Dietary Biomarkers of Vegetable and Fruit Intake in Asians: An Epidemiological Systematic Review
Abstract
1. Introduction
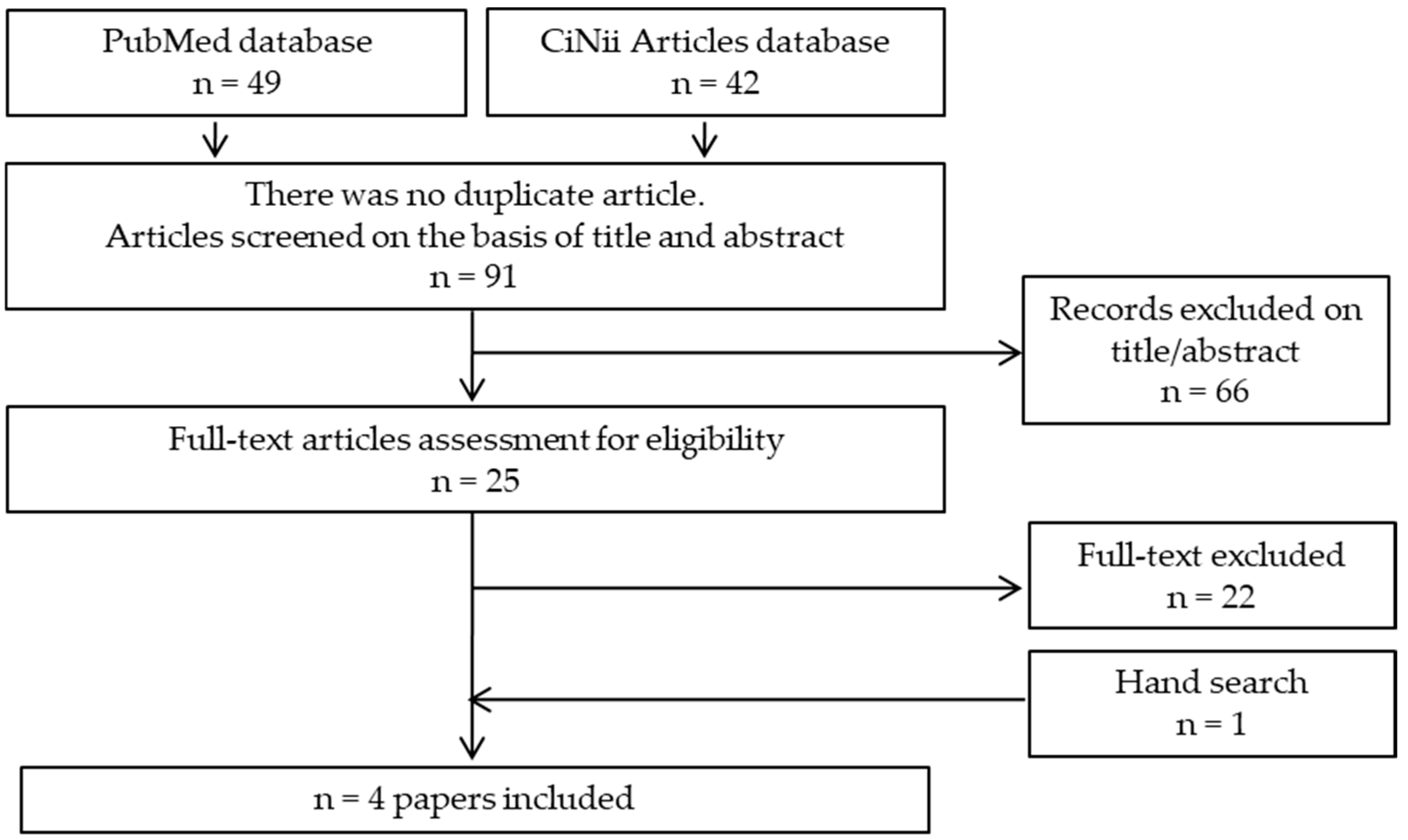
2. Materials and Methods
2.1. Protocol
2.2. Search Strategy
2.3. PECOS Criteria
2.4. Inclusion Criteria
- Studies involved men and women aged over 20 years in Asia;
- Studies written in Japanese or English;
- Studies wherein the intake of vegetables and fruits could be ascertained by weight;
- Interventional, observational, or studies published before or on 31 December 2021 on biomarkers (vitamin C, α-carotene, β-carotene, β-cryptoxanthin, lutein, and lycopene) of vegetable and fruit intake using blood specimens;
- Studies on healthy or unhealthy individuals including those with a high risk of CVD and impaired glucose metabolism;
- Studies wherein health outcomes, including surrogate markers, were measured (e.g., blood cholesterol levels in the case of hyperlipidemia).
