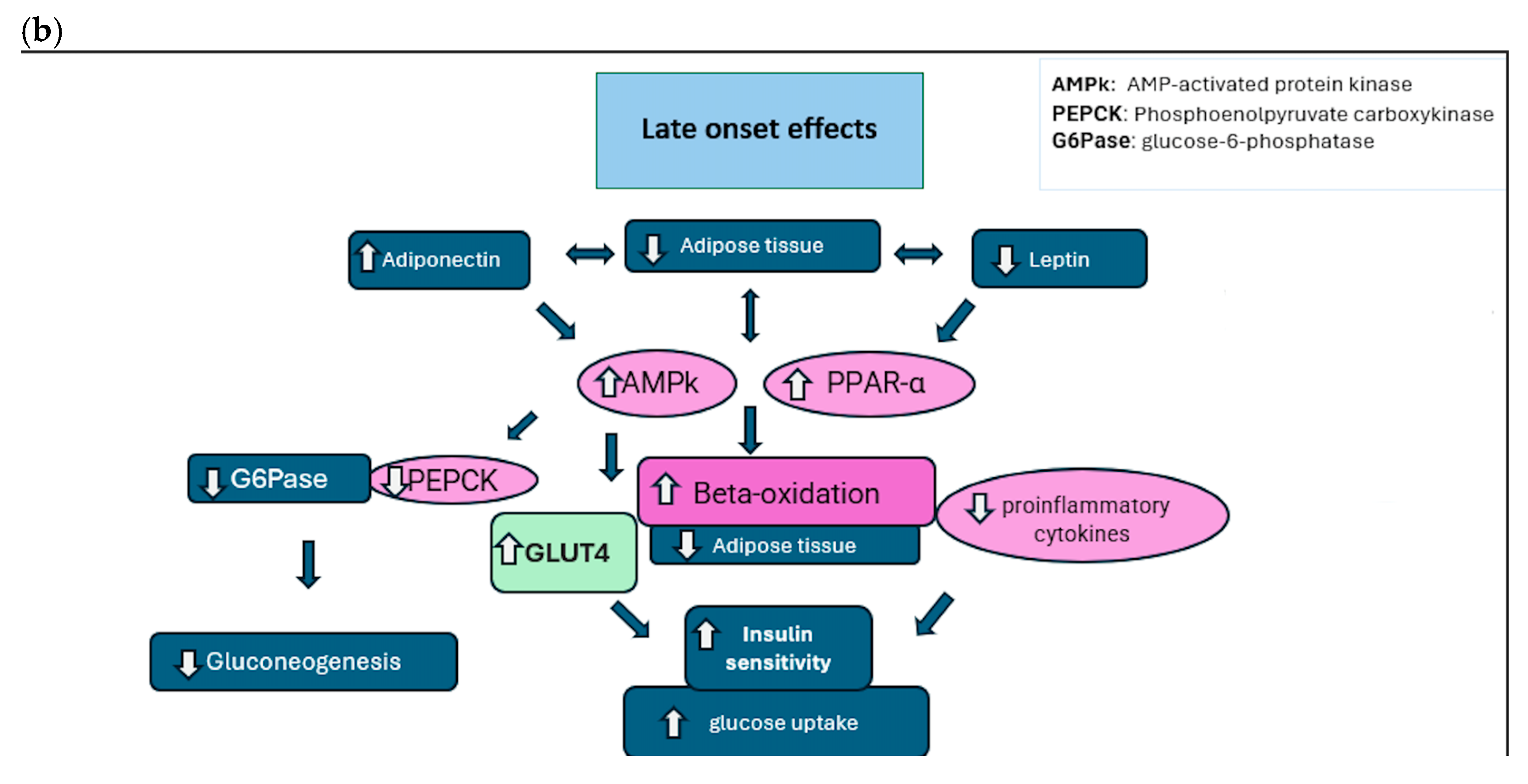
7.2. Type 2 Diabetes Mellitus
- Improvement of insulin secretion: A rapid increase in insulin secretion in the first few days/weeks after surgery, regardless of weight loss. The effect is linked to changes in the flow of nutrients through the gastrointestinal tract.
- Improved insulin sensitivity: Increased cellular responses to insulin, improving blood glucose management.
- Changes to the gastrointestinal tract: An alteration of gastrointestinal physiology (e.g., gastric bypass and SG), which promotes better glucose regulation thanks to changes in hormonal signals.
- Hormonal changes (GLP-1, PYY): Increased production of hormones such as GLP-1 and PYY, which improve insulin sensitivity and stimulate insulin secretion.
- Reduction in hepatic glucose production: A reduction in excessive glucose production by the liver, contributing to improved blood sugar levels.
- Adipokines (e.g., adiponectin): Increased circulation of adiponectin, which improves insulin sensitivity and reduces inflammation. In addition, there is a decrease in pro-inflammatory interleukins (IL-1, IL-6, and IL-8).
- Myokines: Muscles exhibit an enhanced capacity to utilize nutrients, leading to a decrease in inflammation and better glucose metabolism.
- Hepatokines: The liver experiences improved lipid utilization and reduced hepatic inflammation, aiding in metabolic regulation.
- Synergistic interactions: Metabolic adaptations result from the interaction between adipokines, myokines, and hepatokines, which work together to improve metabolic regulation and reduce inflammation.
