Abstract
Selenium (Se) has important anti-inflammatory and antioxidant activities, plays an important role in the immune system through redox balance, and is part of selenoproteins. In patients who are critically ill, Se supplementation causes alterations in inflammatory markers such as procalcitonin, leukocyte count, albumin, prealbumin, C-reactive protein (CRP), inflammatory cytokines, and cholesterol. The decrease in Se levels leads to a reduction in the levels of various selenoenzymes, in particular glutathione peroxidase and selenoprotein P. These antioxidant selenoproteins play a protective role against the lipoperoxidation of cell membranes and also participate in the process of regulating the inflammatory response. Currently, there are no conclusive data that allow us to affirm the existence of a significant reduction in mortality with the use of Se in intensive care. Selenium nanoparticles (SeNPs) can be used as dietary supplements or therapeutic agents due to their low toxicity and better bioavailability compared to traditional Se supplementation. In this review, we focus on the current state of research on SeNPs and their anti-inflammatory and antioxidant properties as a therapy for patients who are seriously ill, without the toxic effects of other Se species.
Keywords:
selenium; critical illness; inflammation; oxidative stress; selenoproteins; nutrients; nanoparticles; sepsis 1. Introduction
Selenium (Se) is a mineral found in the soil that occurs naturally in water and some foods. Se is a reactive nonmetal or metalloid located in Block P, Period IV, and Group 16 of the Mendeleev periodic table of elements, with atomic number 34 and a molecular weight of 78.971 Da. The oxidation states of Se are −2, 0, +4, and +6, implying that it is prone to releasing or accepting pairs of electrons. Se exists in two different forms: organic and inorganic. Organic forms of Se are present as selenocysteine and selenomethionine in the human body. Inorganic forms such as selenite and selenate accumulate in plants through the soil. Selenite is predominantly found in acidic soils, while the oxidized form, selenate, is found in alkaline soils. Selenate is considered the most soluble form and is easily absorbed by plants [1]. Se has the ability to combine with other minerals and elements such as sulfur, copper, silver, nickel, and lead. Se ranks 67th among the most abundant elements in the Earth’s crust. Se dietary figure can be found in inorganic, organic, and Se-enriched yeast forms, so absorption and metabolism will differ from one form to another. The bioavailability of inorganic Se may be lower than that of organic Se (Figure 1).
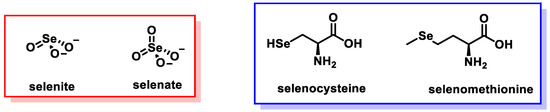
Figure 1.
Se ingested in the diet.
The human body cannot synthesize Se; therefore, daily supplementation is necessary to meet the body’s Se needs, because it is one of the most necessary micronutrients for humans and animals. Se is co-translationally incorporated into the polypeptide chain as part of the naturally occurring amino acid number 21, selenocysteine, which is encoded in the mRNA by a UGA triplet. This is an important difference between Se and most trace elements, which serve their function as enzymatic cofactors. Inorganic Se accumulates primarily in plants through the sulfur assimilation pathway, while animals and humans consume it in the form of vegetables, meats, and dietary supplements. Se has antioxidant, antimutagenic, and anticancer properties, can act against microbes and parasites, has anti-inflammatory effects, participates in metabolism, growth, and development, protects organs from oxidative stress, affects immune function, and improves fertility (Figure 2).
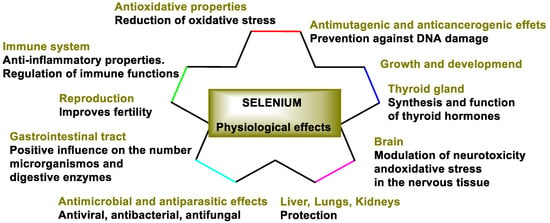
Figure 2.
Physiological effects of Se.
1.1. Se Deficiency
Se deficiency causes cardiovascular diseases, infertility, myodegenerative diseases, and cognitive impairment, among others [2,3]. The levels of Se in the body are highest in the kidneys, followed by the liver and spleen [4]. Daily intake of Se is highly recommended to maintain natural metabolism and homeostasis in the human body. A low Se content in the diet can lead to a selenoprotein deficiency, leading to human diseases such as Keshan disease, Kashin–Beck disease, endemic myxedematous cretinism, and male infertility [5,6,7,8,9,10]. Most Se-containing enzymes use the nucleophilic and reducing properties of the selenolate (Sec-Se–) form of selenocysteine to perform redox reactions. After oxidizing, the oxidation state of the resulting selenic acid (Sec-SeOH) is usually returned to selenolate by reduction with glutathione or a Cys residue that resolves into the enzyme.
The recommended intake levels of Se according to the European Food Safety Authority are as follows: women (60 μg/day), men (70 μg/day), pregnant women (65 μg/day), and lactating women (75 μg/day) [11]. Seafood and meats are the first to be included among Se-rich foods. Other Se-rich foods include muscle meats, cereals, grains, and dairy products. The amount of Se in drinking water is not nutritionally significant. Se-enriched functional foods, such as milk, meat and even eggs, are now commercially available in more than 25 countries around the world [12,13].
1.2. Manifestations of Se Toxicity
The excess of Se compounds can accumulate in cells and perform redox cycles with intracellular thiols, which leads to a state of oxidative stress (OS) evidenced by the manifestation of toxic effects. The most common symptoms of Se poisoning are alopecia, brittle and discolored nails, dermatitis if topical exposure took place, peripheral neuropathy, nausea, diarrhea, fatigue, irritability, and garlic-like odor of the breath [1]. The Food and Nutrition Board of the Institute of Medicine suggests that the upper limit of Se intake be 400 μg/day.
For the application of Se supplementation to patients, serum Se measurements should be performed before, during, and after Se supplementation. An excessive concentration of tyrosine hydroxylase (TH) does not directly cause endocrine disruptions in its own synthesis or increase the risk of type 2 diabetes. However, TH plays a crucial role in catecholamine biosynthesis, which can indirectly affect insulin secretion and glucose metabolism [14,15]. Excessive concentration of tyrosine hydroxylase (TH) is not directly related to selenium (Se) toxicity. In fact, research suggests that selenium deficiency, rather than toxicity, can lead to increased TH activity and expression in the nigrostriatal system [16].
1.3. Significant Chemical Differences Between Sulfur and Se
Sulfur and Se have very similar physical and chemical properties [17], but almost all chemical reactions involving Se are faster than those with sulfur. Se can act as a nucleophile and electrophile and shows better redox properties than sulfur [18]. The outer valence electrons of Se are looser than those of sulfur, and for this reason, Se is a better nucleophile and reacts with reactive oxygen species (ROS) faster than sulfur, but not forming the π bond in the Se-O bond implies that Se oxide can be reduced much more easily than S-oxides. The combination of these properties means that replacing sulfur with Se in nature results in a Se-containing biomolecule that resists oxidation.
Another important difference is found when comparing selenocysteine and cysteine: the pKa value of Cys is 8.3, while that of Sec is 5.2, which implies that, at a neutral pH, the thiol of Cys is mostly in its neutral form (protonated), while the selenol group of Sec is negatively charged, as selenolate (Figure 3). Enzymes containing Sec in the active site are catalytically more efficient than homologues containing Cys [19].
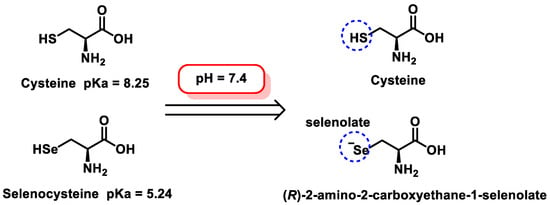
Figure 3.
Selenocysteine and cysteine at neutral pH values.
2. Se Metabolites
The biological activity of Se depends on its metabolites and their availability, accumulation, absorption capacity, and excretion in the body. The Se obtained through one’s diet has to be transformed by the body mainly in the form of SeMet. It is introduced into the human body or in animals through the following soil–plant–food chain. Plants absorb Se from the soil in the form of selenate or selenite and synthesize SeMet. Vertebrates obtain Se from the diet in the form of SeMet and other Se amino acids, including Sec and its methylated forms, depending on its content in the food/feed components [20]. The absorption of Se in the human body depends on the chemical nature of the compounds consumed. This absorption occurs primarily in the duodenum and small intestine. The metabolic pathways between different types of Se compounds are different, and this gives rise to several Se metabolites, which determine the efficacy and use of Se (Figure 4).
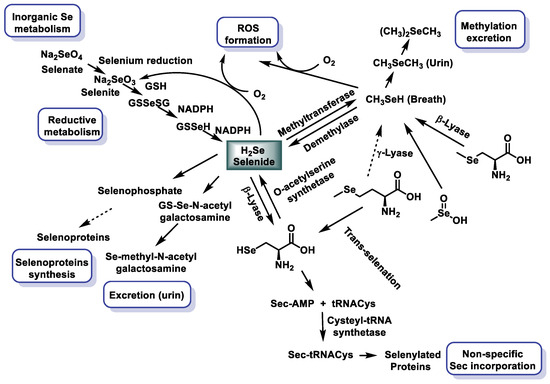
Figure 4.
Se metabolism of the most studied Se compounds [21,22].
Plant-derived SeMet is used directly for protein synthesis. The Se released from SeMet and other compounds participates in the synthesis of Sec that will generate the selenoproteins that contain Sec. H2Se is one of the fundamental intermediates in the generation of Sec-tRNA. The excess of Se produced is excreted from the body in the urine as trimethylSe ion [23] and Se sugars [24]; the other possibility of elimination is through the respiratory tract in the form of dimethylselenide formed by the methylation of Se [25].
3. Selenoproteins in Humans
Se is a key element that acts as a cofactor and coenzyme at the catalytically active site of numerous selenoproteins and enzymes in the human body, protecting cells and tissues from oxidative damage and stress [26,27]. Selenoenzymes inhibit pro-inflammatory cellular metabolisms and protect cellular components against oxidation [28]. Selenoproteins are of fundamental importance for optimal human and animal health. The selenoprotein family, in humans, includes the following members: glutathione peroxidases (GPX1–GPX4 and GPX6), thioredoxin reductases (TXNRD1–2), thioredoxin–glutathione reductase (TXNRD3), iodothyronine deiodases (DIO1–3), selenophosphate synthetase (SEPHS2), 15 kDa selenoprotein (SELENOF), selenoprotein H (SELENOH), selenoprotein I (SELENOI), selenoprotein K (SELENOK), selenoprotein M (SELENOM), selenoprotein N (SELENON), selenoprotein O (SELENOO), selenoprotein P (SELENOP), methionine sulfoxide reductase B1 (MSRB1), selenoprotein S (SELENOS), selenoprotein T (SELENOT), selenoprotein V (SELENOV), and selenoprotein W (SELENOW) [29,30] (Figure 5).
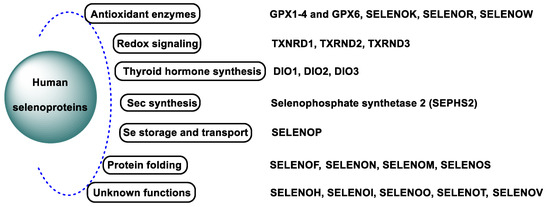
Figure 5.
Human selenoproteins.
Se is essential for life and is necessary for the proper enzymatic function of selenoproteins [31].
3.1. Antioxidant Selenoproteins
Se is a crucial component of various selenoenzymes, including glutathione peroxidases (GPXs), thioredoxin reductases (TrxRs), and deiodinases (DIOs), which are employed in biochemical processes involved in cellular defense mechanisms, especially those with antioxidant functions [32]. The human genome encodes 25 selenoprotein genes, collectively known as the selenoproteome. These selenoproteins are of central importance for the maintenance of redox homeostasis and cellular antioxidant defense systems and thus influence numerous ROS-dependent biological processes such as signal transduction, cell proliferation, differentiation, aging, ferroptosis, and the regulation of the immune response. The most important antioxidant and redox-active selenoproteins include glutathione peroxidases (GPxs), thioredoxin reductases (Txnrds), and methionine sulphoxide reductase (MsrB1). In addition, the selenoproteins P, S, and T contribute to modulating the ROS level. Of these enzymes, GPx, DIO, TrxR, and Msr are the best studied.
Glutathione Peroxidases (GPxs)
The GPX family consists of eight members (GPX1–GPX8) [33,34], five of which incorporate Se (GPx1–GPx4 and GPx6). These enzymes play a key role in mitigating oxidative damage by catalyzing the reduction of hydroperoxides and hydrogen peroxide to less harmful products. Most vertebrate GPX enzymes utilize selenocysteine (SeCys) at their active sites for catalytic activity. These selenoproteins reduce H2O2 or organic hydroperoxides into water or corresponding alcohols [35,36], limiting their toxicity and maintaining cellular redox balance [37]. All GPX enzymes work via a ping–pong mechanism characterized by sequential oxidation and reduction steps. In the first phase, the reduced form of the enzyme (GPX-SeH) is oxidized to selenic acid (GPX-SeOH) by reaction with hydroperoxide substrates [38]. In the subsequent reduction phase, the selenic acid is regenerated into its active selenol form by interaction with two equivalents of reduced glutathione (GSH). The first GSH molecule reacts with GPX-SeOH to form a Se-glutathione intermediate, producing water [39]. The second GSH molecule then reduces this intermediate to regenerate selenol, forming glutathione disulfide (GSSG). (Figure 6).
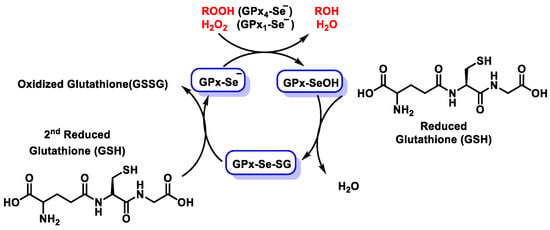
Figure 6.
Diagram of the catalytic mechanism of glutathione peroxidases.
Interestingly, three members of the GPX family (GPX5, GPX7, and GPX8) lack selenocysteine in their active sites and instead rely on cysteine residues to be active. Among the GPX enzymes, GPX1 is particularly notable for its efficiency in peroxide detoxification in the cytosol of mammalian cells [40]. Se availability is a critical factor for GPX1 expression and activity, as Se deficiency leads to a marked decrease in mRNA, protein concentration and enzymatic activity in cultured cells and tissues [41,42]. In 2020, an extensive review was published comparing the history, biochemistry, genetics, and biomedical relevance of each GPX enzyme [39].