2.5. Exclusion Criteria
- Interventional studies wherein the intervention consisted of dietary advice or counseling;
- Studies that altered dietary profiles (e.g., low-fat diet) through the additional intake of fruits and vegetables. This criterion excluded the possibility that changes in biomarkers were the result of dietary changes in foods other than fruits and vegetables;
- Interventional studies wherein not all fruits and vegetables were provided or were provided as supplements, juices, or extracts;
- Studies conducted in children, adolescents, institutionalized older populations, or pregnant or lactating women;
- Studies conducted in individuals with impaired micronutrient metabolism or vitamin deficiency.
2.6. Data Extraction
2.7. Quality Assessment
3. Results
3.1. Relationship between Vegetable Intake and Their Biomarkers
3.2. Relationship between Fruit Intake and Their Biomarkers
3.3. Article Quality in Each Study Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Z.; Sun, T.Y.; He, Y.; Gou, W.; Zuo, L.S.; Fu, Y.; Miao, Z.; Shuai, M.; Xu, F.; Xiao, C.; et al. Dietary fruit and vegetable intake, gut microbiota, and type 2 diabetes: Results from two large human cohort studies. BMC Med. 2020, 18, 371. [Google Scholar] [CrossRef] [PubMed]
- Madsen, H.; Sen, A.; Aune, D. Fruit and vegetable consumption and the risk of hypertension: A systematic review and meta-analysis of prospective studies. Eur. J. Nutr. 2023, 62, 1941–1955. [Google Scholar] [CrossRef] [PubMed]
- Van’t Veer, P.; Jansen, M.C.; Klerk, M.; Kok, F.J. Fruits and vegetables in the prevention of cancer and cardiovascular disease. Public Health Nutr. 2000, 3, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- World Health Organization. Fruit and Vegetables for Health. In Proceedings of the Report of a Joint FAO/WHO Workshop, Kobe, Japan, 1–3 September 2004; Available online: https://www.fao.org/3/y5861e/y5861e.pdf (accessed on 17 January 2024).
- Herforth, A.; Arimond, M.; Álvarez-Sánchez, C.; Coates, J.; Christianson, K.; Muehlhoff, E. A Global Review of Food-Based Dietary Guidelines. Adv. Nutr. 2019, 10, 590–605. [Google Scholar] [CrossRef]
- Ministry of Health, Labour, and Welfare. Health Japan 21 (the Second Term). Available online: https://www.nibiohn.go.jp/eiken/kenkounippon21/en/kenkounippon21/ (accessed on 10 January 2024).
- Yoshiike, N.; Hayashi, F.; Takemi, Y.; Mizoguchi, K.; Seino, F. A new food guide in Japan: The Japanese food guide Spinning Top. Nutr. Rev. 2007, 65, 149–154. [Google Scholar] [CrossRef]
- Ministry of Health, Labour, and Welfare. National Health and Nutrition Survey. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/r1-houkoku_00002.html (accessed on 7 February 2024).