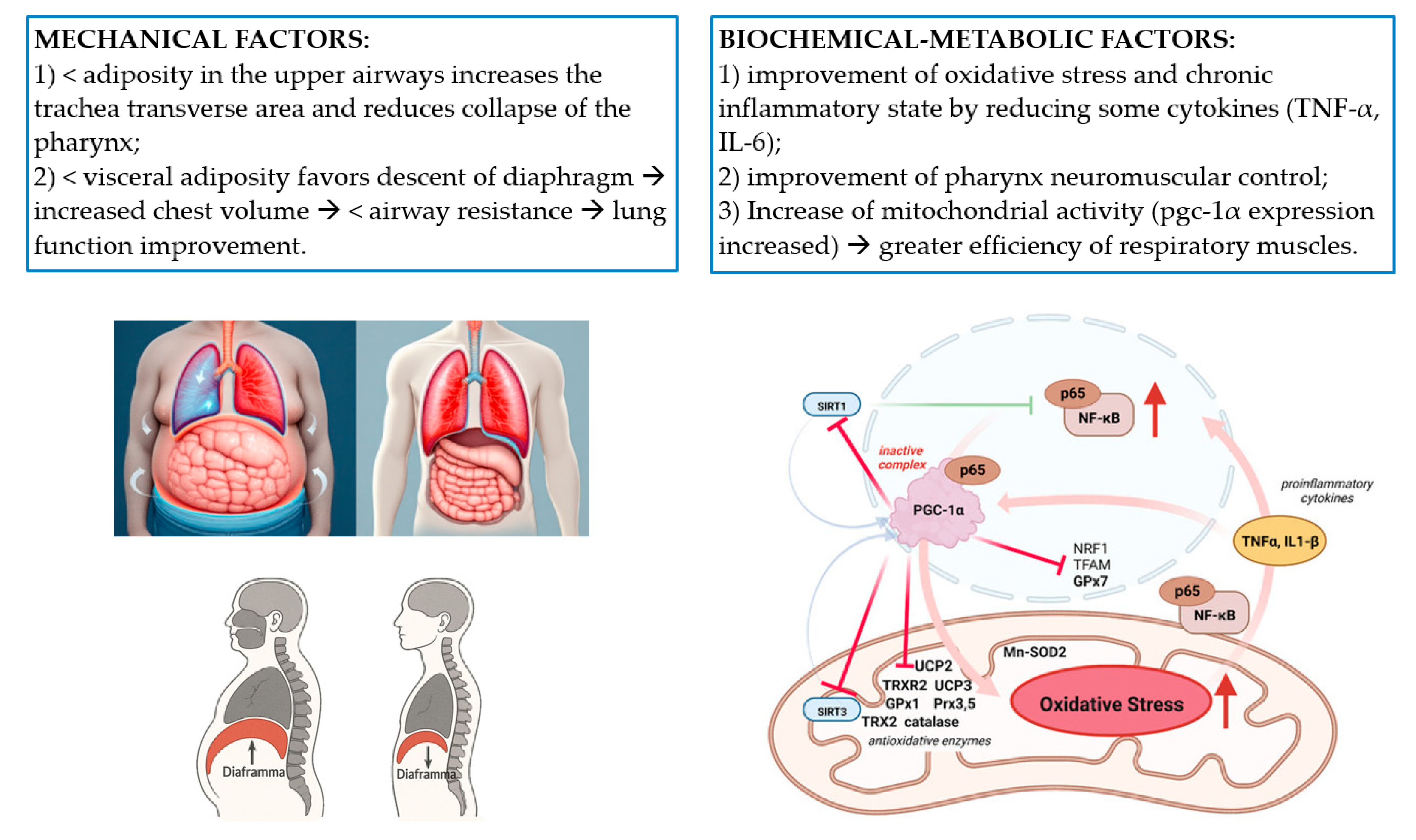
7.3. Obstructive Sleep Apnea Syndrome (OSAS)
- Reduces adiposity in the upper airway by increasing the transverse area of the trachea and reducing pharyngeal collapse;
- Reduces visceral adiposity that is associated with lower intra-abdominal pressure, diaphragm descent, increased thoracic volume, and lower airway resistance, resulting in improved lung function.
- Amelioration of oxidative stress and the chronic inflammatory state typical of obesity through the reduction in certain cytokines (e.g., TNF-α and IL-6). A reduction in these mediators could contribute to improved neuromuscular control of the pharynx and have an effect at the CNS level;
- Increased mitochondrial activity compared to preoperative levels, alleviating the symptoms of hypoxia [29].
8. Complications
8.1. Deficiency of Vitamins and Minerals
- Iron: Its deficiency is one of the most common nutritional complications post-bariatric surgery. Limited absorption capacity, combined with a possible reduced dietary intake of iron-rich foods, can result in sideropenic anemia, a condition that may manifest with fatigue, weakness, and, in severe cases, cardiac changes. Oral iron supplementation (30 mg/day) is frequently necessary, but in the presence of reduced intestinal surface area, this may be inadequate or insufficient. In these cases, intravenous iron supplementation is calculated as follows: (Kg body weight × ↑ desired Hb × 2.5 + 10 mg pro Kg body weight).
- Folic acid: Its deficiency exposes individuals to megaloblastic anemia. Regular supplementation is essential to prevent deficiency (0.4–0.6 mg/day per os).
- Vitamin B12: Anatomical alterations can impair intestinal absorption of vitamin B12, leading to megaloblastic anemia, peripheral neuropathy, and cognitive deficits. Treatment involves oral supplements or intramuscular injections of vitamin B12 (per os: 250–1000 mcg/day, i.m.: 1000 mcg/3 months).
- Vitamin D: Reduced intestinal absorption of vitamin D, combined with limited sun exposure and reduced physical activity, may promote vitamin D deficiency, with an increased risk of osteoporosis and fractures. Vitamin D oral supplementation is often necessary to maintain adequate levels.
- Calcium: Bariatric surgery can alter calcium absorption, increasing the risk of osteopenia and osteoporosis. Supplementation with calcium supplements and regular monitoring of serum levels are essential.
- Vitamins A, E, and K: Deficiencies of these fat-soluble vitamins are less common but can occur, especially in the presence of lipid malabsorption.
- Vitamin B1 or thiamine: Deficiency of this vitamin can lead to a neurological syndrome known as Wernicke’s encephalopathy (confusion, amnestic deficits, tinnitus, dizziness, and hearing loss).
8.2. Dumping Syndrome and Reactive Hypoglycemia
8.3. Lipid Malabsorption and Chronic Diarrhea
8.4. Gastrointestinal and Digestive Disorders
8.5. Protein-Calorie Malnutrition
8.6. Gut Dysbiosis and Microbiota Alterations
9. Weight Regain
- Anatomical and surgical: Enlargement of the gastric sac and site of anastomosis, as well as gastro–gastric fistula formation, is among the main structural causes of weight regain (WR);
- Endocrine and metabolic: Changes in hormones levels such as ghrelin, GLP-1, and peptide YY, which regulate appetite and satiety, may contribute to WR;
- Behavioral and lifestyle: Patients may resume unhealthy eating habits and reduce physical activity, negatively affecting maintained weight loss;
- Psychological: Untreated eating disorders or psychological disorders during follow-up such as binge eating disorder, anxiety, depression, and other mental health problems are associated with an increased likelihood of WR.
- Lifestyle modifications: Programs combining diet, physical activity, and behavioral support can improve post-surgical outcomes;
- Pharmacotherapy: The use of medications that reduce appetite and improve satiety is an effective option for patients with WR (GLP-1 RA);
- Sequential bariatric surgery: It may be necessary in cases of WR.