There are three human iodothyronine deiodases (DIO1, DIO2, and DIO3) that contain Sec [43], are found in the thyroid gland and other tissues, and catalyze the removal of iodine atoms in the phenolic ring (activation pathway) or in the tyrosyl ring (inactivation pathway) from T4 to T3 [44] (Figure 7). The human iodothyronine deiodases (DIO1, DIO2, and DIO3) are selenoproteins that facilitate thyroid hormone metabolism by catalyzing the removal of iodine atoms. These reactions take place either on the phenol ring (activation pathway) or on the tyrosyl ring (inactivation pathway) and convert thyroxine (T4) into the active hormone triiodothyronine (T3). All three deiodases require selenocysteine for their catalytic function. DIO1 and DIO3 are localized at the plasma membrane, while DIO2 is associated with the endoplasmic reticulum [45]. Functionally, DIO1 and DIO3 are expressed in tissues such as the thyroid gland, liver, and kidneys and mediate both activation and inactivation pathways. In contrast, DIO2 exclusively catalyzes the deiodination of phenol rings and contributes to T3 production in the nervous system [46].
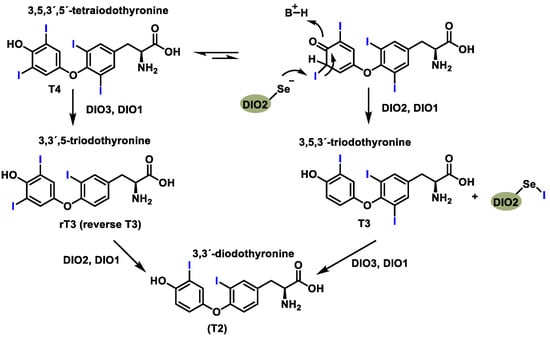
Figure 7.
Reactions catalyzed by specific deiodinase isoforms.
The human thioredoxin reductase (TrxR) family comprises three selenoproteins: a cytosolic form, a mitochondrial form, and a testis-specific enzyme. TrxR plays a central role in maintaining thiol-disulfide redox homeostasis by catalyzing the reduction of thioredoxin (Trx), a small redox-active protein [47]. As a selenoprotein, TrxR is sensitive to Se availability, and its activity is directly influenced by dietary Se intake (Figure 8).
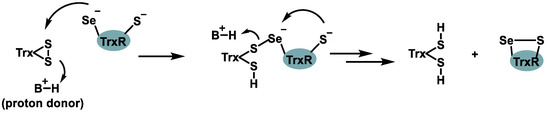
Figure 8.
Chemical process mediated by TrxR.
In organs such as the liver, kidneys, and lungs, TrxR activity decreases under conditions of Se deficiency and increases with high dietary Se intake. In contrast, enzymatic activity in the brain remains relatively unaffected by Se deficiency because, unlike other tissues, Se concentration in the brain is tightly regulated by robust homeostatic mechanisms.
The human methionine sulfoxide reductase (Msr) family comprises four enzymes, only one of which contains selenocysteine (MsrB1) [48]. MsrB1 is stereospecific for methionine R-sulfoxide and contributes to actin polymerization by catalyzing its reduction on actin [49]. The other members of this family, MsrB2 and MsrB3, use cysteine residues instead of selenocysteine at their active sites.
4. Critically Ill Patients: Sepsis
Severe inflammatory response syndrome (SIRS) is characterized by the increased production of ROS, which contributes to tissue destruction [50], oxidative damage, and the activation of macrophages. This condition is associated with reduced Se levels [51]. Patients who are severely ill, particularly those with sepsis, often have low Se levels, which have been associated with increased tissue damage and organ dysfunction [52,53]. In contrast, a short observational study reported that Se supplementation improved clinical outcomes, mitigated oxidative damage, and increased glutathione peroxidase (GPX) and thioredoxin reductase (TRXR) activity [54]. The presence of selenocysteine at the active sites of GPX and TRXR enables these selenoproteins to facilitate simultaneous oxidation/reduction reactions [55].
Sepsis-related organ dysfunction remains the leading cause of mortality in intensive care units (ICUs). Sepsis is a multifaceted clinical syndrome with physiologic, pathologic, and biochemical disturbances caused by an infectious agent, leading to a dysregulated immune response resulting in life-threatening organ dysfunction. It is a major global health problem that affects people of all ages and contributes significantly to morbidity and mortality. If untreated or left untreated, SIRS can progress through the stages of sepsis and severe sepsis to septic shock [56,57] (Figure 9).
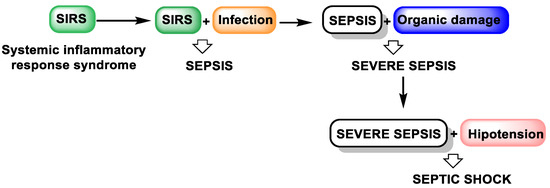
Figure 9.
The evolution of untreated SIRS.
Over the years, the definition of sepsis has evolved to reflect its complex pathophysiology and biochemical dysregulation [58]. According to the third international consensus (Sepsis-3), sepsis is defined as “life-threatening organ dysfunction caused by a dysregulated host response to infection” [59]. This condition is associated with widespread changes in organ function at the cellular and molecular level (Figure 10).
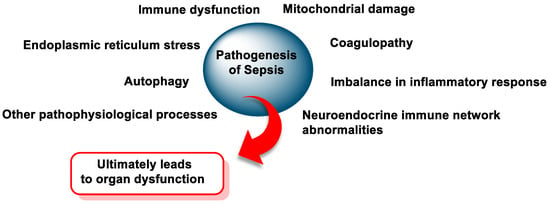
Figure 10.
The pathogenesis of sepsis.
Critical illnesses such as sepsis and systemic inflammatory response syndrome (SIRS) [60] are characterized by oxidative stress, hyperinflammation, and dysfunction of the mitochondria or immune system [61]. Oxidative stress (OS) plays an important role in the development and complications of these diseases [62]. Sepsis, in particular, is characterized by uncontrolled inflammation and excessive production of reactive oxygen and nitrogen species (RONS), which the host immune system is unable to neutralize [59,63,64]. This dysregulation leads to cell and tissue damage, immune system dysfunction, and, ultimately, the development of multiple organ failure syndrome [65,66,67,68].
Sepsis is recognized worldwide as a life-threatening condition with a high mortality rate. Current treatment strategies include the administration of broad-spectrum antibiotics, fluid resuscitation, and organ support through mechanical ventilation and hemodynamic stabilization [69,70]. Despite these measures, clinical management remains suboptimal due to challenges such as an unbalanced inflammatory response, the rise of antibiotic resistance, and a lack of specific therapeutic interventions.
There is, therefore, an urgent need to develop effective treatments for sepsis. Research efforts are primarily focused on (i) modulating the systemic inflammatory response, (ii) treating coagulopathy and immune dysfunction, (iii) restoring the balance between pro-inflammatory and anti-inflammatory mechanisms, and (iv) improving patient outcomes and prognosis.
4.1. Biomarkers of Sepsis
In septic patients, and those with SIRS and COVID-19 disease, a decrease in Se levels has been described [71,72]. The effects of Se supplementation on inflammatory markers (Figure 11), including procalcitonin, leukocyte count, albumin, prealbumin, CRP, inflammatory cytokines, and cholesterol, in critically ill patients is highly important.
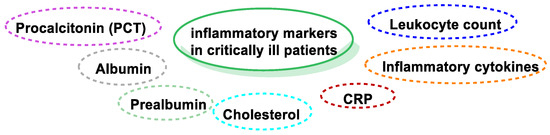
Figure 11.
Inflammatory and oxidative markers in critically ill patients.
In the intensive care unit (ICU), procalcitonin (PCT) is used as a biomarker for the diagnosis of sepsis, severe sepsis, and septic shock. In healthy patients, plasma levels of PCT have a concentration of ≤0.2 ng/mL, and the increase in plasma levels to values ≥ 0.5 ng/mL is interpreted as abnormal and suggestive of sepsis. In these patients, PCT is more accurate and may be preferable to CRP, since the specificity and sensitivity of CRP are lower, and peak levels may not correspond to the severity of inflammation [73,74].
High-dose Se supplementation in sepsis provided a more rapid decrease in PCT levels between the baseline and days 7 and 14 [75]. Treatment with 1000 μg of seleniobel on the first day and continuing up to 200 μg after one week has been found to decrease PCT levels on day 10 in patients with SIRS/sepsis [76]. In contrast, Sakr et al., in 2014, indicated elevated PCT levels in severe sepsis after receiving 1000 μg of sodium selenite intravenously [77]; this might have been due to pro-oxidant properties at high doses of sodium selenite [78]. Woth et al., in 2014, found no significant change in PCT values after treatment with sodium selenite (1000 μg/2 h) in patients with severe sepsis [79].
4.2. Se Supplementation in Critically Ill Conditions
In recent years, the effects of Se compounds have been evaluated in ICU patients, particularly those with systemic inflammation, sepsis, and severe sepsis [80].
Critically ill patients are at an increased risk of plasma micronutrient deficiency, which may result from decreased plasma carrier proteins [81]. Se has anti-inflammatory and antioxidant effects, and a link has been found between Se deficiency and the severity of critical illness. Decreased Se levels have been observed in septic patients, as well as in those with SIRS and coronavirus disease. To meet the daily needs of Se, additional supplementation with Se is necessary, especially for those who are deficient. Se compounds, both inorganically and organically (selenite, selenomethionine, and Se-methylselenocysteine), have been used for decades to prevent Se deficiency in animals and humans [82,83]. However, these Se supplements, especially the inorganic ones, are often toxic when taken above their nutritional dose.
Clinical results in patients with sepsis treated with Se supplementation are contradictory. Li et al., in 2022, found that Se supplementation correlated with a reduced duration of vasopressor therapy time, a shorter length of stay in the ICU, a shorter hospital stay time, and fewer respiratory tract infections [84]. Djalalinia et al., in 2021, detected that supplemental Se with a daily dose of 200 μg/d in patients with metabolic diseases could be clearly connected with a decrease in high-sensitivity C-reactive protein, indicating a reduced degree of inflammation in the body [85]. In the same study, an increase in the serum CRP level was found in patients who received regular daily Se supplements.
Puze et al. concluded, in their study, that there was no evidence of Se being associated with better outcomes among patients in intensive care units [86].
There are studies evaluating the efficacy of daily oral or intravenous Se supplementation in adult patients in intensive care units (ICUs). The results found indicate that Se supplementation (i) did not improve or decrease total mortality and (ii) did not decrease the risk of new infectious complications, (iii) the length of hospital stay in the ICU, (iv) the occurrence of new renal dysfunction events, (v) overall survival, (vi) nor the days of mechanical ventilation [87,88].
5. Se and Antioxidant Markers
As mentioned above, Se, when incorporated into selenoproteins, plays a crucial role in two major antioxidant systems: (i) the thiol-redox system, which includes glutathione (GSH), glutaredoxin, GSH reductase, and glutathione peroxidase (GSH-Px), and (ii) the thioredoxin (Trx) system, which includes thioredoxin, Trx peroxidase, and thioredoxin reductase (TrxR) [89].
Glutathione peroxidase (GSH-Px) plays an important role in scavenging free radicals and detoxifying lipid hydrogen peroxide and hydroperoxides [90]. Changes in GSH-Px levels are closely related to the extent of oxidative damage to tissues [91]. In diseases such as systemic inflammatory response syndrome (SIRS) and sepsis, a reduced Se level is often associated with a decrease in GSH-Px activity. These findings suggest an inverse correlation between these markers and the severity of clinical outcomes, highlighting their potential predictive value in SIRS [52,92]. Se supplementation has been shown to increase GSH-Px levels, highlighting its efficacy as a micronutrient in restoring antioxidant defenses and mitigating complications associated with critical illness.
Supplementation with organic Se compounds increases the activity of members of the glutathione peroxidase family, enhancing antioxidant activity [93]. While most GPX enzymes reduce small organic hydroperoxides, they are generally ineffective against lipid hydroperoxides or cholesterol. GPX4 is unique in the GPX family in that it is able to reduce lipid hydroperoxides and complex cholesterols [94], even when these substrates are embedded in biological membranes [95]. Although glutathione (GSH) serves as the primary reducing substrate for mammalian GPX enzymes, GPX4 has the special ability to utilize other thiol-containing proteins in addition to GSH [96].
6. Nanoparticles for Sepsis Treatment
For the diagnosis and treatment of sepsis, nanoparticles (NPs) have been proposed. NPs have adequate surface area, composition and size, a longer half-life and a differential biodistribution profile compared to the homologous free drug; in addition, NP surfaces can also be functionalized with multiple ligands to interact with a specific type of cells, all of which help their longer half-life and biodistribution, customized according to the specificity of the target, compared to conventional dosage forms [97,98,99,100] (Figure 12). All of this means that NPs can be used to prevent and treat sepsis.
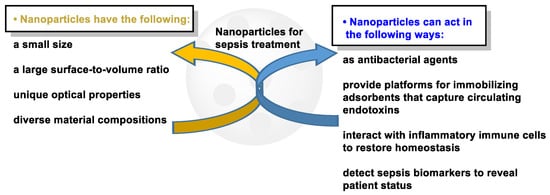
Figure 12.
Characteristics and performance of nanoparticles in the treatment of sepsis.
6.1. SeNPs
The benefit of nano-Se (Nano-Se) is the potential to use Se in its zero-oxidation state (Se0). Se compounds exist in nature in four degrees of oxidation: selenate (Se6+), selenite (Se4+), selenide (Se2−), and elemental Se (Se0) [101]. The first three forms are toxic even at low concentrations, and only the last one is insoluble in water and, in essence, non-toxic [102]. To achieve the desired therapeutic effect, Se-based nanoparticles can be created, which can alleviate toxicity issues. When comparing the use of traditional organic and inorganic Se compounds, the NPs of SeNP show numerous advantages, such as low toxicity, high degradability, and important anticancer, antimicrobial, and antiviral activities [26,103,104,105,106]. SeNPs could potentially be useful in suppressing viral epidemics, such as COVID-19, in addition to their antibacterial and antiparasitic uses.
Se nanoparticles (SeNPs) have attracted interest for their use as food supplements or therapeutic agents. In vitro and in vivo studies have demonstrated the therapeutic power of SeNPs, which reduce the toxicity of Se and could thus improve its therapeutic properties [107]. SeNPs are three times less toxic than the amino acid selenomethionine and seven times less toxic than sodium selenite [108]. NPs of Se exhibit excellent stability and drug encapsulation capacity. Therefore, they serve as powerful nanocarriers for the treatment of cancer, inflammation, and infections.
The physical characteristics of SeNPs include the following: (i) they are elemental particles of Se at the nanometer scale, (ii) possess a bright red color, and have aroused worldwide attention due to their unique properties and excellent biological activities [83,109,110,111]. TheSeNPs can scavenge free radicals in vitro, serum oxidant state, and Se concentration in vivo. They also increase the activities of selenoenzymes, and SeNPs are able to inhibit the growth of microorganisms [112].
The antioxidant effect exerted by SeNPs is mediated by the enhancement of GPx, superoxide dismutase (SOD), and catalase (CAT) activities, as well as by the direct scavenging activity of free radicals. In addition, SeNPs inhibit lipid peroxidation by decreasing TBAR thiobarbituric acid [113].