- Hebert, J.R.; Hurley, T.G.; Peterson, K.E.; Resnicow, K.; Thompson, F.E.; Yaroch, A.L.; Ehlers, M.; Midthune, D.; Williams, G.C.; Greene, G.W.; et al. Social desirability trait influences on self-reported dietary measures among diverse participants in a multicenter multiple risk factor trial. J. Nutr. 2008, 138, 226s–234s. [Google Scholar] [CrossRef]
- Kipnis, V.; Midthune, D.; Freedman, L.; Bingham, S.; Day, N.E.; Riboli, E.; Ferrari, P.; Carroll, R.J. Bias in dietary-report instruments and its implications for nutritional epidemiology. Public Health Nutr. 2002, 5, 915–923. [Google Scholar] [CrossRef]
- Naska, A.; Lagiou, A.; Lagiou, P. Dietary assessment methods in epidemiological research: Current state of the art and future prospects. F1000Research 2017, 6, 926. [Google Scholar] [CrossRef]
- Leclercq, C.; Valsta, L.M.; Turrini, A. Food composition issues—Implications for the development of food-based dietary guidelines. Public Health Nutr. 2001, 4, 677–682. [Google Scholar] [CrossRef]
- Liang, S.; Nasir, R.F.; Bell-Anderson, K.S.; Toniutti, C.A.; O’Leary, F.M.; Skilton, M.R. Biomarkers of dietary patterns: A systematic review of randomized controlled trials. Nutr. Rev. 2022, 80, 1856–1895. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, C.; Tinker, L.F.; Neuhouser, M.L.; Prentice, R.L. Biomarker-Based Methods and Study Designs to Calibrate Dietary Intake for Assessing Diet-Disease Associations. J. Nutr. 2022, 152, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.; Luben, R.; Welch, A.; Low, Y.L.; Khaw, K.T.; Wareham, N.; Day, N. Associations between dietary methods and biomarkers, and between fruits and vegetables and risk of ischaemic heart disease, in the EPIC Norfolk Cohort Study. Int. J. Epidemiol. 2008, 37, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Pennant, M.; Steur, M.; Moore, C.; Butterworth, A.; Johnson, L. Comparative validity of vitamin C and carotenoids as indicators of fruit and vegetable intake: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 1331–1340. [Google Scholar] [CrossRef]
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 10 January 2024).
- Frankenfeld, C.L.; Lampe, J.W.; Shannon, J.; Gao, D.L.; Li, W.; Ray, R.M.; Chen, C.; King, I.B.; Thomas, D.B. Fruit and vegetable intakes in relation to plasma nutrient concentrations in women in Shanghai, China. Public Health Nutr. 2012, 15, 167–175. [Google Scholar] [CrossRef]
- Shibutami, E.; Ishii, R.; Harada, S.; Kurihara, A.; Kuwabara, K.; Kato, S.; Iida, M.; Akiyama, M.; Sugiyama, D.; Hirayama, A.; et al. Charged metabolite biomarkers of food intake assessed via plasma metabolomics in a population-based observational study in Japan. PLoS ONE 2021, 16, e0246456. [Google Scholar]
- Sugiura, M.; Matsumoto, H.; Kato, M.; Ikoma, Y.; Yano, M.; Nagao, A. Multiple linear regression analysis of the seasonal changes in the serum concentration of beta-cryptoxanthin. J. Nutr. Sci. Vitaminol. 2004, 50, 196–202. [Google Scholar] [CrossRef]
- Tsugane, S.; Fahey, M.T.; Kobayashi, M.; Sasaki, S.; Tsubono, Y.; Akabane, M.; Gey, F. Four food-frequency categories of fruit intake as a predictor of plasma ascorbic acid level in middle-aged Japanese men. Ann. Epidemiol. 1998, 8, 378–383. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef] [PubMed]