10. Public vs. Private
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| T2DM | Type 2 diabetes mellitus |
| OSAS | Obstructive sleep apnea syndrome |
| BMI | Body mass index |
| WHO | World Health Organization |
| GLP-1 | Glucagon-like peptide-1 |
| GLP-1 RA | Glucagon-like peptide-1 receptor agonist |
| GIP | Gastric inhibitory polypeptide |
| BMS | Bariatric–metabolic surgery |
| SG | Sleeve gastrectomy |
| BPD-DS | Biliary pancreatic diversion with duodenal switch |
| RYGB | Roux-en-Y gastric bypass |
| OAGB | One-anastomosis gastric bypass |
| LGB | Laparoscopic gastric banding |
| SADI-S | Single-anastomosis duodenal–ileal bypass with sleeve gastrectomy |
| CCK | Cholecystokinin |
| PYY | Peptide YY |
| MMPI | Minnesota Multiphasic Personality Inventory |
| SF 36 | Short-Form Health Survey 36 |
| BITE | Bulimic Investigatory Test Edinburgh |
| BIA | Bioelectrical impedance analysis |
| EGDS | Esophagogastroduodenoscopy |
| PFTs | Pulmonary function tests |
| TWL | Total weight loss |
| PCOS | Polycystic ovary syndrome |
| ACE | Angiotensin-converting enzyme |
| AHI | Apnea–hypopnea index |
| CNS | Central nervous system |
| GRD | Gastroesophageal reflux disease |
| Iv | Intravenous |
| Im | Intramuscular |
| BMD | Bone mineral density |
| PTH | Parathyroid hormone |
| WR | Weight regain |
References
- Williamson, K.; Blane, D.N.; Lean, M.E. Challenges in obtaining accurate anthropometric measures for adults with severe obesity: A community-based study. Scand. J. Public Health 2023, 51, 935–943. [Google Scholar] [CrossRef]
- World Obesity Atlas. 2024. Available online: https://www.sportesalute.eu/studiedatidellosport/blog-studi-e-dati-dello-sport/world-obesity-atlas-report-2024.html (accessed on 1 February 2025).
- Seventh IFSO Global Registry Report. 2022. IFSO Registry|International Federation for the Surgery of Obesity and Metabolic Disorders. Available online: https://www.ifso.com/ifso-registry.php (accessed on 1 February 2025).
- Shilton, H. Bariatric surgery. Aust. J. Gen. Pract. 2025, 54, 202–206. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef]
- Walter, E.; Langer, F.B.; Beckerhinn, P.; Hoffer, F.; Prager, G. Impact of metabolic surgery on cost and long-term health outcome: A cost-effectiveness approach. Surg. Obes. Relat. Dis. 2022, 18, 260–270. [Google Scholar]
- SICOB Guidelines 2016–2023. Available online: https://www.sicob.org/03_attivita/pubblicazioni_linee_guida.aspx (accessed on 1 February 2025).
- Neff, K.J.; Olbers, T.; Le Roux, C.W. Bariatric surgery: The challenges with candidate selection, individualizing treatment and clinical outcomes. BMC Med. 2013, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Salas-Parra, R.D.; Smolkin, C.; Choksi, S.; Pryor, A.D. Bariatric Surgery: Current Trends and Newer Surgeries. Gastrointest. Endosc. Clin. N. Am. 2024, 34, 609–626. [Google Scholar] [CrossRef]
- Gentileschi, P.; Sensi, B.; Siragusa, L.; Sorge, R.; Rispoli, E.; Angrisani, L.; Galfrascoli, E.; Bianciardi, E.; Giusti, M.P.; De Luca, M.; et al. Evolution of Bariatric Surgery in Italy in the Last 11 Years: Data from the SICOB Yearly National Survey. Obes. Surg. 2023, 33, 930–937. [Google Scholar] [CrossRef]
- Pennestrì, F.; Sessa, L.; Prioli, F.; Salvi, G.; Gallucci, P.; Ciccoritti, L.; Greco, F.; De Crea, C.; Raffaelli, M. Single anastomosis duodenal-ileal bypass with sleeve gastrectomy (SADI-S): Experience from a high-bariatric volume center. Langenbeck’s Arch. Surg. 2022, 407, 1851–1862. [Google Scholar] [CrossRef]
- Balamurugan, G.; Leo, S.J.; Sivagnanam, S.T.; Prasad, S.B.; Ravindra, C.; Rengan, V.; Arora, E.; Bindal, V. Comparison of Efficacy and Safety Between Roux-en-Y Gastric Bypass (RYGB) vs One Anastomosis Gastric Bypass (OAGB) vs Single Anastomosis Duodeno-ileal Bypass with Sleeve Gastrectomy (SADI-S): A Systematic Review of Bariatric and Metabolic Surgery. Obes. Surg. 2023, 33, 2194–2209. [Google Scholar] [CrossRef] [PubMed]
- Elsaigh, M.; Awan, B.; Marzouk, M.; Khater, M.H.; Asqalan, A.; Szul, J.; Mansour, D.; Naim, N.; Saleh, O.S.; Jain, P.; et al. Comparative Safety and Efficacy of Roux-en-Y Gastric Bypass Versus One-Anastomosis Gastric Bypass: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Cureus 2024, 16, e71193. [Google Scholar] [CrossRef] [PubMed]
- Clapp, B.; Mosleh, K.A.; Corbett, J.; Hage, K.; Moore, R.L.; Billy, H.; Ponce, J.; Ghanem, O.M. One Anastomosis Gastric Bypass Versus Single Anastomosis Duodenoileostomy with Sleeve: Comparative Analysis of 30-Day Outcomes Using the MBSAQIP. Obes. Surg. 2023, 33, 720–724. [Google Scholar] [CrossRef] [PubMed]
- The Australia and New Zealand Bariatric Surgery Registry. Annual Report—2023. Report No. 11. Version 1.0. School of Translational Medicine, Monash University. 2024. Available online: www.monash.edu/__data/assets/pdf_file/0007/3757876/Bariatric_Surgery_Registry_-Annual_Report_2023.pdf (accessed on 30 January 2025).