Comparing Au and Ag metal NPs with SeNPs, the latter are more economical and can be integrated with other biological agents to improve their biological properties. For example, Vahdati et al., in 2020, demonstrated the synergistic antimicrobial effect of SeNPs and lysozymes [114].
6.2. SeNP Synthesis Methods
The design and synthesis of SeNPs require the control of a number of characteristics, such as size, shape, surface functionality, and composition, which can directly affect the properties of the NPs. This goal can be achieved by choosing the synthesis method, controlling the concentration of precursors, the pH, the reaction temperature, and the brew time.
There are many ways to synthesize SeNPs, which can be summarized in three major techniques: physical, chemical, and biological [115,116,117] (Figure 13).
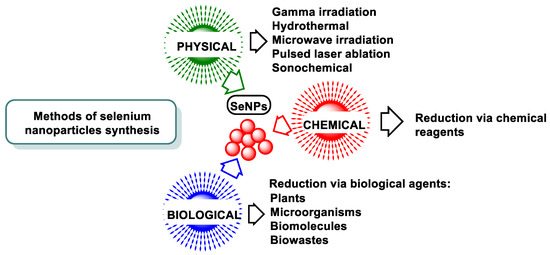
Figure 13.
Various techniques for the synthesis of SeNPs.
6.2.1. Chemical Methods
Chemical reducing agents are used in the synthesis of SeNPs by chemical reduction. This method can be classified according to the source of energy or the apparatus used for the reaction. Researchers have mainly reported hydrothermal, microwave, and sonochemical methods.
The advantages of chemical methods are (i) a large production in a relatively short time and (ii) the control of shape, size, and distribution. The main disadvantages are the use of harmful reducing agents and the generation of chemical pollution caused by these reactions, which has limited their large-scale production and use for the synthesis of metallic NPs [118,119,120,121] (Figure 14). Depending on the reducing agent used, SeNPs have different morphology and, therefore, different antibacterial and antioxidant properties [122].
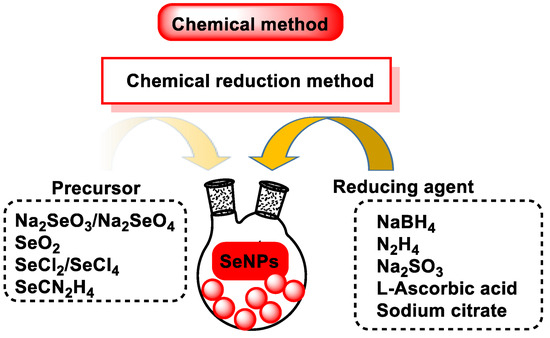
Figure 14.
Schematic representation of the experimental procedure used to make SeNPs.
The most general chemical method of preparing SeNPs is the reduction of a solution of selenious acid with ascorbic acid (Figure 15).
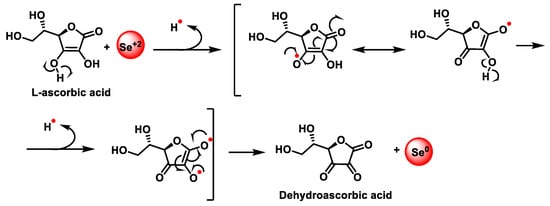
Figure 15.
Redox reaction for the synthesis of SeNPs. Mechanism of reduction of sodium selenite by ascorbic acid.
6.2.2. Physical Synthesis
The physical methods for SeNP synthesis are carried out by exciting and releasing electrons such as microwave or gamma irradiation, sonochemistry, hydrothermal, UV radiation, and laser ablation of crystalline Se pellets [26,123]. Physical methods are not widely used to produce SeNPs. The disadvantages of physical techniques include (i) high energy consumption, (ii) the ease with which samples are contaminated and (iii) the creation of highly variable particle sizes. The advantage of physical synthesis over chemical synthesis is that it is environmentally friendly.
6.2.3. Biological Synthesis
The synthesis of SeNPs by biological reduction methods includes the reduction of various organic/inorganic Se compounds by biological agents such as bacteria, fungi, algae, and higher plants. It is an environmentally friendly alternative to chemical and physical methods, produces NPs with diverse biological properties, and does not require high energy costs, the use of hazardous and toxic substances, or complex equipment [124,125,126,127]. Biological synthesis can be carried out directly in living organisms or with the help of bioreagents extracted from them (Figure 16). These biocomposites can also act as glazing agents capable of (i) preventing agglomeration, (ii) providing functional groups for drug binding [128,129], (iii) stabilizing NPs, and (iv) presenting a specific effect.
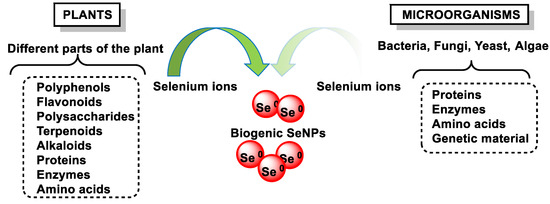
Figure 16.
Synthesis of SeNPs using microorganisms and plant-based materials.
Biogenic SeNPs are biocompatible and stable due to the natural coatings provided by biomolecules that avoid the use of chemical stabilizers, prevent the aggregation of particles, improve their pharmacological activity, and protect them against physical and chemical degradation [130,131].
The use of plant extracts is even more cost-effective than the use of bacteria or fungi, as bacterial culture and isolation require techniques, expertise, special equipment, and the increased costs associated with maintaining cell cultures.
Synthesis methods based on the use of plant-derived extracts is a green, one-step, eco-friendly, bioreductive, and cost-effective approach, requiring shorter reaction times and the use of smaller amounts of solvents. A significant number of plants have been used for the synthesis of SeNPs, such as hawthorn fruit [132], lemon leaf [133], Hibiscus sabdariffa leaf [134], Clausena dentata leaf extract [135], Theobroma cacao L. bean husk extract [136], and gallic acid (GA) from various fruits and plants [137].
Plant extracts contain different terpenes, flavonoids, tannins, coumarins, cinnamic acid, phenolic acid, vitamins, sterols, polysaccharides, enzymes, proteins, etc., which can act as reducing and stabilizing agents. Recently, some review articles highlighted the importance of plant-based SeNPs, their simplicity in synthesis, and their feasibility for biological applications [124,126,138].
7. Biomedical Applications of SeNPs
The therapeutic potential of SeNPs has been studied in several immunological and oxidative stress-related diseases. They can perform their biological function as anticancer, antioxidant, anti-inflammatory, and antidiabetic agents. In addition, SeNP can be used as carriers for targeted drug delivery to the desired tissue [139,140,141,142,143,144,145].
SeNPs have great potential for biomedical applications due to their remarkable properties and have many useful therapeutic properties (Figure 17).
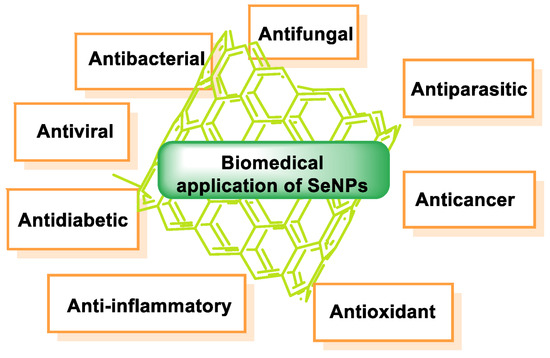
Figure 17.
Biomedical application of SeNPs.
8. SeNP Stabilization and Functionalization Approaches
8.1. Capping Agents for SeNPs
The application of SeNPs is limited by the following facts: (i) economical and environmentally friendly ways are needed to synthesize SeNPs [146,147,148,149], and (ii) naked SeNPs are normally enlarged, aggregated due to high surface energy, and eventually transformed into gray/black analogs that are thermodynamically stable but biologically inert [150,151]. An increase in the size of SeNPs decreases the activities of the SeNPs in terms of the removal of multiple radical species in vitro [152], the enhancement of Se accumulation, and the activity of GSH S-transferase in vivo [153].
Protective agents can modify the characteristics of NPs and make them appropriate candidates for biomedical use. These agents play a fundamental role in the prevention of overgrowth and aggregation of NPs, which is one of the main obstacles in the preparation of NPs. Protective agents stabilize the NP interface, where it is in contact with the surrounding environment, and are responsible for the physicochemical and biological properties of NPs. Compounds from different sources can be used as protective agents for the synthesis of SeNPs to improve their pharmacokinetics and pharmacodynamics. These templates contain a large number of hydroxyl, carbonyl, and other functional groups [127], allowing the templates to interact with SeNPs. Therefore, it is of great importance to develop stable and effective Se supplements.
The modification and functionalization of the surface of SeNPs has been carried out, for example, with polyphenols [154,155,156], polysaccharides [157,158,159,160,161,162,163], proteins [164], and surfactants [165].
Protective agents, based on their sources, are divided into bio-based and synthetic protective agents. Synthetic polymers and surfactants are used to stabilize NPs, but due to environmental problems and difficulties in disposal, bio-based protective agents and the biological synthesis of NPs have gained interest instead [166]. Natural stabilizers include carbohydrates (glucose, lactose, sucrose, fructose, cellulose, starch, and chitosan), proteins (collagen, enzymes, and albumin), amino acids (proteins and non-proteins), lipids, honey, nucleic acid (DNA), and biological extracts (plants, bacteria, viruses, and fungal extracts [167]).
8.1.1. Synthetic Capping Agents
Water-Soluble Synthetic Polymers
To prevent SeNP aggregation, water-soluble synthetic polymers such as polyvinyl alcohol (PVA), polyvinylpyrrolidone (PVP), polyethylene glycol 200 (PEG-200), polyethylenimine (PEI), and polyacrylic acid (PAA) have been commonly used as stabilizers for SeNPs [168,169,170] (Figure 18).
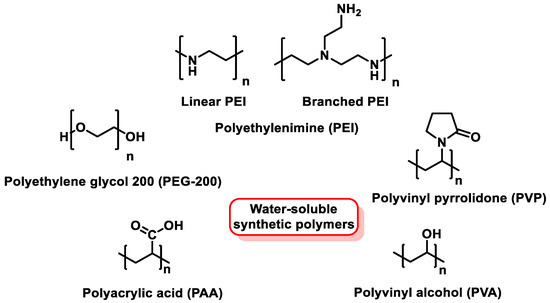
Figure 18.
Chemical structure of synthetic polymers used as SeNP stabilizers.
Shah et al. prepared SeNPs with sizes between 100 and 300 nm using PVA as a stabilizer [171]. They found that, in the absence of PVA, a black precipitate of Se was formed, which highlighted the efficacy of PVA as a stabilizer for the synthesis of SeNPs. They also found that increasing the concentration of PVA limited the aggregation of SeNPs and reduced their size. Selmani et al. used PVP as a stabilizer to prepare water-dispersible SeNPs, with a size of 70 to 80 nm [172] (Figure 19).
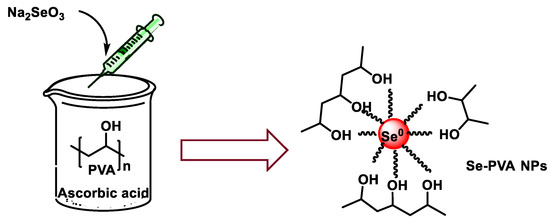
Figure 19.
Synthesis scheme of SeNPs stabilized with polymer vinyl alcohol (PVA).
These PVA-coated SeNPs (SeNP-PVAs) are stable and can be easily synthesized [173]. PEG is one of the most widely used polymers in the synthesis of NPs because it has high solubility in aqueous and organic solvents and can reduce the toxicity and immunogenicity of NPs [128]. The same solubility is found in polyvinylpyrrolidone (PVP).
Surfactant
Surfactants are compounds with high surface activity that can reduce the surface tension between a liquid and a solid. Chauhan et al., in 2019, modified the surface of SeNPs using three surfactants of variable nature, cationic (CTAB), anionic (SDS) and nonionic (Brij 58) [174]. The role of these surfactants is to prevent the tendency of synthesized NPs to agglomerate. Bartůněk et al., in 2015, prepared amorphous Se antimicrobial NPs stabilized with an odor-suppressing surfactant and non-toxic polysorbate 20 [175] (Figure 20).
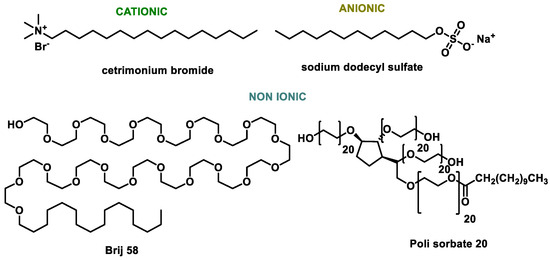
Figure 20.
Surfactant capping agents for SeNPs.
8.1.2. Bio-Based Capping Agents for SeNPs
The properties of SeNPs are highly influenced by the associated biomolecules [176]. In the biological synthesis of SeNPs, with the help of microorganisms and plants, the coating layer formed on the surface of NPs is the mixture of biomolecules containing proteins, polysaccharides, lipids, biosurfactants, nitrate reducers, and coenzymes. Bioactive polysaccharides derived from mushrooms, fruits, vegetables, traditional Chinese medicines, and some aquatic animals are among the most promising stabilizers of SeNPs due to their excellent biocompatibility and high bioactivity [177,178,179,180].
Polyphenol-Functionalized SeNPs
The antioxidant properties of SeNPs can be enhanced by combining them with polyphenols, giving SeNPs properties inherent to polyphenols, such as adhesion. Wang et al., in 2017, found that epigallocatechin gallate-modified SeNPs had improve oral stability and availability [181]. EGCG in EGCG-SeNPs has good stability as a dispersant.
Wang et al., in 2019, used gallic acid (GA) to reduce and modify SeNPs, with improved broad-spectrum antibacterial activity [182]. Kumari et al., in 2017, synthesized SeNPs loaded with curcumin (Cur@SeNPs) that presented a uniform size [183] (Figure 21).
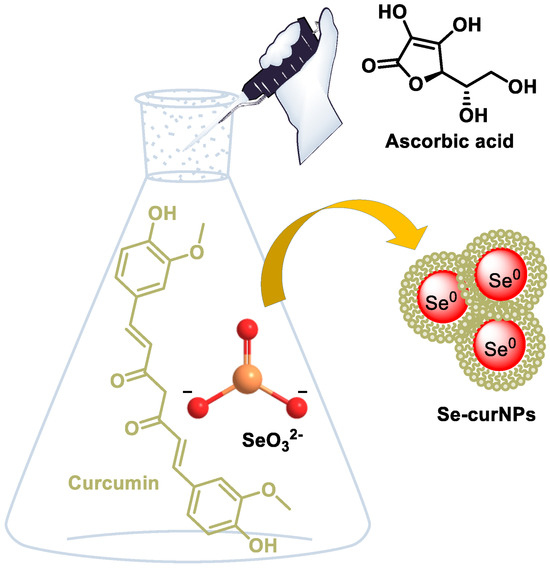
Figure 21.