- Ward, M. Homocysteine, folate, and cardiovascular disease. Int. J. Vitam. Nutr. Res. 2001, 71, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.; Na, X.; Zhao, A. β-Carotene Supplementation and Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 1284. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, T.; Zheng, Y.; Muka, T.; Troup, J.; Hu, F.B. Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e003768. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Wee, C.C.; Kovell, L.C.; Plante, T.B.; Miller, E.R., 3rd; Appel, L.J.; Mukamal, K.J.; Juraschek, S.P. Effects of Diet on 10-Year Atherosclerotic Cardiovascular Disease Risk (from the DASH Trial). Am. J. Cardiol. 2023, 187, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Coyle, D.H.; Trieu, K.; Neal, B.; Mozaffarian, D.; Marklund, M.; Wu, J.H.Y. Healthy Food Prescription Programs and their Impact on Dietary Behavior and Cardiometabolic Risk Factors: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 1944–1956. [Google Scholar] [CrossRef]
- Maurer, J.; Taren, D.L.; Teixeira, P.J.; Thomson, C.A.; Lohman, T.G.; Going, S.B.; Houtkooper, L.B. The psychosocial and behavioral characteristics related to energy misreporting. Nutr. Rev. 2006, 64 Pt 1, 53–66. [Google Scholar] [CrossRef]
- Salvini, S.; Hunter, D.J.; Sampson, L.; Stampfer, M.J.; Colditz, G.A.; Rosner, B.; Willett, W.C. Food-based validation of a dietary questionnaire: The effects of week-to-week variation in food consumption. Int. J. Epidemiol. 1989, 18, 858–867. [Google Scholar] [CrossRef]
- Strassburg, A.; Eisinger-Watzl, M.; Krems, C.; Roth, A.; Hoffmann, I. Comparison of food consumption and nutrient intake assessed with three dietary assessment methods: Results of the German National Nutrition Survey II. Eur. J. Nutr. 2019, 58, 193–210. [Google Scholar] [CrossRef]
- Ministry of Education; Culture Sports, Science and Technology Council for Science and Technology Resource Research Sub-committee. Standard Tables of Food Composition in Japan 2020 Edition (8th Edition). Available online: https://www.mext.go.jp/a_menu/syokuhinseibun/mext_01110.html (accessed on 10 January 2024). (In Japanese)
- Ministry of Health, Labour and Welfare. Dietary Reference Intakes for Japanese (2020). Available online: https://www.mhlw.go.jp/content/001151422.pdf (accessed on 17 January 2014).
- Sauberlich, H.E.; Hodges, R.E.; Wallace, D.L.; Kolder, H.; Canham, J.E.; Hood, J.; Raica, N., Jr.; Lowry, L.K. Vitamin A metabolism and requirements in the human studied with the use of labeled retinol. Vitam. Horm. 1974, 32, 251–275. [Google Scholar]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- Cifelli, C.J.; Green, J.B.; Wang, Z.; Yin, S.; Russell, R.M.; Tang, G.; Green, M.H. Kinetic analysis shows that vitamin A disposal rate in humans is positively correlated with vitamin A stores. J. Nutr. 2008, 138, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Thürmann, P.A.; Steffen, J.; Zwernemann, C.; Aebischer, C.P.; Cohn, W.; Wendt, G.; Schalch, W. Plasma concentration response to drinks containing beta-carotene as carrot juice or formulated as a water dispersible powder. Eur. J. Nutr. 2002, 41, 228–235. [Google Scholar] [PubMed]
- Hartmann, D.; Thürmann, P.A.; Spitzer, V.; Schalch, W.; Manner, B.; Cohn, W. Plasma kinetics of zeaxanthin and 3’-dehydro-lutein after multiple oral doses of synthetic zeaxanthin. Am. J. Clin. Nutr. 2004, 79, 410–417. [Google Scholar] [CrossRef]
- Souverein, O.W.; de Vries, J.H.; Freese, R.; Watzl, B.; Bub, A.; Miller, E.R.; Castenmiller, J.J.; Pasman, W.J.; van Het Hof, K.; Chopra, M.; et al. Prediction of fruit and vegetable intake from biomarkers using individual participant data of diet-controlled intervention studies. Br. J. Nutr. 2015, 113, 1396–1409. [Google Scholar] [CrossRef]