- Hage, K.; Teixeira, A.F.; Surve, A.; Lind, R.; Jawad, M.A.; Ghanem, M.; Abi Mosleh, K.; Kendrick, M.L.; Cottam, D.; Ghanem, O.M. Single anastomosis duodenal switch versus Roux-en-Y gastric bypass in patients with BMI ≥ 50 kg/m2: A multi-centered comparative analysis. Surg. Endosc. 2024, 38, 2657–2665. [Google Scholar]
- Capoccia, D. The long-term follow-up of metabolic surgery. Endocrinologist 2023, 24, 1–6. [Google Scholar]
- Beck, T.; Aviran, E.; Cohn, S.; Goitein, D. Perioperative Morbidity and Long-term Outcomes of Bariatric Surgery in Patients with Severe Obesity. Isr. Med. Assoc. J. 2023, 25, 612–616. [Google Scholar] [PubMed]
- Alghamdi, S.; Mirghani, H.; Alhazmi, K.; Alatawi, A.M.; Brnawi, H.; Alrasheed, T.; Badoghaish, W. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy effects on obesity comorbidities: A systematic review and meta-analysis. Front. Surg. 2022, 9, 953804. [Google Scholar] [CrossRef]
- Sargsyan, N.; Chen, J.Y.; Aggarwal, R.; Fadel, M.G.; Fehervari, M.; Ashrafian, H. The effects of bariatric surgery on cardiac function: A systematic review and meta-analysis. Int. J. Obes. 2024, 48, 166–176. [Google Scholar] [CrossRef]
- Van Veldhuisen, S.L.; Gorter, T.M.; Van Woerden, G.; De Boer, R.A.; Rienstra, M.; Hazebroek, E.J.; Van Veldhuisen, D.J. Bariatric surgery and cardiovascular disease: A systematic review and meta-analysis. Eur. Heart J. 2022, 43, 1955–1969. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Z.; Ilyas, I.; Little, P.J.; Kamato, D.; Sahebka, A.; Chen, Z.; Luo, S.; Zheng, X.; Weng, J.; et al. GLP-1 receptor agonists (GLP-1RAs): Cardiovascular actions and therapeutic potential. Int. J. Biol. Sci. 2021, 17, 2050–2068. [Google Scholar] [CrossRef]
- Dastjerdi, P.; Pourfaraji, S.M.; Shayesteh, H.; Maghsoudi, M.; Saeidi, S.; Narimani Davani, D.; Masouri, M.M.; Parhizkar Roudsari, P.; Ojaghi Shirmard, F.; Ebrahimi, P.; et al. The role of bariatric surgery in hypertension control: A systematic review and meta-analysis with extended benefits on metabolic factors. BMC Cardiovasc. Disord. 2025, 25, 213. [Google Scholar] [CrossRef]
- Ashrafian, H.; le Roux, C.W. Metabolic surgery and gut hormones—A review of bariatric entero-humoral modulation. Physiol. Behav. 2009, 97, 620–631. [Google Scholar] [CrossRef]
- Faramia, J.; Ostinelli, G.; Drolet-Labelle, V.; Picard, F.; Tchernof, A. Metabolic adaptations after bariatric surgery: Adipokines, myokines and hepatokines. Curr. Opin. Pharmacol. 2020, 52, 67–74. [Google Scholar] [CrossRef]
- Alqunai, M.S.; Alrashid, F.F. Bariatric surgery for the management of type 2 diabetes mellitus-current trends and challenges: A review article. Am. J. Transl. Res. 2022, 14, 1160–1171. [Google Scholar]
- Sandoval, D.A.; Patti, M.E. Glucose metabolism after bariatric surgery: Implications for T2DM remission and hypoglycaemia. Nat. Rev. Endocrinol. 2023, 19, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.D.; Borradaile, K.E.; Sanders, M.H.; Millman, R.; Zammit, G.; Newman, A.B.; Wadden, T.A.; Kelley, D.; Wing, R.R.; Pi-Sunyer, F.X.; et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: The Sleep AHEAD study. Arch. Intern. Med. 2009, 169, 1619–1626. [Google Scholar] [CrossRef]