Schematic representation for the synthesis of Se-curNPs.
Modifying SeNPs with resveratrol not only endowed the SeNPs with antioxidant activity but also improved their binding affinity to amyloid-β (Aβ) [184].
Polysaccharide-Functionalized SeNPs
Polysaccharides have different molecular weights, substitution of functional groups, and chain conformation, which makes them play an important role in the properties of SeNPs (Figure 22).
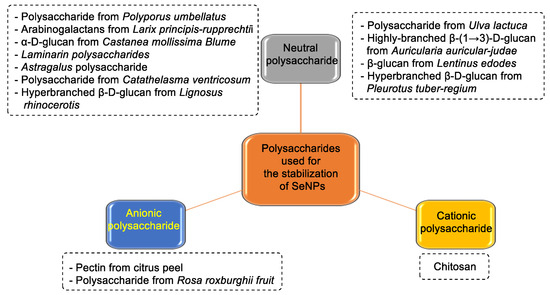
Figure 22.
Different polysaccharides used as protection in the synthesis of SeNPs.
Chen et al., in 2015, studied the antioxidant properties of SeNPs stabilized with chitosan and carboxymethylchitosan. The synthesis of CS-SeNPs and CCS-SeNPs was performed using sodium selenite as a precursor, ascorbic acid as a reducer, and potassium iodide as a stabilizer, under safe conditions [185]. The SeNP samples, after being synthesized, were purified by washing with water and ethanol, and, subsequently, the surface of SeNP was functionalized with CS and CCS (Figure 23).
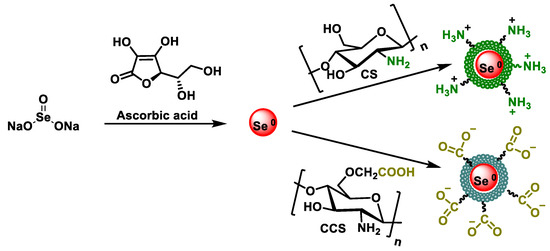
Figure 23.
Mechanism for the synthesis of SeNPs modified with chitosan (CS) and carboxymethyl chitosan (CCS).
Other procedures for preparing chitosan-modified SeNPs (polysaccharides) are shown in Figure 24A, where a solution of Na2SeO3 is added to the polysaccharide suspension, and then ascorbic acid is added to the mixture [186,187,188,189,190]. In other cases, such as in Figure 24B, the chitosan (polysaccharide) solution is first mixed with ascorbic acid, and only then is the Na2SeO3 solution added [191,192,193] (Figure 24).
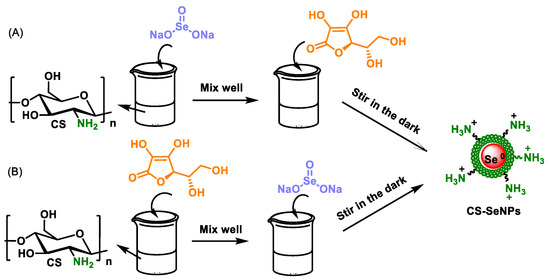
Figure 24.
Other procedures to prepare SeNP and chitosan (polysaccharide) compounds. (A) Na2SeO3 solution was first added into the chitosan solution, well mixed, and then ascorbic acid was added to reduce the precursor SeO32− to Se0 atoms (B) The solution of Na2SeO3 is subsequently added to the mixture of chitosan and ascorbic acid.
Zhang et al., in 2015, demonstrated that chitosan-SeNPs (CTS-SeNPs) with a particle size of 80 to 120 nm exhibited remarkable physicochemical stability after 30 days of storage [150]. Similarly, Yu et al., in 2012, reported that CTS-SeNPs with particle sizes below 180 nm remained stable for up to 60 days [194]. Hageman et al., in 2017, investigated the influence of pH (6–9) and temperature (20–50 °C) on the structure, morphology, and stability of biogenic SeNPs using advanced analytical techniques, including scanning electron microscopy, X-ray diffraction, and optical microscopy [195].
Chitosan, the only naturally occurring polysaccharide with a positive charge, is characterized by its excellent biodegradability and biocompatibility. Bai et al., in 2017, developed and synthesized a novel SeNP system called SeNP-M in a two-step process: (1) SeNP were synthesized in the presence of chitosan, and (2) the resulting CTS-SeNP were encapsulated in chitosan microspheres using a spray-drying technique to produce SeNP-M [196].
β-Glucan, another water-soluble polysaccharide derived from sources such as oats, barley, bacteria, yeast, algae, and fungi, exhibits physicochemical properties that depend on its origin. Bioactive templates have been used to develop simple, efficient, and environmentally friendly methods for dispersing and stabilizing SeNPs [197]. For example, Jia et al., in 2015, synthesized SeNPs stabilized with lentinan, which exhibited a more uniform particle size distribution and improved antitumor activity [198]. Sun et al., in 2023, investigated the anti-inflammatory properties of SeNPs stabilized with amino yeast glucan [199].
The interaction of polyphenols and polysaccharides with the surface of SeNPs increases the structural stability. Zhou et al., in 2022, used a water-soluble glucan derived from natural corn starch in combination with rosmarinic acid as a stabilizer to produce glucan-RA-SeNPs. This formulation showed high stability under different pH and temperature conditions [156] (Figure 25).
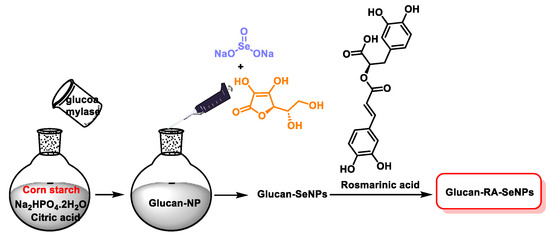
Figure 25.
Scheme of the preparation of glucan-RA-SeNPs.
Amino Acid
Li et al., in 2010, used l-cysteine to coat SeNPs. In their study, l-cysteine acted as a Na2SeO3-reducing agent and surface modifier. They obtained monodisperse and homogeneous spherical SeNPs [147]. Another amino acid used was l-asparagine. Yu et al., in 2016, showed that l-asparagine could produce spherical and monodisperse SeNPs with high antioxidant activity [200].
The interaction of the -NH3+ groups of the amino acids with the negatively charged SeNPs could be the main dispersion force of the NPs, thus controlling particle size, shape and agglomeration [201]. In addition, amino acids enhance the antioxidant properties and water dispersibility of nanomaterials, which are very important for their biological applications [202].
Proteins
Proteins are ideally suited as glazing agents for the production of highly stable SeNPs due to their various functional groups and their ability to form strong interactions with the surface of the NPs [203]. Commonly used proteins include albumin, keratin, fibroin, gelatin, and collagen, which are obtained from various animal and fungal sources. Due to its high content of sulfhydryl (-SH), amino (-NH2), and hydroxyl (-OH) groups, albumin can form strong bonds with SeNPs that stabilize particles and prevent aggregation [204]. Keratin, derived from wool, feathers, and other animal sources, provides structural strength and stability to NPs through its disulfide bonds and high amino acid content [205]. Fibroin, a silk protein obtained from silkworms or spiders, contributes to the stabilization of SeNPs through its crystalline structure and strong hydrophobic and hydrophilic domains [206]. Gelatine, derived from animal collagen by hydrolysis, is a flexible and biocompatible vitrification agent that can bind SeNPs via its reactive side groups. Its versatility makes it a widely used stabilizer in food and biomedical applications [207].
Collagen is a structural protein found in connective tissues and provides stability to SeNPs through interactions between its peptide chains and the surface of the NPs [208].
β-Lactoglobulin (Blg), a whey protein found mainly in the milk of cows and other ruminants, stands out as an exceptional in situ stabilizer for SeNPs. Its functional groups, including amino (-NH2) and hydroxyl (-OH) groups, interact strongly with the SeNP surface and form a robust stabilizing shell [209]. Blg provides biocompatibility, improves colloidal stability, and prevents the aggregation of NPs. Blg is also beneficial due to its amphiphilic nature, which facilitates interaction with both hydrophilic and hydrophobic environments [210]. This makes Blg-coated SeNPs particularly effective for biomedical applications, such as drug delivery and antioxidant therapies. In addition, the spherical structure enables the encapsulation of other bioactive molecules alongside the SeNPs, further enhancing their utility as multifunctional therapeutic agents [211].
Proteins derived from fungi, such as fungal mycelial proteins, are emerging as environmentally friendly and sustainable alternatives for SeNP stabilization. These proteins have a high affinity to SeNPs due to their different functional groups and their biocompatibility. Like animal proteins, fungal proteins contribute to the increased stability and antioxidant activity of SeNPs [212].
8.2. Polypeptides
Peptides and polypeptides serve as effective stabilizers and vitrifying agents for SeNPs, as they increase stability and confer antimicrobial properties. Huang et al., in 2020, synthesized SeNPs coated with the antimicrobial polypeptide ε-poly-l-lysine (ε-PL) in a one-step procedure [213] (Figure 26). ε-PL consists of 25–30 residues of L-lysine and is often used as a food additive due to its broad-spectrum antimicrobial activity [214]. The resulting SeNP-ε-PL showed improved colloidal stability and robust antimicrobial activity against both bacterial and fungal pathogens. Importantly, the risk of microbial resistance development was significantly lower compared to conventional antibiotics, likely due to the synergistic effect of SeNPs and the positively charged ε-PL coating.
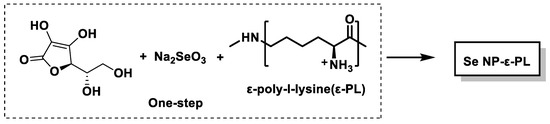
Figure 26.
Schematic of the preparation of Se NP-ε-PL.
This synergy results from the ability of ε-PL to disrupt microbial membranes through electrostatic interactions, which is further enhanced by the antimicrobial activity of SeNPs. The positively charged ε-PL coating facilitates binding to the negatively charged microbial cell membranes, enhancing the antimicrobial effect.
The antimicrobial peptide (AMP) UBI29-41, which is derived from the antimicrobial domain of ubiquicidin, a naturally occurring peptide found in humans and animals, has also been used to stabilize SeNPs, seeing as UBI29-41 can bind to the anionic regions of microbial cell membranes, significantly improving the stability and antimicrobial properties of SeNPs [215]. This AMP-coated SeNP formulation showed good biocompatibility and selective activity at infection sites, making it a promising candidate for combating bacterial infections.
Cyclic peptides were also used in SeNP synthesis. For example, NPs coated with a cyclic peptide containing five tryptophan (W), four arginine (R), and one cysteine (C) residues (W5R4C) have been developed as efficient drug transporters [216]. This peptide stabilizes the SeNPs through hydrophobic and electrostatic interactions, while its amphipathic nature enhances the NPs ability to penetrate microbial membranes.
In addition, tilapia polypeptide 1 (TP1), which is derived from tilapia fish, has been previously used to form pH-stabilized SeNPs (TP1-SeNPs) without the need for additional reducing agents. The stability of these NPs resulted from the electrostatic and hydrophobic interactions between TP1 and the SeNPs, enabling the production of biocompatible and environmentally friendly antimicrobial agents [217].
9. Treatment Effects of SeNPs in Sepsis
Excess ROS is the main factor leading to high mortality in local inflammation and sepsis. An efficient ROS scavenger needs to be developed in pro-inflammatory immune cells for the therapy of local inflammatory injury and sepsis. Excess ROS will selectively attack major organs and lead to organ dysfunction and even death [218,219,220]. Gao et al., in 2002, found that SeNPs have the capacity to eliminate hydroxyl radicals (⋅OH) [221].
At pH = 8.0, the redox potential of OH is more positive than SeO32−, meaning that HO can react with Se0 [222] (Figure 27).
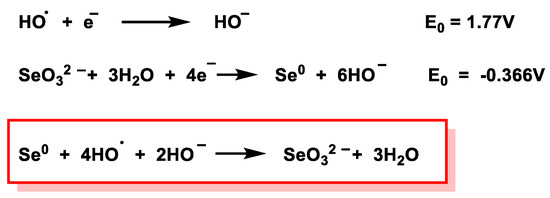
Figure 27.
Values of the redox potentials of HO. and Se0 at pH = 8.0.
Mesoporous silica NPs (MSNs), characterized by their large surface area, are used as a delivery system for the transport of Se compounds with GPx enzymatic activity. Chen et al., in 2021, engineered a mesoporous Se-hyaluronic acid nanoenzyme (MSe-HA NP) for local inflammatory injury and sepsis by targeting ROS clearance in inflammatory macrophages and found that HA effectively aided the NP MSe in the removal of ROS [223]. By injecting these mesoporous SeNPs into mice with sepsis, they found a higher survival rate and an improvement in organ dysfunction.
Huang et al., in 2018, synthesized nanocompound Se@pDA by combining Se and dopamine components, achieving synergistic antioxidation, and they were able to mimic intracellular enzymatic and non-enzymatic antioxidants [224]. The Se nanocomponent could serve as a model for mimicking an antioxidant enzyme, while polydopamine was able to mimic non-enzymatic antioxidant biomolecules in living systems. Se@pDAs were first obtained with SeNPs functionalized with polyvinylpyrrolidone (PVP-SeNP), and then polydopamine was added (Figure 28).
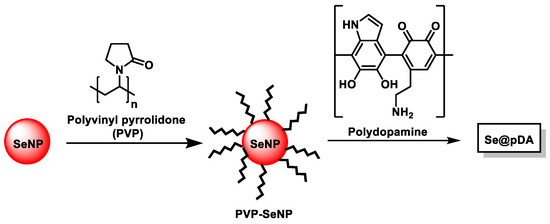
Figure 28.
Se@pDA synthesis.
Several nanozymes with enzyme-mimicking activities have been developed for the treatment of ROS-related diseases. Nanozymes combine the physicochemical properties of nanomaterials with the catalytic activity of natural enzymes. Nanoenzymes that mimic GPX and SOD may be a possible treatment to avoid the inflammatory microenvironment of sepsis.
For example, the mesoporous Se-hyaluronic acid nanozyme therapeutic system was developed to treat localized inflammation and sepsis by removing ROS [225].
Liu et al. developed multifunctional tannic acid-Zn-coated SeNPs (TZn@CSes), observing that they exhibited enzymatic activities similar to superoxide dismutase and glutathione peroxidase, to effectively neutralize several types of sepsis-induced ROS [225] (Figure 29).
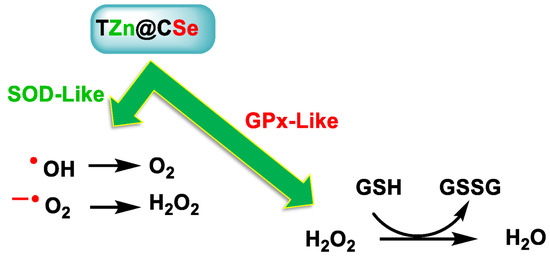
Figure 29.
TZn@CSe possesses enzymatic activity similar to SOD and GPX and removes various types of ROS.