- Duthie, S.J.; Duthie, G.G.; Russell, W.R.; Kyle, J.A.M.; Macdiarmid, J.I.; Rungapamestry, V.; Stephen, S.; Megias-Baeza, C.; Kaniewska, J.J.; Shaw, L.; et al. Effect of increasing fruit and vegetable intake by dietary intervention on nutritional biomarkers and attitudes to dietary change: A randomised trial. Eur. J. Nutr. 2018, 57, 1855–1872. [Google Scholar] [CrossRef]
- Baldrick, F.R.; Elborn, J.S.; Woodside, J.V.; Treacy, K.; Bradley, J.M.; Patterson, C.C.; Schock, B.C.; Ennis, M.; Young, I.S.; McKinley, M.C. Effect of fruit and vegetable intake on oxidative stress and inflammation in COPD: A randomised controlled trial. Eur. Respir. J. 2012, 39, 1377–1384. [Google Scholar] [CrossRef]
- Broekmans, W.M.; Klöpping-Ketelaars, I.A.; Schuurman, C.R.; Verhagen, H.; van den Berg, H.; Kok, F.J.; van Poppel, G. Fruits and vegetables increase plasma carotenoids and vitamins and decrease homocysteine in humans. J. Nutr. 2000, 130, 1578–1583. [Google Scholar] [CrossRef]
- van het Hof, K.H.; Tijburg, L.B.; Pietrzik, K.; Weststrate, J.A. Influence of feeding different vegetables on plasma levels of carotenoids, folate and vitamin C. Effect of disruption of the vegetable matrix. Br. J. Nutr. 1999, 82, 203–212. [Google Scholar] [CrossRef]
- Wallace, I.R.; McEvoy, C.T.; Hunter, S.J.; Hamill, L.L.; Ennis, C.N.; Bell, P.M.; Patterson, C.C.; Woodside, J.V.; Young, I.S.; McKinley, M.C. Dose-response effect of fruit and vegetables on insulin resistance in people at high risk of cardiovascular disease: A randomized controlled trial. Diabetes Care 2013, 36, 3888–3896. [Google Scholar] [CrossRef]
- Watzl, B.; Kulling, S.E.; Möseneder, J.; Barth, S.W.; Bub, A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am. J. Clin. Nutr. 2005, 82, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, D.; Donato, P.; Dugo, P.; Mondello, L. Recent analytical techniques advances in the carotenoids and their derivatives determination in various matrixes. J. Agric. Food Chem. 2018, 66, 3302–3307. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.M.; Canela-Garayoa, R. Analytical tools for the analysis of carotenoids in diverse materials. J. Chromatogr. A. 2012, 1224, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Ludwig, I.A.; Tomatis, V.B.; Acharjee, A.; Calani, L.; Rosi, A.; Brighenti, F.; Ray, S.; Griffin, J.L.; Bluck, L.J.; et al. Inter-individual variability in the production of flavan-3-ol colonic metabolites: Preliminary elucidation of urinary metabotypes. Eur. J. Nutr. 2019, 58, 1529–1543. [Google Scholar] [CrossRef] [PubMed]
- Englard, S.; Seifter, S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 1986, 6, 365–406. [Google Scholar] [CrossRef]
- Lang, R.; Lang, T.; Bader, M.; Beusch, A.; Schlagbauer, V.; Hofmann, T. High-Throughput Quantitation of Proline Betaine in Foods and Suitability as a Valid Biomarker for Citrus Consumption. J. Agric. Food Chem. 2017, 65, 1613–1619. [Google Scholar] [CrossRef]
- Saenger, T.; Hübner, F.; Lindemann, V.; Ganswind, K.; Humpf, H.U. Urinary Biomarkers for Orange Juice Consumption. Mol. Nutr. Food Res. 2021, 65, e2000781. [Google Scholar] [CrossRef]
- Ministry of Internal Affairs and Communications. Expenditure, Purchase Quantity, and Average Price per Household by City Class, Region, and Prefectural City. Household Survey. 2021. Available online: https://www.stat.go.jp/data/kakei/5.html (accessed on 19 January 2024).