- Ge, X.; Wang, Z.; Song, Y.; Meng, H. Effect of bariatric surgery on mitochondrial remodeling in human skeletal muscle: A narrative review. Front. Endocrinol. 2024, 15, 1488715. [Google Scholar] [CrossRef] [PubMed]
- Al Oweidat, K.; Toubasi, A.A.; Abu Tawileh, R.B.; Abu Tawileh, H.B.; Hasuneh, M.M. Bariatric surgery and obstructive sleep apnea: A systematic review and meta-analysis. Sleep Breath 2023, 27, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Gulinac, M.; Miteva, D.G.; Peshevska-Sekulovska, M.; Novakov, I.P.; Antovic, S.; Peruhova, M.; Snegarova, V.; Kabakchieva, P.; Assyov, Y.; Vasilev, G.; et al. Long-term effectiveness, outcomes and complications of bariatric surgery. World J. Clin. Cases 2023, 11, 4504–4512. [Google Scholar] [CrossRef]
- Aguas-Ayesa, M.; Yárnoz-Esquíroz, P.; Olazarán, L.; Gómez-Ambrosi, J.; Frühbeck, G. Precision nutrition in the context of bariatric surgery. Rev. Endocr. Metab. Disord. 2023, 24, 979–991. [Google Scholar] [CrossRef]
- Budny, A.; Janczy, A.; Szymanski, M.; Mika, A. Long-Term Follow-Up After Bariatric Surgery: Key to Successful Outcomes in Obesity Management. Nutrients 2024, 16, 4399. [Google Scholar] [CrossRef]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg. Obes. Relat. Dis. 2017, 13, 727–741. [Google Scholar] [CrossRef]
- Rajabi, M.R.; Rezaei, M.; Abdollahi, A.; Gholi, Z.; Mokhber, S.; Mohammadi-Farsani, G.; Abdoli, D.; Mousavi, S.D.; Amini, H.; Ghandchi, M. Long-term systemic effects of metabolic bariatric surgery: A multidisciplinary perspective. Heliyon 2024, 10, e34339. [Google Scholar] [CrossRef]
- Athanasiadis, D.I.; Martin, A.; Kapsampelis, P.; Monfared, S.; Stefanidis, D. Factors associated with weight regain post-bariatric surgery: A systematic review. Surg. Endosc. 2021, 35, 4069–4084. [Google Scholar] [CrossRef]
- Hatami, M.; Pazouki, A.; Hosseini-Baharanchi, F.S.; Kabir, A. Bariatric Surgeries, from Weight Loss to Weight Regain: A Retrospective Five-Years Cohort Study. Obes. Facts 2023, 16, 540–547. [Google Scholar] [CrossRef]
- Noria, S.F.; Shelby, R.D.; Atkins, K.D.; Nguyen, N.T.; Gadde, K.M. Weight Regain After Bariatric Surgery: Scope of the Problem, Causes, Prevention, and Treatment. Curr. Diabetes Rep. 2023, 23, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Duran, P.; Garrido, B.; Parra, H.; Hernández, M.; Cano, C.; Añez, R.; García-Pacheco, H.; Cubillos, G.; Vasquez, N.; et al. Weight Regain after Metabolic Surgery: Beyond the Surgical Failure. J. Clin. Med. 2024, 13, 1143. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Klemensberg, J.; Klein, L.V.; Urbach, D.; Bell, C.M. Comparison of public and private bariatric surgery services in Canada. Can. J. Surg. 2011, 54, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Rodrigues, D.; Gomes, L.; Carrilho, F. Short- and long-term mortality after bariatric surgery: A systematic review and meta-analysis. Diabetes Obes. Metab. 2017, 19, 1223–1232. [Google Scholar] [CrossRef]