TZn@CSes were prepared from SeNPs dispersed in chitosan and then coated with a metal–phenolic network (MNP) formed by tannic acid and zinc ions [225,226,227] (Figure 30).
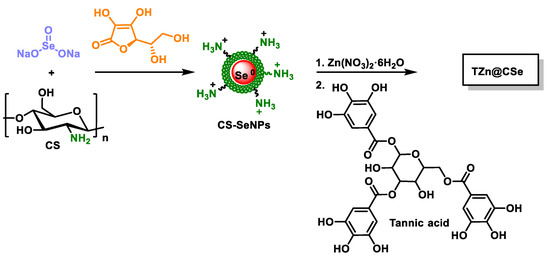
Figure 30.
Schematic representation for the synthesis of TZn@CSes.
Se-based multifunctional polyphenolic metal NPs (TZn@CSes) prevent pathological interactions between ROS and inflammation in sepsis. In addition, in vivo studies were conducted showing that sepsis survival rates using TZn@CSe increased by 50%, outperforming the first-line clinical drug Xuebijing (XBJ).
SeNPs not only possess enzymatic activities similar to SOD and GPX—i.e., are capable of removing various types of ROS and reducing intracellular OS levels—but they also alleviate cytokine storms in sepsis by reversing macrophage M1 polarization and reducing the release of inflammatory cytokines from sepsis.
10. SeNPs as a New Source of Se in the Human Diet
The selection of a suitable particle size and morphology of SeNPs is of crucial importance in the development of dietary products. Nano-sized Se particles offer significant advantages over the conventional Se compounds used in human nutrition [110]. These include high surface activity, a large specific surface area, a strong adsorption capacity, and numerous surfactant centers. SeNPs exhibit a pronounced size-dependent effect, with smaller particles exhibiting higher biological activity. With decreasing particle size, changes in the physical, chemical, and biological properties of SeNP are observed [228,229]. These size differences have been shown to influence the efficiency of free radical scavenging and DNA protection in vitro [152]. A size-dependent effect has also been observed in cellular uptake, where NPs with a size of 1 μm and 10 μm are absorbed 2.5 and 6 times less effectively, respectively, than those with a size of 100 nm [230,231].
The absorption of SeNPs in the gastrointestinal tract is influenced by physical and chemical barriers. These NPs cross the intestinal epithelium via two primary routes: intercellular (between neighboring cells) and transcellular (through the cells). Factors such as electrical charge, surface hydrophobicity, and particle size significantly influence gastrointestinal absorption. Hydrophobic SeNPs exhibit a higher absorption efficiency compared to hydrophilic particles, as the lipid composition of epithelial cell membranes favors hydrophobic interactions. In particular, 100 nm SeNPs are absorbed 15 to 250 times more efficiently than particles several micrometres in size [26,231,232,233].
The interaction of SeNPs with proteins in the gastrointestinal tract can alter their surface chemistry, leading to changes in charge and agglomeration state. In addition, pH fluctuations in the digestive environment can influence the agglomeration of NPs. For example, the incubation of SeNPs under simulated gastrointestinal conditions in both enzymatic and non-enzymatic media with pH values between 2 and 7.0 leads to size changes. Such transformations underscore the importance of understanding the physicochemical behavior of SeNPs under nutritional and gastrointestinal conditions.
11. Toxicity of SeNPs
SeNPs exhibit prooxidant properties similar to elemental Se and are therefore capable of generating ROS at high concentrations. Bioaccumulation can exacerbate this effect in various tissues, with the liver being particularly susceptible [234]. To date, toxicological evaluations of SeNPs have mainly focused on their effects on the antioxidant system, body weight, and bioaccumulation in critical organs such as the liver, kidneys, and heart. However, the interactions of SeNPs with the immune system, gastrointestinal tract, muscle bioaccumulation, and other indirect targets have only been explored to a limited extent [21].
SeNPs and NPs, in general, exhibit increased reactivity and better biodistribution compared to other forms of Se due to their large surface area and nanoscale dimensions. Toxicity studies consistently show that SeNPs are less harmful than inorganic Se compounds such as sodium selenite. In addition, their nanoscale properties ensure higher bioavailability [235]. Data published in the literature suggest that the toxicity of SeNPs depends on several interrelated factors, including particle size, chemical composition, dosage, and duration of exposure, all of which influence the biological response of the organism. Toxicological evaluations have shown that the toxicity of SeNPs is not only due to their pro-oxidant activities but also to their interactions with metabolic and molecular signaling pathways, such as those regulating apoptosis. In addition, the ability of small NPs to penetrate various tissues contributes to their biological effects [236].
The synthesis method also plays a decisive role in determining the toxicity of SeNPs. Biosynthesized SeNPs have shown lower toxicity in mouse models than chemically synthesized SeNPs, underlining the importance of production techniques in influencing their biological effects [237,238].
12. Conclusions
Se is an essential trace element in the diet. It is important for the functioning of the immune system and is a fundamental component of the active catalytic sites of several selenoproteins and enzymes in the body. It is also an antioxidant that protects cells from oxidative damage caused by reactive oxygen and nitrogen species (ROS and RNS) such as superoxide, hydrogen peroxide, hydroxyl radicals, nitric oxide, and peroxynitrite, while also playing an important role in iodine metabolism.
Se is an essential trace element that plays a crucial role in various physiological activities and has a major impact on human health. A deficiency in Se can lead to the development of various diseases, while its excessive consumption can cause toxicity.
Patients who are severely ill and have systemic inflammatory response and multiple organ dysfunction are characterized by an inflammatory state with OS and a decrease in serum levels of Se and selenoenzymes GPx and SePP. Several clinical trials have been conducted in which the effects of parenteral Se supplementation using different doses of Se were investigated. The results in terms of mortality, infections, and days of mechanical ventilation and hospitalization were inconclusive.
SeNPs represent the best way to regulate supplementation as they have low toxicity. are readily available and absorbed by the system, and have high antioxidant activity. All these properties make them suitable for numerous applications. In the search to improve the efficacy and reduce the toxicity of SeNPs, extensive research on SeNPs has been promoted to take advantage of their therapeutic properties. Current in vitro and in vivo studies have demonstrated the therapeutic effect of SeNPs due to their antidiabetic, anticancer, and antimicrobial properties. SeNPs can be modified in a variety of ways, including biomolecule conjugation, size control, and surface functionalization.
The synthesis of SeNPs using ecological methods and bio-based materials is more acceptable than chemical methods in terms of their safety. For instance, biomaterials can act as reducing agents and stabilizers. Se nanoenzymes have shown to be excellent ROS scavengers. Mimicking GPX and SOD would constitute an effective treatment to avoid the inflammatory microenvironment of sepsis.
Finally, there is a need to explore effective therapies that focus on combating inflammation and ROS to increase the survival rate of sepsis patients.
Author Contributions
Conceptualization, C.M.C.A. and C.A.J.; investigation, E.B.M., C.M.C.A. and C.A.J.; writing—review and editing, E.B.M., C.M.C.A., C.A.J., J.M.P.d.l.L. and E.P.-L.; and supervision, E.B.M., C.M.C.A., C.A.J. and J.M.P.d.l.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Nuttall, K.L. Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006, 36, 409–420. [Google Scholar] [PubMed]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef]
- Shreenath, A.P.; Hashmi, M.F.; Dooley, J. Selenium Deficiency. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Zachara, B.A.; Pawluk, H.; Bloch-Boguslawska, E.; Sliwka, K.M.; Korenkiewicz, J.; Skok, Z.; Ryć, K. Tissue level, distribution, and total body selenium content in healthy and diseased humans in Poland. Arch. Environ. Health 2001, 56, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, T.; Li, Q.; Li, D. Prevention of Keshan Disease by Selenium Supplementation: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2018, 186, 98–105. [Google Scholar] [CrossRef]
- Liu, H.; Yu, F.; Shao, W.; Ding, D.; Yu, Z.; Chen, F.; Geng, D.; Tan, X.; Lammi, M.J.; Guo, X. Associations Between Selenium Content in Hair and Kashin-Beck Disease/Keshan Disease in Children in Northwestern China: A Prospective Cohort Study. Biol. Trace Elem. Res. 2018, 184, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J. Keshan disease, selenium deficiency, and the selenoproteome. N. Engl. J. Med. 2014, 370, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Polymorphism of antioxidant selenoprotein genes and kashin-beck disease susceptibility, a systematic review and meta-analysis. Osteoarthr. Cartil. 2017, 25, S213–S214. [Google Scholar] [CrossRef][Green Version]
- Ventura, M.; Melo, M.; Carrilho, F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int. J. Endocrinol. 2017, 2017, 1297658. [Google Scholar] [CrossRef]
- Agarwal, A.; Nallella, K.P.; Allamaneni, S.S.; Said, T.M. Role of antioxidants in treatment of male infertility: An overview of the literature. Reprod. Biomed. Online 2004, 8, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.D.; Tian, Y.Q.; Wu, J.L.; Wang, S.Y. Synthesis, characterization, and biological activity of selenium nanoparticles conjugated with polysaccharides. Crit. Rev. Food Sci. Nutr. 2021, 61, 2225–2236. [Google Scholar] [CrossRef]
- Sigrist, M.; Brusa, L.; Campagnoli, D.; Beldoménico, H. Determination of selenium in selected food samples from Argentina and estimation of their contribution to the Se dietary intake. Food Chem. 2012, 134, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Fisinin, V.I.; Papazyan, T.T.; Surai, P.F. Producing selenium-enriched eggs and meat to improve the selenium status of the general population. Crit. Rev. Biotechnol. 2009, 29, 18–28. [Google Scholar] [CrossRef]
- Schomburg, L. The other view: The trace element selenium as a micronutrient in thyroid disease, diabetes, and beyond. Hormones 2020, 19, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Xu, X.; Ye, H.; Jin, L.; Zhang, X.; Zhu, Y. High levels of plasma selenium are associated with metabolic syndrome and elevated fasting plasma glucose in a Chinese population: A case-control study. J. Trace Elem. Med. Biol. 2015, 32, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ramos, M.; Venero, J.L.; Cano, J.; Machado, A. Low selenium diet induces tyrosine hydroxylase enzyme in nigrostriatal system of the rat. Mol. Brain Res. 2000, 84, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Wessjohann, L.A.; Schneider, A.; Abbas, M.; Brandt, W. Selenium in chemistry and biochemistry in comparison to sulfur. Biol. Chem. 2007, 388, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, T.C. Selenocysteine. Annu. Rev. Biochem. 1996, 65, 83–100. [Google Scholar] [CrossRef]
- Rayman, M.P. The use of high-selenium yeast to raise selenium status: How does it measure up? Br. J. Nutr. 2004, 92, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta BBA—Gen. Subj. 2015, 1850, 1642–1660. [Google Scholar] [CrossRef] [PubMed]
- Minich, W.B. Selenium metabolism and biosynthesis of selenoproteins in the human body. Biochemistry 2022, 87, S168–S177. [Google Scholar] [CrossRef]
- Nahapetian, A.T.; Janghorbani, M.; Young, V.R. Urinary trimethylselenonium excretion by the rat: Effect of level and source of selenium-75. J. Nutr. 1983, 113, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Ogra, Y.; Ishiwata, K.; Takayama, H.; Aimi, N.; Suzuki, K.T. Selenosugars are key and urinary metabolites for selenium excretion within the required to low-toxic range. Proc. Natl. Acad. Sci. USA 2002, 99, 15932–15936. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, B.S.; Palmer, I.S.; Dwivedi, C. Selenium detoxification by methylation. Res. Commun. Mol. Pathol. Pharmacol. 1995, 90, 133–142. [Google Scholar] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Z.; Liu, H.; Zhang, L.; Gao, P.; Li, D. Biosynthesis and structural characteristics of selenium nanoparticles by Pseudomonas alcaliphila. Colloids Surf. B Biointerfaces 2011, 88, 196–201. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef]
- Santhosh Kumar, B.; Priyadarsini, K.I. Selenium nutrition: How important is it? Biomed. Prev. Nutr. 2014, 4, 333–341. [Google Scholar] [CrossRef]
- Lothrop, A.P.; Snider, G.W.; Ruggles, E.L.; Hondal, R.J. Why is mammalian thioredoxin reductase 1 so dependent upon the use of selenium? Biochemistry 2014, 53, 554–565. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Toppo, S.; Vanin, S.; Bosello, V.; Tosatto, S.C. Evolutionary and structural insights into the multifaceted glutathione peroxidase (Gpx) superfamily. Antioxid. Redox Signal. 2008, 10, 1501–1514. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, O.H.; Degnan, S.M. Antioxidant enzymes that target hydrogen peroxide are conserved across the animal kingdom, from sponges to mammals. Sci. Rep. 2023, 13, 2510. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, B.O. Reduction of linolenic acid hydroperoxide by a glutathione peroxidase. Biochim. Biophys. Acta 1969, 176, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Little, C.; Olinescu, R.; Reid, K.G.; O’Brien, P.J. Properties and regulation of glutathione peroxidase. J. Biol. Chem. 1970, 245, 3632–3636. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zong, W.; Zhang, H.; Liu, R. Kidney Toxicity and Response of Selenium Containing Protein-glutathione Peroxidase (Gpx3) to CdTe QDs on Different Levels. Toxicol. Sci. 2019, 168, 201–208. [Google Scholar] [CrossRef]