| Author | Year | Participants | Study Design | N | Dietary Survey Method | Dietary Factor | Blood Biomarkers | Analytical Methods | Major Findings |
|---|---|---|---|---|---|---|---|---|---|
| Frankenfeld et al. [21] | 2012 | Textile workers in China | Randomized trial | 2031 | FFQ | Vegetables and fruits | β-carotene, α-carotene, lycopene, β-cryptoxanthin, lutein + zeaxanthin, retinol, retinol palmitate, α-tocopherol, γ-tocopherol, ascorbic acid | Ascorbic acid: colorimetric methodOthers: HPLC |
|
| Shibutami et al. [22] | 2021 | Local residents in Japan | Cross-sectional study | 7012 | Semi-quantitative FFQ | Vegetables (carotenoid-rich and other vegetables) and fruits | Citrate, creatine, cystine, galactarate, hippurate, lysine, proline betaine, threonate, tyrosine | CE-TOF-MS |
|
| Sugiura et al. [23] | 2004 | Local residents in Japan | Cross-sectional study | 27 | FFQ (Satsuma mandarin and other fruits and vegetables) | Fruits (Satsuma mandarin, mandarin juice, and other fruits) and vegetables (green-yellow and others) | β-cryptoxanthin | HPLC |
|
| Tsugane et al. [24] | 1998 | Local residents in Japan | Longitudinal study | FFQ: 621, Combined with a DR: 203 | FFQ combined with a 3-day DR | Vegetables (yellow, green leafy, other, fresh, and pickled vegetables) and fruits | Ascorbic acid | Fluorometric method |
|
| Criteria and Questions | Frankenfeld et al. [21] | Shibutani et al. [22] | Sugiura et al. [23] | Tsugane et al. [24] | ||
|---|---|---|---|---|---|---|
| 1 | Research question: was the research question or objective in this paper clearly stated? | Research question | Y | Y | Y | Y |
| 2 | Study population: was the study population clearly specified and defined? | Selection bias | Y | Y | Y | Y |
| 3 | Study population: was the participation rate of eligible persons at least 50%? | Selection bias | Y | Y | NR | Y |
| 4 | Groups recruited from the same population and uniform eligibility criteria: were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Selection bias | Y | Y | Y | Y |
| 5 | Sample size justification: was a sample size justification, power description, or variance and effect estimates provided? | Selection bias | N | N | N | N |
| 6 | Exposure assessed prior to outcome measurement: for the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | Causality | N | N | N | N |
| 7 | Sufficient timeframe to see an effect: was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | Causality | N | N | N | N |
| 8 | Different levels of the exposure of interest: for exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | Information bias | Y | N | N | Y |
| 9 | Exposure measures and assessment: were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Information bias | Y | Y | Y | Y |
| 10 | Repeated exposure assessment: was the exposure(s) assessed more than once over time? | Information bias | N | N | Y | Y |
| 11 | Outcome measures: were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Information bias | Y | Y | Y | Y |
| 12 | Blinding of outcome assessors: were the outcome assessors blinded to the exposure status of participants? | Information bias | NA | NA | NA | NA |
| 13 | Follow-up rate: was loss to follow-up after baseline 20% or less? | Selection bias | NA | NA | NA | NA |
| 14 | Statistical analyses: were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | Confounding bias | Y | Y | Y | Y |
| Ratio of YES * (%) | 66.7 | 58.3 | 63.6 | 75.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tousen, Y.; Ikaga, R.; Yasudomi, A.; Sasaki, N.; Kobori, T.; Kobori, M.; Takimoto, H. Dietary Biomarkers of Vegetable and Fruit Intake in Asians: An Epidemiological Systematic Review. Dietetics 2024, 3, 409-422. https://doi.org/10.3390/dietetics3040030
Tousen Y, Ikaga R, Yasudomi A, Sasaki N, Kobori T, Kobori M, Takimoto H. Dietary Biomarkers of Vegetable and Fruit Intake in Asians: An Epidemiological Systematic Review. Dietetics. 2024; 3(4):409-422. https://doi.org/10.3390/dietetics3040030
Chicago/Turabian StyleTousen, Yuko, Reina Ikaga, Ai Yasudomi, Naho Sasaki, Toshiro Kobori, Masuko Kobori, and Hidemi Takimoto. 2024. "Dietary Biomarkers of Vegetable and Fruit Intake in Asians: An Epidemiological Systematic Review" Dietetics 3, no. 4: 409-422. https://doi.org/10.3390/dietetics3040030
APA StyleTousen, Y., Ikaga, R., Yasudomi, A., Sasaki, N., Kobori, T., Kobori, M., & Takimoto, H. (2024). Dietary Biomarkers of Vegetable and Fruit Intake in Asians: An Epidemiological Systematic Review. Dietetics, 3(4), 409-422. https://doi.org/10.3390/dietetics3040030