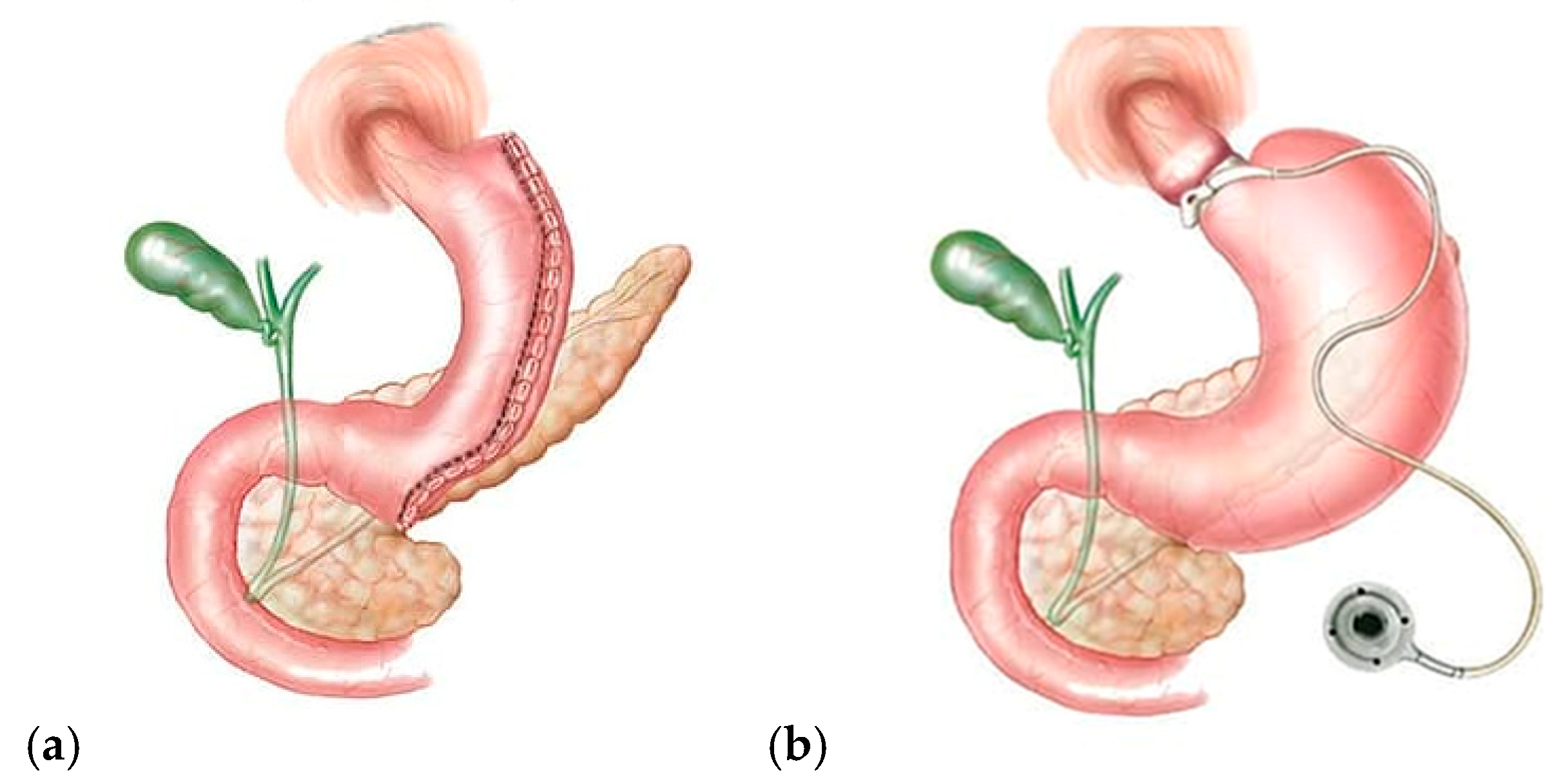
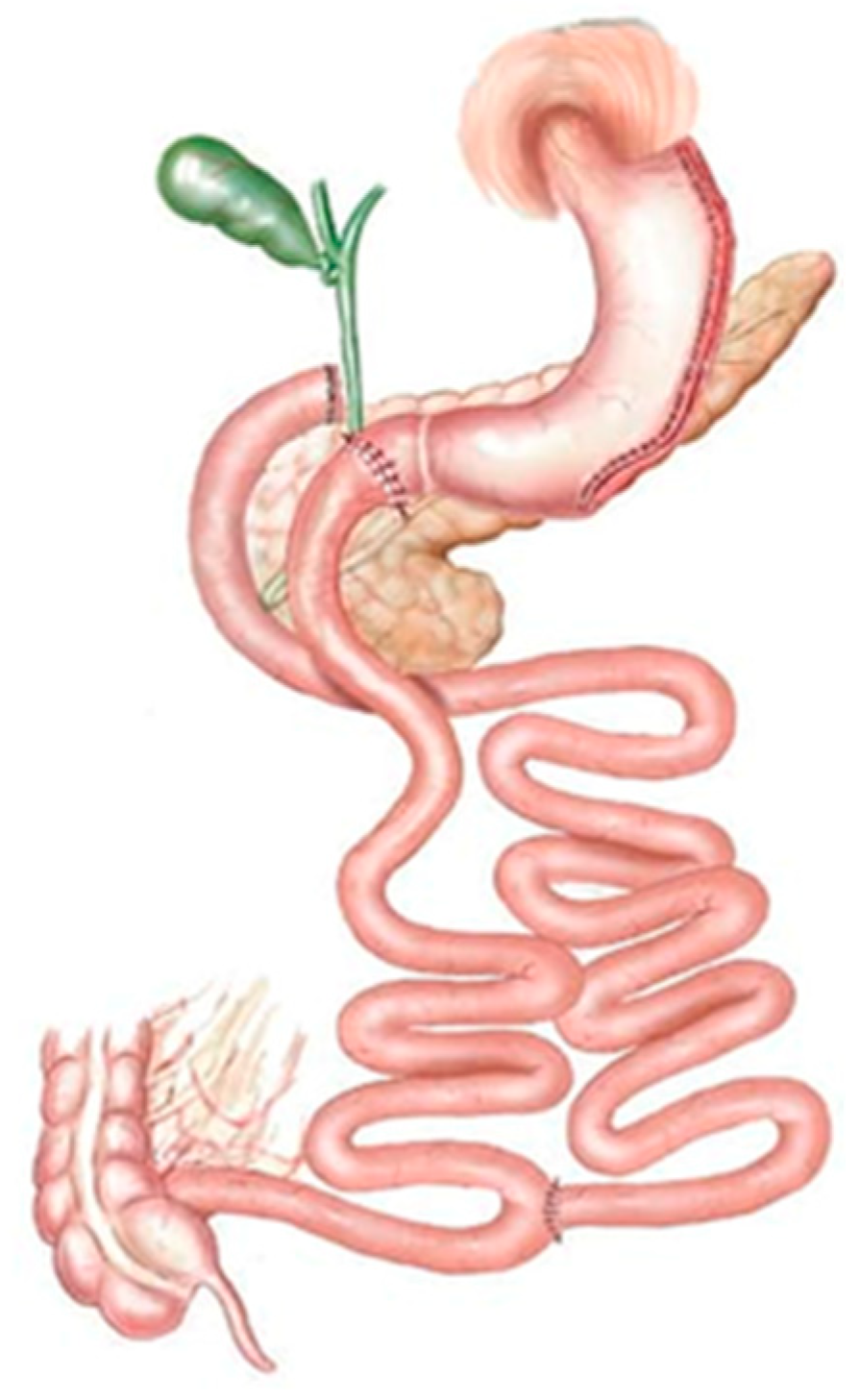
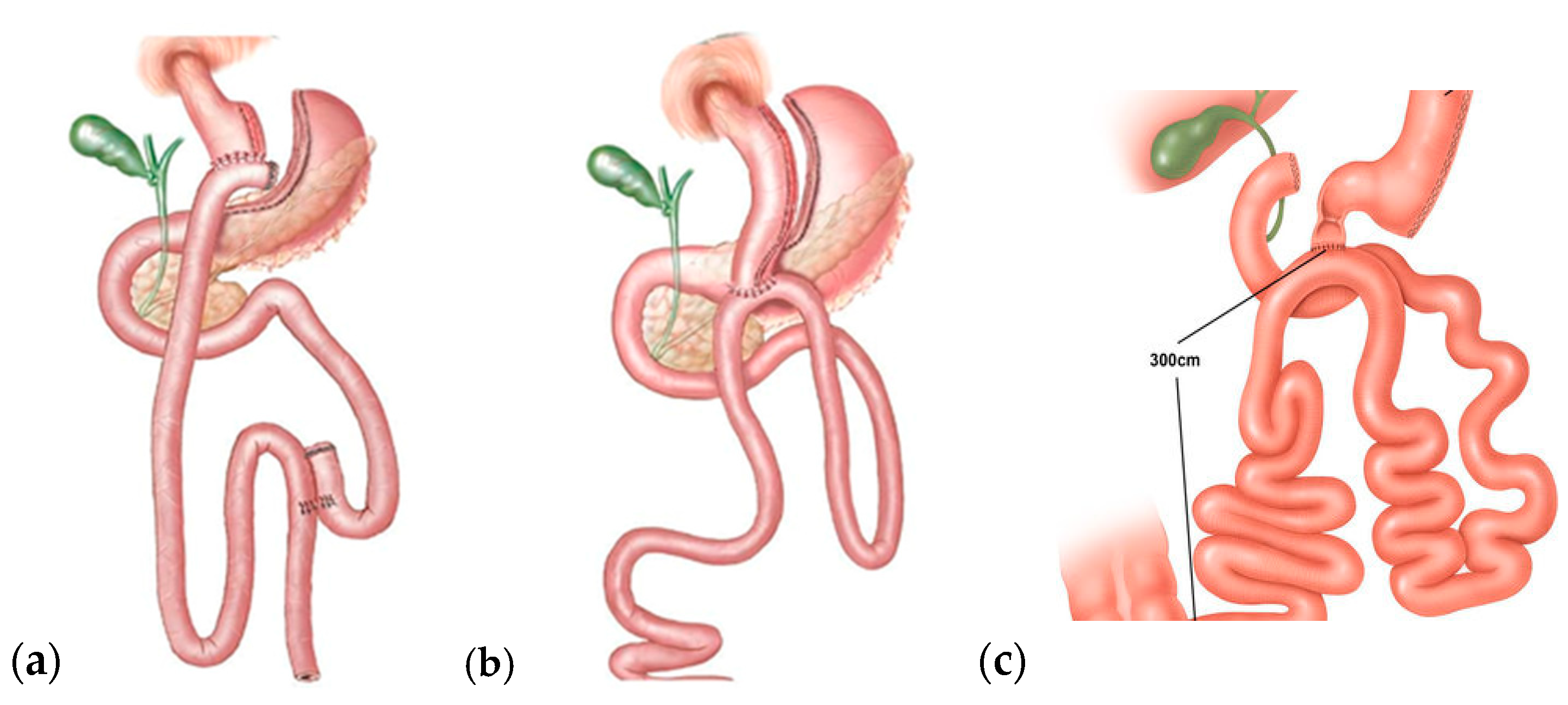
| Restrictive | Malabsorptive | Restrictive–Malabsorptive |
|---|---|---|
| Sleeve gastrectomy (SG) | Biliary pancreatic diversion with duodenal switch (BPD-DS) | Roux-en-Y Gastric Bypass (RYGB) One-Anastomosis Gastric Bypass (OAGB) |
| Laparoscopic gastric banding (LGB) | Ileal jejunal bypass | Single-Anastomosis Duodenal–ileal Bypass with Sleeve Gastrectomy (SADI-S). |
| Procedure | Mechanisms | Benefits | Complications |
|---|---|---|---|
| Sleeve Gastrectomy (SG) | Restriction: resection of a significant portion of the stomach, leaving a tubular structure, similar to a “sleeve,” which drastically reduces gastric capacity. Hormonal effect: a reduction in ghrelin and an increase in GLP-1, CCK, and PYY. | Simpler and less invasive procedure. Lower risk of short- and long-term complications. | Vomiting. Regurgitation or gastroesophageal reflux. Esophagitis. Suture dehiscence. |
| Roux-en-Y Gastric Bypass (RYGB) | Gastric partitioning with ileogastric and jejunoileal anastomosis (variable common segment): gastric restriction, malabsorption, dumping syndrome, and a reduction in ghrelin and appetite. | Significant and lasting weight loss. Improvement in comorbidities (T2DM, hypertension, and OSAS) | Infections, bleeding, intestinal obstructions, and protein malnutrition. Risk of vitamin and mineral deficiencies. Bone demineralization, diarrhea, flatulence, and chronic malabsorption. Vomiting and regurgitation. |
| One-Anastomosis Gastric Bypass (OAGB) | Tubular gastrectomy (approximately 60 mL capacity) with a single gastro-jejunal anastomosis (duodenal bypass) usually 150–200 cm from the duodenum (non-standardized measurement): gastric restriction, malabsorption, and effects on metabolism (increases insulin sensitivity). | Technically simpler. Shorter procedure times. Faster recovery. Effective weight loss. Improvement in obesity-related conditions, such as T2DM. | Comparable to RYGB in the long term; inferior in the post-operative period. |
| Single-Anastomosis Duodenal–Ileal Bypass with Sleeve Gastrectomy (SADI-S) | It combines sleeve gastrectomy and the duodeno–ileal bypass: vertical gastric resection, resection of the duodenum at the post-pyloric level, and anastomosis of the first duodenal segment with the ileum 300 cm from the ileocecal valve. Mainly indicated for the treatment of “complex” bariatric patients (BMI ≥ 50 kg/m2, metabolic patients, and revision surgery). | Billroth II duodenojejunal anastomosis results in a lower incidence of bile reflux thanks to the post-pyloric reconstruction of the single anastomosis. The presence of a single anastomosis reduces operating time, anesthesia time, and the likelihood of post-operative complications. | A low rate of early complications, provided that it is performed in referral centers. The common segment of 300 cm is associated with fewer long-term nutritional and malabsorption complications. |
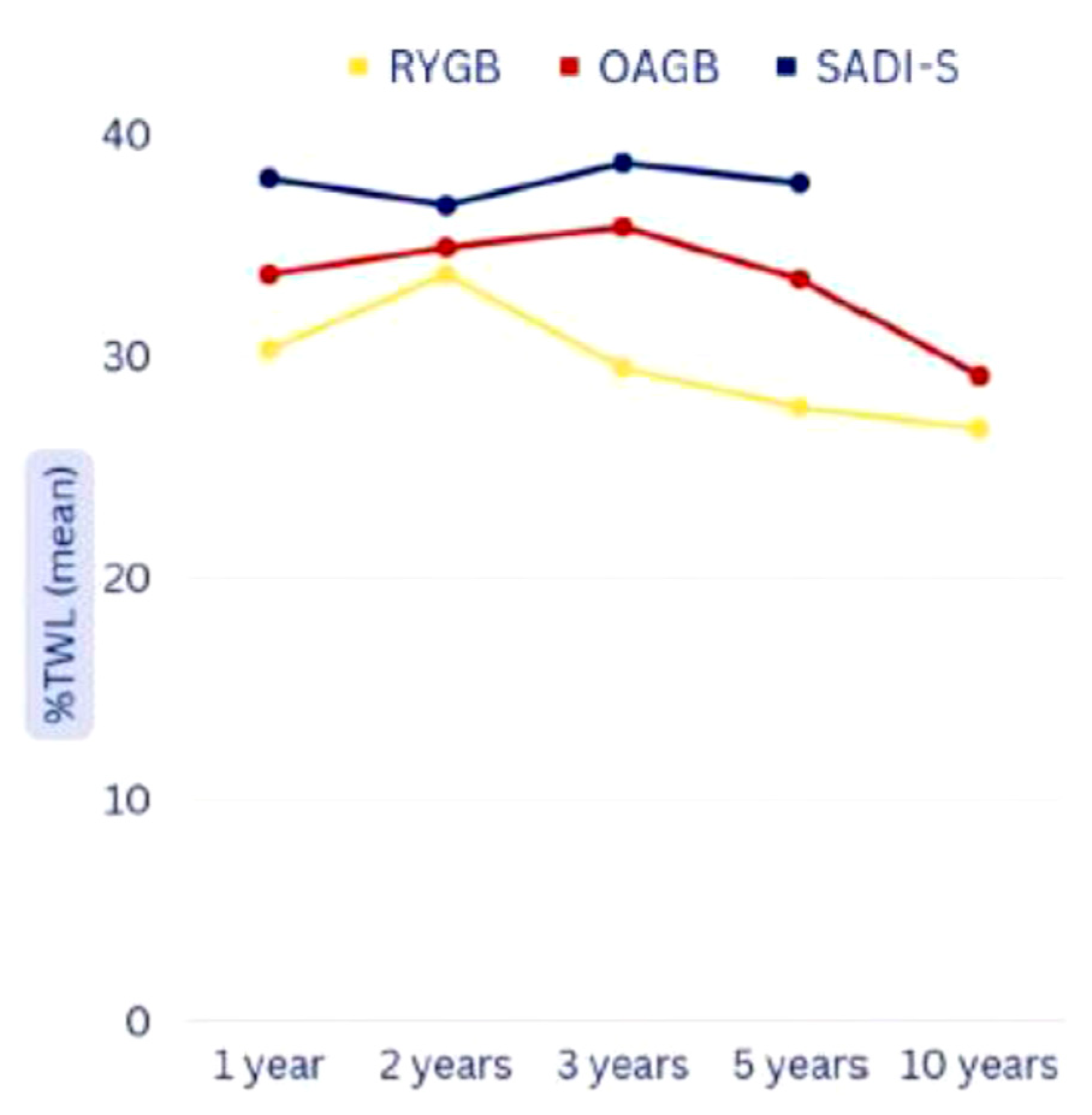