- Labrecque, C.L.; Fuglestad, B. Electrostatic Drivers of GPx4 Interactions with Membrane, Lipids, and DNA. Biochemistry 2021, 60, 2761–2772. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Flohé, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Saedi, M.S.; Smith, C.G.; Frampton, J.; Chambers, I.; Harrison, P.R.; Sunde, R.A. Effect of selenium status on mRNA levels for glutathione peroxidase in rat liver. Biochem. Biophys. Res. Commun. 1988, 153, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.D.; Baker, S.S.; LaRosa, K.; Whitney, C.; Newburger, P.E. Selenium regulation of glutathione peroxidase in human hepatoma cell line Hep3B. Arch. Biochem. Biophys. 1993, 304, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Darras, V.M.; Van Herck, S.L. Iodothyronine deiodinase structure and function: From ascidians to humans. J. Endocrinol. 2012, 215, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Gereben, B.; Zavacki, A.M.; Ribich, S.; Kim, B.W.; Huang, S.A.; Simonides, W.S.; Zeöld, A.; Bianco, A.C. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 2008, 29, 898–938. [Google Scholar] [CrossRef]
- Gereben, B.; Zeöld, A.; Dentice, M.; Salvatore, D.; Bianco, A.C. Activation and inactivation of thyroid hormone by deiodinases: Local action with general consequences. Cell. Mol. Life Sci. 2008, 65, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, L.; Vassalle, C.; Del Seppia, C.; Iervasi, G. Deiodinases and the Three Types of Thyroid Hormone Deiodination Reactions. Endocrinol. Metab. 2021, 36, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S. Focus on mammalian thioredoxin reductases--important selenoproteins with versatile functions. Biochim. Biophys. Acta 2009, 1790, 495–526. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y. The methionine sulfoxide reduction system: Selenium utilization and methionine sulfoxide reductase enzymes and their functions. Antioxid. Redox Signal. 2013, 19, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Péterfi, Z.; Hoffmann, F.W.; Moore, R.E.; Kaya, A.; Avanesov, A.; Tarrago, L.; Zhou, Y.; Weerapana, E.; Fomenko, D.E.; et al. MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation. Mol. Cell 2013, 51, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Kaushal, N.; Gandhi, U.H.; Nelson, S.M.; Narayan, V.; Prabhu, K.S. Selenium and inflammation. In Selenium: Its Molecular Biology and Role in Human Health; Springer: New York, NY, USA, 2012; pp. 443–456. [Google Scholar]
- Sakr, Y.; Reinhart, K.; Bloos, F.; Marx, G.; Russwurm, S.; Bauer, M.; Brunkhorst, F. Time course and relationship between plasma selenium concentrations, systemic inflammatory response, sepsis, and multiorgan failure. Br. J. Anaesth. 2007, 98, 775–784. [Google Scholar] [CrossRef]
- Angstwurm, M.W.; Engelmann, L.; Zimmermann, T.; Lehmann, C.; Spes, C.H.; Abel, P.; Strauss, R.; Meier-Hellmann, A.; Insel, R.; Radke, J.; et al. Selenium in Intensive Care (SIC): Results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit. Care Med. 2007, 35, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Marshall, J.C.; Namendys-Silva, S.A.; François, B.; Martin-Loeches, I.; Lipman, J.; Reinhart, K.; Antonelli, M.; Pickkers, P.; Njimi, H.; et al. Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir. Med. 2014, 2, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Sartelli, M.; Kluger, Y.; Ansaloni, L.; Hardcastle, T.C.; Rello, J.; Watkins, R.R.; Bassetti, M.; Giamarellou, E.; Coccolini, F.; Abu-Zidan, F.M. Raising concerns about the Sepsis-3 definitions. World J. Emerg. Surg. 2018, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Angstwurm, M.W.; Schottdorf, J.; Schopohl, J.; Gaertner, R. Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Crit. Care Med. 1999, 27, 1807–1813. [Google Scholar] [CrossRef]
- Allingstrup, M.; Afshari, A. Selenium supplementation for critically ill adults. Cochrane Database Syst. Rev. 2015, 2015, Cd003703. [Google Scholar] [CrossRef] [PubMed]
- Hamishehkar, H.; Beigmohammadi, M.T.; Abdollahi, M.; Ahmadi, A.; Mahmoodpour, A.; Mirjalili, M.R.; Abrishami, R.; Khoshayand, M.R.; Eslami, K.; Kanani, M.; et al. Identification of enhanced cytokine generation following sepsis. Dream of magic bullet for mortality prediction and therapeutic evaluation. Daru 2010, 18, 155–162. [Google Scholar] [PubMed]
- Crunkhorn, S. New route to sepsis therapy. Nat. Rev. Drug Discov. 2019, 18, 251. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Zhang, L.; You, Y.; Zheng, L.; Ren, J.; Qu, X. An Enzyme-Mimicking Single-Atom Catalyst as an Efficient Multiple Reactive Oxygen and Nitrogen Species Scavenger for Sepsis Management. Angew. Chem. Int. Ed. Engl. 2020, 59, 5108–5115. [Google Scholar] [CrossRef] [PubMed]
- Toledo, A.G.; Golden, G.; Campos, A.R.; Cuello, H.; Sorrentino, J.; Lewis, N.; Varki, N.; Nizet, V.; Smith, J.W.; Esko, J.D. Proteomic atlas of organ vasculopathies triggered by Staphylococcus aureus sepsis. Nat. Commun. 2019, 10, 4656. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef]
- Silversides, J.A.; Major, E.; Ferguson, A.J.; Mann, E.E.; McAuley, D.F.; Marshall, J.C.; Blackwood, B.; Fan, E. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: A systematic review and meta-analysis. Intensive Care Med. 2017, 43, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Je, Y.S.; Baek, J.; Chung, M.H.; Kwon, H.Y.; Lee, J.S. Nutritional status of patients with COVID-19. Int. J. Infect. Dis. 2020, 100, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Belsky, J.B.; Wira, C.R.; Jacob, V.; Sather, J.E.; Lee, P.J. A review of micronutrients in sepsis: The role of thiamine, l-carnitine, vitamin C, selenium and vitamin D. Nutr. Res. Rev. 2018, 31, 281–290. [Google Scholar] [CrossRef]
- Meisner, M. Update on procalcitonin measurements. Ann. Lab. Med. 2014, 34, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Assink-de Jong, E.; de Lange, D.W.; van Oers, J.A.; Nijsten, M.W.; Twisk, J.W.; Beishuizen, A. Stop Antibiotics on guidance of Procalcitonin Study (SAPS): A randomised prospective multicenter investigator-initiated trial to analyse whether daily measurements of procalcitonin versus a standard-of-care approach can safely shorten antibiotic duration in intensive care unit patients–calculated sample size: 1816 patients. BMC Infect. Dis. 2013, 13, 178. [Google Scholar] [CrossRef]
- Valenta, J.; Brodska, H.; Drabek, T.; Hendl, J.; Kazda, A. High-dose selenium substitution in sepsis: A prospective randomized clinical trial. Intensive Care Med. 2011, 37, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Montoya, G.C.; Hernández, L.A.; Villalobos, S.J.A.; Olvera, G.C.; Aguirre, S.J.; Franco, G.J. Efecto antiinflamatorio del selenio en pacientes sépticos. Rev. Asoc. Mex. Med. Crit. Y Ter. Int. 2009, 23, 199–205. [Google Scholar]
- Sakr, Y.; Maia, V.P.; Santos, C.; Stracke, J.; Zeidan, M.; Bayer, O.; Reinhart, K. Adjuvant selenium supplementation in the form of sodium selenite in postoperative critically ill patients with severe sepsis. Crit. Care 2014, 18, R68. [Google Scholar] [CrossRef] [PubMed]
- Olson, O. Selenium toxicity in animals with emphasis on man. J. Am. Coll. Toxicol. 1986, 5, 45–70. [Google Scholar] [CrossRef]
- Woth, G.; Nagy, B.; Mérei, Á.; Ernyey, B.; Vincze, R.; Kaurics, Z.; Lantos, J.; Bogár, L.; Mühl, D. The effect of Na-selenite treatment on the oxidative stress-antioxidants balance of multiple organ failure. J. Crit. Care 2014, 29, 883.e7–883.e11. [Google Scholar] [CrossRef]
- Manzanares, W.; Langlois, P.L.; Heyland, D.K. Pharmaconutrition with selenium in critically ill patients: What do we know? Nutr. Clin. Pract. 2015, 30, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A. Selenium in intensive care: Probably not a magic bullet but an important adjuvant therapy. Crit. Care Med. 2007, 35, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Zhang, J.; Spallholz, J.E. Toxicity of selenium compounds and nano-selenium particles. In General, Applied and Systems Toxicology; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Nazari, N.; Niazvand, F.; Chamkouri, N.; Amoori, N.; Asl, M.S. Role of zinc, selenium, vitamin D and vitamin C in boosting respiratory system: A meta-analysis approach. Med. Sci. 2022, 26, ms77e1837. [Google Scholar] [CrossRef]
- Djalalinia, S.; Hasani, M.; Asayesh, H.; Ejtahed, H.S.; Malmir, H.; Kasaeian, A.; Zarei, M.; Baygi, F.; Rastad, H.; Mahdavi Gorabi, A.; et al. The effects of dietary selenium supplementation on inflammatory markers among patients with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 2021, 20, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, B.; Huang, Y.; Li, J.; Cao, D.; Chen, Z.; Li, J.; Ran, B.; Yang, J.; Wang, R. Selenium intake and multiple health-related outcomes: An umbrella review of meta-analyses. Front. Nutr. 2023, 10, 1263853. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, M.A.; Saghaleini, S.H.; Mahmoodpoor, A.; Ghojazadeh, M.; Mousavi, S.N. Daily parenteral selenium therapy in critically ill patients: An updated systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2021, 41, 49–58. [Google Scholar] [CrossRef]
- Manzanares, W.; Lemieux, M.; Elke, G.; Langlois, P.L.; Bloos, F.; Heyland, D.K. High-dose intravenous selenium does not improve clinical outcomes in the critically ill: A systematic review and meta-analysis. Crit. Care 2016, 20, 356. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Sarıkaya, E.; Doğan, S. Glutathione peroxidase in health and diseases. In Glutathione System and Oxidative Stress in Health and Disease; IntechOpen: London, UK, 2020; Volume 49. [Google Scholar]
- Xie, W.; Huang, X.; Chen, R.; Chen, R.; Li, T.; Wu, W.; Huang, Z. Esomeprazole alleviates the damage to stress ulcer in rats through not only its antisecretory effect but its antioxidant effect by inactivating the p38 MAPK and NF-κB signaling pathways. Drug Des. Dev. Ther. 2019, 13, 2969–2984. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, W.; Biestro, A.; Galusso, F.; Torre, M.H.; Mañay, N.; Pittini, G.; Facchin, G.; Hardy, G. Serum selenium and glutathione peroxidase-3 activity: Biomarkers of systemic inflammation in the critically ill? Intensive Care Med. 2009, 35, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Mamgain, R.; Kostic, M.; Singh, F.V. Synthesis and antioxidant properties of organoselenium compounds. Curr. Med. Chem. 2023, 30, 2421–2448. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.; Skouta, R. The Selenoprotein Glutathione Peroxidase 4: From Molecular Mechanisms to Novel Therapeutic Opportunities. Biomedicines 2022, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 19, e1800311. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar] [PubMed]
- Green, M.R.; Manikhas, G.M.; Orlov, S.; Afanasyev, B.; Makhson, A.M.; Bhar, P.; Hawkins, M.J. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann. Oncol. 2006, 17, 1263–1268. [Google Scholar] [CrossRef]
- Yuk, S.A.; Sanchez-Rodriguez, D.A.; Tsifansky, M.D.; Yeo, Y. Recent advances in nanomedicine for sepsis treatment. Ther. Deliv. 2018, 9, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Saha, I.; Bag, N.; Roy, S.; Ullah, Z.; Bardhan, S.; Karmakar, P.; Das, S.; Guo, B. The state-of-the-art therapeutic paradigms against sepsis. Smart Mater. Med. 2024, 5, 425–446. [Google Scholar] [CrossRef]
- Rasche, B.; Amin, H.M.A.; Clarke, S.J.; Compton, R.G. Polyselenides on the route to electrodeposited selenium. J. Electroanal. Chem. 2019, 835, 239–247. [Google Scholar] [CrossRef]
- Malyugina, S.; Skalickova, S.; Skladanka, J.; Slama, P.; Horky, P. Biogenic Selenium Nanoparticles in Animal Nutrition: A Review. Agriculture 2021, 11, 1244. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef]
- Huang, W.; Wang, M.; Hu, L.; Wang, C.; Xie, Z.; Zhang, H. Recent advances in semiconducting monoelemental selenium nanostructures for device applications. Adv. Funct. Mater. 2020, 30, 2003301. [Google Scholar] [CrossRef]
- Ruiz-Fresneda, M.A.; Staicu, L.C.; Lazuén-López, G.; Merroun, M.L. Allotropy of selenium nanoparticles: Colourful transition, synthesis, and biotechnological applications. Microb. Biotechnol. 2023, 16, 877–892. [Google Scholar] [CrossRef]
- Sharma, V.K.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.K.; Pettine, M.; Zboril, R. Assessment of toxicity of selenium and cadmium selenium quantum dots: A review. Chemosphere 2017, 188, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Qiu, W.-Y.; Sun, L.; Ding, Z.-C.; Yan, J.-K. Preparation, characterization, and antioxidant capacities of selenium nanoparticles stabilized using polysaccharide–protein complexes from Corbicula fluminea. Food Biosci. 2018, 26, 177–184. [Google Scholar] [CrossRef]
- Chhabria, S.; Desai, K. Selenium nanoparticles and their applications. Encycl. Nanosci. Nanotechnol. 2016, 20, 1–32. [Google Scholar]
- Zhang, J.; Wang, H.; Yan, X.; Zhang, L. Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci. 2005, 76, 1099–1109. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Yu, H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007, 42, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xun, W.; Yue, W.; Zhang, C.; Ren, Y.; Shi, L.; Wang, Q.; Yang, R.; Lei, F. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011, 96, 49–52. [Google Scholar] [CrossRef]
- Tran, P.A.; Webster, T.J. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomed. 2011, 6, 1553–1558. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Basu, A.; Sen, T.; Biswas, J.; Bhattacharya, S. Nano-Se as a novel candidate in the management of oxidative stress related disorders and cancer. Nucleus 2017, 60, 137–145. [Google Scholar] [CrossRef]
- Vahdati, M.; Tohidi Moghadam, T. Synthesis and Characterization of Selenium Nanoparticles-Lysozyme Nanohybrid System with Synergistic Antibacterial Properties. Sci. Rep. 2020, 10, 510. [Google Scholar] [CrossRef]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar] [CrossRef]
- Sampath, S.; Sunderam, V.; Manjusha, M.; Dlamini, Z.; Lawrance, A.V. Selenium Nanoparticles: A Comprehensive Examination of Synthesis Techniques and Their Diverse Applications in Medical Research and Toxicology Studies. Molecules 2024, 29, 801. [Google Scholar] [CrossRef] [PubMed]