| Weight Loss | Duration | RYGB (N Studies) | OAGB (N Studies) | SADI-S (N Studies) |
|---|---|---|---|---|
| %TWL (median—SD) | 1 year | 30.28 ± 4.12 (5) | 33.69 ± 1.35 (4) | 38 (1) |
| 2 years | 33.68 ± 1.67 (5) | 33.68 ± 1.67 (5) | 36.8 (1) | |
| 3 years | 29.45 ± 2.55 (4) | 35.82 ± 1.68 (3) | 38.7 (1) | |
| 5 years | 27.67 ± 3.56 (3) | 33.46 ± 1.26 (2) | 37.8 (1) | |
| 10 years | 26.7 (1) | 29.1 (1) | - |
| Complications | RYGB (N Studies SD) | OAGB (N Studies SD) | SADI-S (N Studies SD) |
|---|---|---|---|
| Early complications | 8.06 ± 0.14 (5) | 8.12 ± 1.50 (4) | 11.8 ± 2.29 (3) |
| Late complications | 48.94 ± 40.96 (3) | 12.39 ± 4.39 (2) | 22.9 (1) |
| Mortality | 0.07 ± 0.11 (11) | 0.09 ± 0.18 (8) | 0.2 ± 0.28 (3) |
| Bariatric Surgery: The State of the Art |
|---|
| The number of surgeries is increasing every year. |
| The performance of RYGB is on a downward trend. |
| Sleeve gastrectomy is the most commonly used procedure, but it is decreasing. |
| OAGB is significantly on the rise. |
| Type of Surgery | Main Complications |
|---|---|
| Sleeve Gastrectomy | Gastroesophageal reflux disease (GRD), vomiting, and dumping syndrome. |
| Roux-en-Y Gastric Bypass (RYGB), One-Anastomosis Gastric Bypass (OAGB), and Single-Anastomosis Duodenal–Ileal Bypass with Sleeve Gastrectomy (SADI-S) | Protein malnutrition. Lipid malabsorption, chronic diarrhea, and flatulence. Bone demineralization. Dumping syndrome and GRD. Iron deficiency anemia or megaloblastic anemia due to folate and/or vitamin B12 deficiency. Dysvitaminosis with Wernicke’s encephalopathy (thiamine deficiency), neuropathy (B-group deficiency), and night blindness (vitamin A deficiency). Intestinal dysbiosis and alterations in the microbiota. |
| Late Complications | Prevention | Treatment |
|---|---|---|
| GRD | Dietary precautions (avoid large meals and limit fatty or spicy foods). | Antisecretory therapy. |
| Dumping syndrome | Dietary and behavioral modifications (small, frequent meals). | Fractionated diet without simple sugars. |
| Protein malnutrition | High-protein diet. | Oral protein supplementation or parenteral nutrition. |
| Lipid malabsorption and chronic diarrhea | Dietary modifications to limit excessive fat intake. | Pancreatic enzymes to improve lipid digestion (pancrelipase). |
| Gut dysbiosis and alterations in microbiota | Dietary adjustments and probiotics. | Probiotics and prebiotics to restore gut microbiota balance. |
| Iron deficiency anemia | Regular intake of a multivitamin supplement specific for bariatric surgery plus periodic oral or intravenous martial supplementation if necessary (18–60 mg/die). | Oral supplementation should be increased to provide 150–200 mg of elemental iron daily to amounts as high as 300 mg 2–3 times daily (intravenous iron infusion if oral administration is insufficient or poorly tolerated). |
| Megaloblastic anemia caused by folate and/or vitamin B12 deficiency | Regular intake of a multivitamin supplement specific for bariatric surgery plus periodic oral or im supplementation if necessary. - B12 supplementation: 350–500 mcg/day per os sublingual; 1000 mcg/month im. - Folic acid supplementation: 0.4–0.8 mg/day per os. | Administration of oral supplements or intramuscular injections of vitamin B complex: cobalamin 1000 mcg/day and/or folate 1000 mcg/day to achieve normal levels and then resume recommended dosages to maintain normal levels. |
| Bone demineralization | Monthly monitoring of ionized calcium, serum 25 OH vitamin D, 24 h calcium, and phosphate excretion; annual BMD evaluation; and serial PTH testing. Supplementation of vitamin D with 3000 IU/day until blood levels of 25 (OH) D are greater than sufficient (30 ng/mL). The appropriate dose of daily calcium from all sources varies by surgical procedure: BPD-DS: 1800–2400 mg/day; LGB, SG, and RYGB: 1200–1500 mg/day. | Administration of vitamin D per os and/or calcium supplementation per os/iv with the following doses: Vitamin D3 at least 3000 IU/day and as high as 6000 IU/day, or 50,000 IU vitamin D2 1–3 times weekly. Repletion of calcium deficiency varies by surgical procedure: BPD-DS: 1800–2400 mg/day calcium; LGB, SG, and RYGB: 1200–1500 mg/day calcium. |
| Dysvitaminosis with Wernicke’s encephalopathy (thiamine deficiency), neuropathy (group-B deficiency), and night blindness (vitamin A deficiency) | Serial monitoring of serum values and regular intake of a multivitamin supplement specific for bariatric surgery. | Corrective intravenous infusion (oral supplementation at the maximum dosage in less severe forms). |
| Public Health | Private Clinics | |
|---|---|---|
| Access to care | Patient choice often not in line with guidelines (GLs). | Compliance with LG. |
| Waiting times | Waiting times in the private sector are generally shorter. | Waiting times are longer. |
| Quality of facilities and care | It does not guarantee a multidisciplinary approach to candidate selection and, above all, to post-operative follow-up. | It ensures a multidisciplinary intervention both before and after surgery. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedesco, S.; Campelli, N.; Lunetti, S.; Nicolai, G.; Marmorale, C.; Nicolai, A.; Taus, M. Bariatric–Metabolic Surgery: The State of the Art and the Management of Complications. Dietetics 2025, 4, 49. https://doi.org/10.3390/dietetics4040049
Tedesco S, Campelli N, Lunetti S, Nicolai G, Marmorale C, Nicolai A, Taus M. Bariatric–Metabolic Surgery: The State of the Art and the Management of Complications. Dietetics. 2025; 4(4):49. https://doi.org/10.3390/dietetics4040049
Chicago/Turabian StyleTedesco, Silvia, Nadia Campelli, Stefano Lunetti, Giulia Nicolai, Cristina Marmorale, Albano Nicolai, and Marina Taus. 2025. "Bariatric–Metabolic Surgery: The State of the Art and the Management of Complications" Dietetics 4, no. 4: 49. https://doi.org/10.3390/dietetics4040049
APA StyleTedesco, S., Campelli, N., Lunetti, S., Nicolai, G., Marmorale, C., Nicolai, A., & Taus, M. (2025). Bariatric–Metabolic Surgery: The State of the Art and the Management of Complications. Dietetics, 4(4), 49. https://doi.org/10.3390/dietetics4040049