- Karthik, K.K.; Cheriyan, B.V.; Rajeshkumar, S.; Gopalakrishnan, M. A review on selenium nanoparticles and their biomedical applications. Biomed. Technol. 2024, 6, 61–74. [Google Scholar] [CrossRef]
- Ranjitha, V.; Rai, V.R. Selenium nanostructure: Progress towards green synthesis and functionalization for biomedicine. J. Pharm. Investig. 2021, 51, 117–135. [Google Scholar] [CrossRef]
- Sowmya, R.; Karthick Raja Namasivayam, S.; Krithika Shree, S. A critical review on nano-selenium based materials: Synthesis, biomedicine applications and biocompatibility assessment. J. Inorg. Organomet. Polym. Mater. 2024, 34, 3037–3055. [Google Scholar] [CrossRef]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of Selenium Nanoparticles From Emblica officinalis Fruit Extract and Exploring Its Biopotential Applications: Antioxidant, Antimicrobial, and Biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, X.; Rider, A.E.; Furman, S.A.; Ostrikov, K. Fast, energy-efficient synthesis of luminescent carbon quantum dots. Green Chem. 2014, 16, 2566–2570. [Google Scholar] [CrossRef]
- Chandramohan, S.; Sundar, K.; Muthukumaran, A. Reducing agents influence the shapes of selenium nanoparticles (SeNPs) and subsequently their antibacterial and antioxidant activity. Mater. Res. Express 2019, 6, 0850i2. [Google Scholar] [CrossRef]
- Chen, N.; Yao, P.; Zhang, W.; Zhang, Y.; Xin, N.; Wei, H.; Zhang, T.; Zhao, C. Selenium nanoparticles: Enhanced nutrition and beyond. Crit. Rev. Food Sci. Nutr. 2023, 63, 12360–12371. [Google Scholar] [CrossRef]
- Korde, P.; Ghotekar, S.; Pagar, T.; Pansambal, S.; Oza, R.; Mane, D. Plant extract assisted eco-benevolent synthesis of selenium nanoparticles-a review on plant parts involved, characterization and their recent applications. J. Chem. Rev. 2020, 2, 157–168. [Google Scholar]
- Pyrzynska, K.; Sentkowska, A. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostruct. Chem. 2022, 12, 467–480. [Google Scholar] [CrossRef]
- Nikam, P.B.; Salunkhe, J.D.; Minkina, T.; Rajput, V.D.; Kim, B.S.; Patil, S.V. A review on green synthesis and recent applications of red nano Selenium. Results Chem. 2022, 4, 100581. [Google Scholar] [CrossRef]
- Abadi, B.; Hosseinalipour, S.; Nikzad, S.; Pourshaikhali, S.; Fathalipour-Rayeni, H.; Shafiei, G.; Adeli-Sardou, M.; Shakibaie, M.; Forootanfar, H. Capping agents for selenium nanoparticles in biomedical applications. J. Clust. Sci. 2023, 34, 1669–1690. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, E.O. Selenium Nanoparticles: Green Synthesis and Biomedical Application. Molecules 2023, 28, 8125. [Google Scholar] [CrossRef]
- Verma, A.; Gautam, S.P.; Bansal, K.K.; Prabhakar, N.; Rosenholm, J.M. Green Nanotechnology: Advancement in Phytoformulation Research. Medicines 2019, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Qiu, W.Y.; Wang, Y.Y.; Wang, W.H.; Yang, Y.; Zhang, H.N. Fabrication and stabilization of biocompatible selenium nanoparticles by carboxylic curdlans with various molecular properties. Carbohydr. Polym. 2018, 179, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Liang, T.; Sun, L.; Meng, L.; Yang, C.; Wang, L.; Liang, T.; Li, Q. Green synthesis of selenium nanoparticles with extract of hawthorn fruit induced HepG2 cells apoptosis. Pharm. Biol. 2018, 56, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.S.; Patel, H.; Patel, T.; Patel, K.; Selvaraj, K. Biosynthesis of Se nanoparticles and its effect on UV-induced DNA damage. Colloids Surf. B Biointerfaces 2013, 103, 261–266. [Google Scholar] [CrossRef]
- Fan, D.; Li, L.; Li, Z.; Zhang, Y.; Ma, X.; Wu, L.; Zhang, H.; Guo, F. Biosynthesis of selenium nanoparticles and their protective, antioxidative effects in streptozotocin induced diabetic rats. Sci. Technol. Adv. Mater. 2020, 21, 505–514. [Google Scholar] [CrossRef]
- Sowndarya, P.; Ramkumar, G.; Shivakumar, M.S. Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Mellinas, C.; Jiménez, A.; Garrigós, M.D.C. Microwave-Assisted Green Synthesis and Antioxidant Activity of Selenium Nanoparticles Using Theobroma cacao L. Bean Shell Extract. Molecules 2019, 24, 4048. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, M.; Liu, Y.; Bai, Y.; Deng, Y.; Liu, J.; Chen, L. Green synthesis of Se/Ru alloy nanoparticles using gallic acid and evaluation of theiranti-invasive effects in HeLa cells. Colloids Surf. B Biointerfaces 2016, 144, 118–124. [Google Scholar] [CrossRef]
- Martínez-Esquivias, F.; Perez-Larios, A.; Guzmán-Flores, J.M. Effect of Administration of Selenium Nanoparticles Synthesized Using Onion Extract on Biochemical and Inflammatory Parameters in Mice Fed with High-Fructose Diet: In Vivo and In Silico Analysis. Biol. Trace Elem. Res. 2024, 202, 558–568. [Google Scholar] [CrossRef]
- Huang, Y.; He, L.; Liu, W.; Fan, C.; Zheng, W.; Wong, Y.S.; Chen, T. Selective cellular uptake and induction of apoptosis of cancer-targeted selenium nanoparticles. Biomaterials 2013, 34, 7106–7116. [Google Scholar] [CrossRef] [PubMed]
- El-Borady, O.M.; Othman, M.S.; Atallah, H.H.; Abdel Moneim, A.E. Hypoglycemic potential of selenium nanoparticles capped with polyvinyl-pyrrolidone in streptozotocin-induced experimental diabetes in rats. Heliyon 2020, 6, e04045. [Google Scholar] [CrossRef] [PubMed]
- Dumore, N.S.; Mukhopadhyay, M. Antioxidant properties of aqueous selenium nanoparticles (ASeNPs) and its catalysts activity for 1, 1-diphenyl-2-picrylhydrazyl (DPPH) reduction. J. Mol. Struct. 2020, 1205, 127637. [Google Scholar] [CrossRef]
- Shahabi, R.; Anissian, A.; Javadmoosavi, S.A.; Nasirinezhad, F. Protective and anti-inflammatory effect of selenium nano-particles against bleomycin-induced pulmonary injury in male rats. Drug Chem. Toxicol. 2021, 44, 92–100. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef]
- Dominiak, A.; Wilkaniec, A.; Wroczyński, P.; Adamczyk, A. Selenium in the Therapy of Neurological Diseases. Where is it Going? Curr. Neuropharmacol. 2016, 14, 282–299. [Google Scholar] [CrossRef] [PubMed]
- Alim, I.; Caulfield, J.T.; Chen, Y.; Swarup, V.; Geschwind, D.H.; Ivanova, E.; Seravalli, J.; Ai, Y.; Sansing, L.H.; Ste Marie, E.J.; et al. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell 2019, 177, 1262–1279.e25. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, C.; Sampath, K.S.; Arunkumar, P.; Kumar, M.S.; Sujatha, V.; Premkumar, K.; Thirunavukkarasu, C. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [CrossRef]
- Li, Q.; Chen, T.; Yang, F.; Liu, J.; Zheng, W. Facile and controllable one-step fabrication of selenium nanoparticles assisted by L-cysteine. Mater. Lett. 2010, 64, 614–617. [Google Scholar] [CrossRef]
- Langi, B.; Shah, C.; Singh, K.; Chaskar, A.; Kumar, M.; Bajaj, P.N. Ionic liquid-induced synthesis of selenium nanoparticles. Mater. Res. Bull. 2010, 45, 668–671. [Google Scholar] [CrossRef]
- Dhanjal, S.; Cameotra, S.S. Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil. Microb. Cell Fact. 2010, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhai, X.; Zhao, G.; Ren, F.; Leng, X. Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydr. Polym. 2015, 134, 158–166. [Google Scholar] [CrossRef]
- Chen, H.; Shin, D.-W.; Nam, J.-G.; Kwon, K.-W.; Yoo, J.-B. Selenium nanowires and nanotubes synthesized via a facile template-free solution method. Mater. Res. Bull. 2010, 45, 699–704. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, J.; Hou, J.; Chen, C. Free radical scavenging efficiency of Nano-Se in vitro. Free Radic. Biol. Med. 2003, 35, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zhang, J.; Liu, Q.; Taylor, E.W. Size effect of elemental selenium nanoparticles (Nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activity. J. Inorg. Biochem. 2007, 101, 1457–1463. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Q.; Zhao, Z.; He, L.; Chan, L.; Liu, Y.; Chen, Y.; Bai, M.; Pan, T.; Qu, Y.; et al. Functionalized Selenium Nanosystem as Radiation Sensitizer of (125)I Seeds for Precise Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 25857–25869. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Su, S.; Chen, Z.; Liang, F.; Zeng, Y.; Cen, W.; Zhang, X.; Xia, Y.; Huang, D. Hyaluronic acid-modified selenium nanoparticles for enhancing the therapeutic efficacy of paclitaxel in lung cancer therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3456–3464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, D.; Lv, X.; Liu, X.; Xu, W.; Chen, L.; Cai, J.; Din, Z.-U.; Cheng, S. Green Synthesis of Robust Selenium Nanoparticles via Polysaccharide–Polyphenol Interaction: Design Principles and Structure–Bioactivity Relationship. ACS Sustain. Chem. Eng. 2022, 10, 2052–2062. [Google Scholar] [CrossRef]
- Liu, H.J.; Qin, Y.; Zhao, Z.H.; Zhang, Y.; Yang, J.H.; Zhai, D.H.; Cui, F.; Luo, C.; Lu, M.X.; Liu, P.P.; et al. Lentinan-functionalized Selenium Nanoparticles target Tumor Cell Mitochondria via TLR4/TRAF3/MFN1 pathway. Theranostics 2020, 10, 9083–9099. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Y.; Hu, Y.; Zhang, D.; Xu, W.; Chen, L.; He, J.; Cheng, S.; Cai, J. Selenium Nanoparticles Synergistically Stabilized by Starch Microgel and EGCG: Synthesis, Characterization, and Bioactivity. Foods 2022, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Jeyabharathi, S.; Sundar, K.; Selvamani, S.; Prasanna, M.; Muthukumaran, A. A novel biocompatible chitosan–Selenium nanoparticles (SeNPs) film with electrical conductivity for cardiac tissue engineering application. Mater. Sci. Eng. C 2018, 92, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, S.; Song, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Chen, T.; Zheng, W.; Huang, Z. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-κB mediated hyper inflammation. J. Nanobiotechnol. 2017, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Dorazilová, J.; Muchová, J.; Šmerková, K.; Kočiová, S.; Diviš, P.; Kopel, P.; Veselý, R.; Pavliňáková, V.; Adam, V.; Vojtová, L. Synergistic Effect of Chitosan and Selenium Nanoparticles on Biodegradation and Antibacterial Properties of Collagenous Scaffolds Designed for Infected Burn Wounds. Nanomaterials 2020, 10, 1971. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Q.; Zheng, Z.; Ma, H. Selenium release kinetics and mechanism from Cordyceps sinensis exopolysaccharide-selenium composite nanoparticles in simulated gastrointestinal conditions. Food Chem. 2021, 350, 129223. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Shen, L.; Yang, E.; Shen, H.; Huang, D.; Wang, R.; Hu, C.; Jin, H.; Cai, H.; Cai, J.; et al. Macrophage-Targeted Isoniazid–Selenium Nanoparticles Promote Antimicrobial Immunity and Synergize Bactericidal Destruction of Tuberculosis Bacilli. Angew. Chem. Int. Ed. 2020, 59, 3226–3234. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tang, G.; Guo, M.; Xu, T.; Chen, H.; Lin, Z.; Li, Y.; Chen, Y.; Zhu, B.; Liu, H.; et al. Silencing KLK12 expression via RGDfC-decorated selenium nanoparticles for the treatment of colorectal cancer in vitro and in vivo. Mater. Sci. Eng. C 2020, 110, 110594. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chauhan, P.; Kumar, R.; Bhasin, K.K. Toxicological responses of surfactant functionalized selenium nanoparticles: A quantitative multi-assay approach. Sci. Total Environ. 2018, 643, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.K.; Ko, F.H.; Huang, P.W.; Wu, C.H.; Chu, T.C. Studying the size/shape separation and optical properties of silver nanoparticles by capillary electrophoresis. J. Chromatogr. A 2005, 1062, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of biogenic capping agents in the synthesis of metallic nanoparticles and evaluation of their therapeutic potential. Front. Nanotechnol. 2022, 3, 801620. [Google Scholar] [CrossRef]
- Jie, Y.; Chen, F. Progress in the Application of Food-Grade Emulsions. Foods 2022, 11, 2883. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhao, M.; Chen, Y.; Hua, L.; Xu, T.; Wang, C.; Li, Y.; Zhu, B. Folate-targeted selenium nanoparticles deliver therapeutic siRNA to improve hepatocellular carcinoma therapy. RSC Adv. 2018, 8, 25932–25940. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, X.; Chen, X.; Liu, Y.; Gong, Y.; Yuan, G.; Liu, J.; Chen, L. Defense and inhibition integrated mesoporous nanoselenium delivery system against tomato gray mold. Environ. Sci. Nano 2020, 7, 210–227. [Google Scholar] [CrossRef]
- Shah, C.P.; Dwivedi, C.; Singh, K.K.; Kumar, M.; Bajaj, P.N. Riley oxidation: A forgotten name reaction for synthesis of selenium nanoparticles. Mater. Res. Bull. 2010, 45, 1213–1217. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Leng, X.; Stoll, S. Embryonic exposure to selenium nanoparticles delays growth and hatching in the freshwater snail Lymnaea stagnalis. Chemosphere 2022, 307, 136147. [Google Scholar] [CrossRef]
- Wdaah, A.H.; Idan Salman, H.; Balakit, A.A. Synthesis and characterization of selenium-polyvinyl alcohol nanoparticles as eco-friendly corrosion inhibitor for carbon steel in 1.0 M H2SO4. Inorg. Chem. Commun. 2024, 165, 112505. [Google Scholar] [CrossRef]
- Chauhan, P.; Chaudhary, S. Role of surface modification on selenium nanoparticles: Enumerating the optical, thermal and structural properties. Opt. Mater. 2019, 97, 109380. [Google Scholar] [CrossRef]
- Bartůněk, V.; Junková, J.; Šuman, J.; Kolářová, K.; Rimpelová, S.; Ulbrich, P.; Sofer, Z. Preparation of amorphous antimicrobial selenium nanoparticles stabilized by odor suppressing surfactant polysorbate 20. Mater. Lett. 2015, 152, 207–209. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Turner, R.J. Stability of biogenic metal(loid) nanomaterials related to the colloidal stabilization theory of chemical nanostructures. Crit. Rev. Biotechnol. 2018, 38, 1137–1156. [Google Scholar] [CrossRef]
- Lin, X.; Mu, J.; Chen, Z.; Zhang, Y.; Ye, X.; Gao, X.; Chen, W.; Luo, Y.; Li, B. Stabilization and functionalization of selenium nanoparticles mediated by green tea and Pu-Erh tea polysaccharides. Ind. Crops Prod. 2023, 194, 116312. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, R.; Zhou, F.; Wu, Y.; Li, S.; Huo, G.; Ye, J.; Hua, C.; Wang, Z. Preparation, physicochemical characterization, and cytotoxicity of selenium nanoparticles stabilized by Oudemansiella raphanipies polysaccharide. Int. J. Biol. Macromol. 2022, 211, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Biofunctionalization of selenium nanoparticles with a polysaccharide from Rosa roxburghii fruit and their protective effect against H2O2-induced apoptosis in INS-1 cells. Food Funct. 2019, 10, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, X.; Ji, T.; Wen, C.; Ye, Z.; Liu, X.; Liang, L.; Liu, G.; Xu, X. Digestion and absorption properties of Lycium barbarum polysaccharides stabilized selenium nanoparticles. Food Chem. 2022, 373, 131637. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.; Li, J.H.; Chen, X.; Li, X.H.; Sun, K. The evaluation of the stability of EGCG-selenium nanoparticles and its effect on selenium absorption and utilization. J. Tea Sci. 2017, 37, 373–382. [Google Scholar]
- Wang, N.; Chu, L.; Xu, M.; Qiao, G.; Zheng, G.; Yang, L. Synthesis and antibacterial activity evaluation of gallic acid modified selenium nanoparticle. Food Sci. Technol. 2019, 44, 302–307. [Google Scholar]
- Kumari, M.; Ray, L.; Purohit, M.P.; Patnaik, S.; Pant, A.B.; Shukla, Y.; Kumar, P.; Gupta, K.C. Curcumin loading potentiates the chemotherapeutic efficacy of selenium nanoparticles in HCT116 cells and Ehrlich’s ascites carcinoma bearing mice. Eur. J. Pharm. Biopharm. 2017, 117, 346–362. [Google Scholar] [CrossRef]
- Yang, L.; Wang, W.; Chen, J.; Wang, N.; Zheng, G. A comparative study of resveratrol and resveratrol-functional selenium nanoparticles: Inhibiting amyloid β aggregation and reactive oxygen species formation properties. J. Biomed. Mater. Res. Part A 2018, 106, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Y.; Yang, S.; Yue, L.; Jiang, Q.; Xia, W. Synthesis and antioxidant properties of chitosan and carboxymethyl chitosan-stabilized selenium nanoparticles. Carbohydr. Polym. 2015, 132, 574–581. [Google Scholar] [CrossRef]
- Cui, D.; Ma, J.; Liang, T.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Selenium nanoparticles fabricated in laminarin polysaccharides solutions exert their cytotoxicities in HepG2 cells by inhibiting autophagy and promoting apoptosis. Int. J. Biol. Macromol. 2019, 137, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.Y.; Wang, Y.Y.; Wang, M.; Yan, J.K. Construction, stability, and enhanced antioxidant activity of pectin-decorated selenium nanoparticles. Colloids Surf. B Biointerfaces 2018, 170, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, T.; Jiang, M.; Huang, C.; Lai, C.; Fan, Y.; Yong, Q. Construction of arabinogalactans/selenium nanoparticles composites for enhancement of the antitumor activity. Int. J. Biol. Macromol. 2019, 128, 444–451. [Google Scholar] [CrossRef]
- Li, H.; Liu, D.; Li, S.; Xue, C. Synthesis and cytotoxicity of selenium nanoparticles stabilized by α-D-glucan from Castanea mollissima Blume. Int. J. Biol. Macromol. 2019, 129, 818–826. [Google Scholar] [CrossRef]
- Zeng, D.; Zhao, J.; Luk, K.-H.; Cheung, S.-T.; Wong, K.-H.; Chen, T. Potentiation of in Vivo Anticancer Efficacy of Selenium Nanoparticles by Mushroom Polysaccharides Surface Decoration. J. Agric. Food Chem. 2019, 67, 2865–2876. [Google Scholar] [CrossRef]
- Hu, S.; Hu, W.; Li, Y.; Li, S.; Tian, H.; Lu, A.; Wang, J. Construction and structure-activity mechanism of polysaccharide nano-selenium carrier. Carbohydr. Polym. 2020, 236, 116052. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, X.; Liu, W.; Chen, T.; Li, Y.; Zheng, W.; Man, C.W.-Y.; Wong, M.-K.; Wong, K.-H. Surface decoration of selenium nanoparticles by mushroom polysaccharides–protein complexes to achieve enhanced cellular uptake and antiproliferative activity. J. Mater. Chem. 2012, 22, 9602–9610. [Google Scholar] [CrossRef]
- Gao, W.; Chen, F.; Wang, X.; Meng, Q. Recent advances in processing food powders by using superfine grinding techniques: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2222–2255. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, Y.; Zheng, W.; Fan, C.; Chen, T. Positive surface charge enhances selective cellular uptake and anticancer efficacy of selenium nanoparticles. Inorg. Chem. 2012, 51, 8956–8963. [Google Scholar] [CrossRef] [PubMed]
- Hageman, S.P.W.; van der Weijden, R.D.; Stams, A.J.M.; Buisman, C.J.N. Bio-production of selenium nanoparticles with diverse physical properties for recovery from water. Int. J. Miner. Process. 2017, 169, 7–15. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; He, J.; Hong, Z.; Tan, R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int. J. Nanomed. 2017, 12, 4527–4539. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Z.; Niu, S.; He, Y.; Wang, G.; Pei, X.; Tao, W.; Wang, M. Preparation, characteristics and feeble induced-apoptosis performance of non-dialysis requiring selenium nanoparticles@chitosan. Mater. Des. 2019, 182, 108024. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Q.; Zou, S.; Xu, X.; Zhang, L. Construction of selenium nanoparticles/β-glucan composites for enhancement of the antitumor activity. Carbohydr. Polym. 2015, 117, 434–442. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, L.; Yi, Y.; Meng, Y.; Peng, K.; Jiang, X.; Wang, H. Synthesis, characterization and anti-inflammatory activity of selenium nanoparticles stabilized by aminated yeast glucan. Int. J. Biol. Macromol. 2023, 245, 125187. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; You, P.; Song, M.; Zhou, Y.; Yu, F.; Zheng, W. A facile and fast synthetic approach to create selenium nanoparticles with diverse shapes and their antioxidation ability. New J. Chem. 2016, 40, 1118–1123. [Google Scholar] [CrossRef]
- Kannan, S.; Mohanraj, K.; Prabhu, K.; Barathan, S.; Sivakumar, G. Synthesis of selenium nanorods with assistance of biomolecule. Bull. Mater. Sci. 2014, 37, 1631–1635. [Google Scholar] [CrossRef]
- Yu, J.-C.; Zhao, F.-G.; Shao, W.; Ge, C.-W.; Li, W.-S. Shape-controllable and versatile synthesis of copper nanocrystals with amino acids as capping agents. Nanoscale 2015, 7, 8811–8818. [Google Scholar] [CrossRef]
- Cox, A.; Vinciguerra, D.; Re, F.; Magro, R.D.; Mura, S.; Masserini, M.; Couvreur, P.; Nicolas, J. Protein-functionalized nanoparticles derived from end-functional polymers and polymer prodrugs for crossing the blood-brain barrier. Eur. J. Pharm. Biopharm. 2019, 142, 70–82. [Google Scholar] [CrossRef]
- Ghaffari-Bohlouli, P.; Jafari, H.; Taebnia, N.; Abedi, A.; Amirsadeghi, A.; Niknezhad, S.V.; Alimoradi, H.; Jafarzadeh, S.; Mirzaei, M.; Nie, L.; et al. Protein by-products: Composition, extraction, and biomedical applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9436–9481. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Mahmood, S.; Saffe, S.; Arifin, M.A.B.; Gupta, A.; Sikkandar, M.Y.; Begum, S.S.; Narasaiah, B. Extraction and application of keratin from natural resources: A review. 3 Biotech 2021, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.R.; Cansian, R.L.; Mello, R.O.; Demiate, I.M.; Kempka, A.P.; Dornelles, R.C.P.; Rodriguez, J.M.L.; Campagnol, P.C.B. Production of Collagens and Protein Hydrolysates with Antimicrobial and Antioxidant Activity from Sheep Slaughter By-Products. Antioxidants 2022, 11, 1173. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Prabhakaran, M.P.; Sireesha, M.; Ramakrishna, S. Collagen in Human Tissues: Structure, Function, and Biomedical Implications from a Tissue Engineering Perspective. In Polymer Composites—Polyolefin Fractionation—Polymeric Peptidomimetics—Collagens; Abe, A., Kausch, H.-H., Möller, M., Pasch, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 173–206. [Google Scholar]
- Aich, R.; Batabyal, S.; Joardar, S.N. Isolation and purification of beta-lactoglobulin from cow milk. Vet. World 2015, 8, 621–624. [Google Scholar] [CrossRef]
- Dabbaghi, A.; Ramazani, A.; Farshchi, N.; Rezaei, A.; Bodaghi, A.; Rezayati, S. Synthesis, physical and mechanical properties of amphiphilic hydrogels based on polycaprolactone and polyethylene glycol for bioapplications: A review. J. Ind. Eng. Chem. 2021, 101, 307–323. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E. Unravelling the in vitro and in vivo potential of selenium nanoparticles in Alzheimer’s disease: A bioanalytical review. Talanta 2024, 269, 125519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Teng, Z.; Yuan, Y.; Zeng, Q.Z.; Lou, Z.; Lee, S.H.; Wang, Q. Development, physicochemical characterization and cytotoxicity of selenium nanoparticles stabilized by beta-lactoglobulin. Int. J. Biol. Macromol. 2018, 107, 1406–1413. [Google Scholar] [CrossRef]
- Huang, T.; Holden, J.A.; Reynolds, E.C.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Multifunctional Antimicrobial Polypeptide-Selenium Nanoparticles Combat Drug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2020, 12, 55696–55709. [Google Scholar] [CrossRef]
- Huang, T.; Linklater, D.; Li, X.; Gamage, S.S.B.; Alkazemi, H.; Farrugia, B.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. One-Step Synthesis of Antimicrobial Polypeptide-Selenium Nanoparticles Exhibiting Broad-Spectrum Efficacy against Bacteria and Fungi with Superior Resistance Prevention. ACS Appl. Mater. Interfaces 2024, 16, 68996–69010. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Chen, X.; Zhu, X.; Xu, M.; Liu, J. Ruthenium complexes/polypeptide self-assembled nanoparticles for identification of bacterial infection and targeted antibacterial research. Biomaterials 2017, 141, 296–313. [Google Scholar] [CrossRef]
- Nasrolahi Shirazi, A.; Tiwari, R.K.; Oh, D.; Sullivan, B.; Kumar, A.; Beni, Y.A.; Parang, K. Cyclic peptide-selenium nanoparticles as drug transporters. Mol. Pharm. 2014, 11, 3631–3641. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.-Y.; Huang, Q.; Wang, Y.-L.; Yang, X.-Q.; Su, D.-X.; He, S.; Tan, J.-C.; Zeng, Q.-Z.; Yuan, Y. Development, structure characterization and stability of food grade selenium nanoparticles stabilized by tilapia polypeptides. J. Food Eng. 2020, 275, 109878. [Google Scholar] [CrossRef]
- Mu, X.; Wang, J.; Li, Y.; Xu, F.; Long, W.; Ouyang, L.; Liu, H.; Jing, Y.; Wang, J.; Dai, H.; et al. Redox Trimetallic Nanozyme with Neutral Environment Preference for Brain Injury. ACS Nano 2019, 13, 1870–1884. [Google Scholar] [CrossRef]
- Chung, M.F.; Chia, W.T.; Wan, W.L.; Lin, Y.J.; Sung, H.W. Controlled Release of an Anti-inflammatory Drug Using an Ultrasensitive ROS-Responsive Gas-Generating Carrier for Localized Inflammation Inhibition. J. Am. Chem. Soc. 2015, 137, 12462–12465. [Google Scholar] [CrossRef] [PubMed]
- Kaukonen, K.M.; Bailey, M.; Pilcher, D.; Cooper, D.J.; Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015, 372, 1629–1638. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, J.; Zhang, L. Hollow sphere selenium nanoparticles: Their in-vitro anti hydroxyl radical effect. Adv. Mater. 2002, 14, 290–293. [Google Scholar] [CrossRef]
- Sharma, V.K.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.K.; Pettine, M.; Zboril, R. Biogeochemistry of selenium. A review. Environ. Chem. Lett. 2015, 13, 49–58. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Gong, Y.; Yuan, G.; Cen, J.; Lie, Q.; Hou, Y.; Ye, G.; Liu, S.; Liu, J. Porous selenium nanozymes targeted scavenging ROS synchronize therapy local inflammation and sepsis injury. Appl. Mater. Today 2021, 22, 100929. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Z.; Liu, C.; Zhang, Y.; Ren, J.; Qu, X. Selenium-Based Nanozyme as Biomimetic Antioxidant Machinery. Chem.–A Eur. J. 2018, 24, 10224–10230. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, S.; Geng, X.; Pei, X.; Xing, H.; Zhang, X.; Chang, J.; Yang, W.; Wu, X. Multifunctional Selenium-Based Metal Polyphenol Nanoparticles Impede the Pathological Cross-talk Between Reactive Oxygen Species and Inflammation in Sepsis. ACS Mater. Lett. 2024, 6, 2434–2445. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Q.; Wu, F.G.; Dai, Y.; Chen, X. Polyphenol-Containing Nanoparticles: Synthesis, Properties, and Therapeutic Delivery. Adv. Mater. 2021, 33, e2007356. [Google Scholar] [CrossRef]
- Lin, G.; Rahim, M.A.; Leeming, M.G.; Cortez-Jugo, C.; Besford, Q.A.; Ju, Y.; Zhong, Q.Z.; Johnston, S.T.; Zhou, J.; Caruso, F. Selective Metal-Phenolic Assembly from Complex Multicomponent Mixtures. ACS Appl. Mater. Interfaces 2019, 11, 17714–17721. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, M.; Li, J.; Chen, Z.; Hu, L.; Zhang, J.; Cai, L.; Qiu, L.; Chen, J. GSH-Activatable Metal-Phenolic Networks for Photothermal-Enhanced Chemotherapy and Chemodynamic Therapy. J. Funct. Biomater. 2023, 14, 436. [Google Scholar] [CrossRef]
- Ball, P.; Garwin, L. Science at the atomic scale. Nature 1992, 355, 761–764. [Google Scholar] [CrossRef]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Labhasetwar, V.; Walter, E.; Levy, R.J.; Amidon, G.L. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 1997, 14, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Buono, C.; Anzinger, J.J.; Amar, M.; Kruth, H.S. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J. Clin. Investig. 2009, 119, 1373–1381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 163–210. [Google Scholar] [CrossRef]
- Plapied, L.; Duhem, N.; des Rieux, A.; Préat, V. Fate of polymeric nanocarriers for oral drug delivery. Curr. Opin. Colloid Interface Sci. 2011, 16, 228–237. [Google Scholar] [CrossRef]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Bano, I.; Skalickova, S.; Arbab, S.; Urbankova, L.; Horky, P. Toxicological effects of nanoselenium in animals. J. Anim. Sci. Biotechnol. 2022, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Ryabova, Y.V.; Sutunkova, M.P.; Minigalieva, I.A.; Shabardina, L.V.; Filippini, T.; Tsatsakis, A. Toxicological effects of selenium nanoparticles in laboratory animals: A review. J. Appl. Toxicol. 2024, 44, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.S.; Abou Baker, D.H.; Ahmed, E.A. Cytotoxicity and antimicrobial efficiency of selenium nanoparticles biosynthesized by Spirulina platensis. Arch. Microbiol. 2021, 203, 523–532. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).