A Latent Class Analysis of Nutrition Impact Symptoms in Cancer Survivors
Abstract
1. Introduction
2. Materials and Methods
2.1. Questionnaires
2.2. Anthropometry and Handgrip Strength
2.3. Proposed Analytical Plan
3. Results
3.1. Descriptive Trends in Indicators
3.2. Fit Indices and Latent Class Analyses
3.3. Differences in Characteristics among the Latent Classes
3.4. Differences in Functioning and Global Health Status (GHS) between the Identified Classes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenthal, D.I.; Mendoza, T.R.; Chambers, M.S.; Burkett, V.S.; Garden, A.S.; Hessell, A.C.; Lewin, J.S.; Ang, K.K.; Kies, M.S.; Gning, I.; et al. The MD Anderson Symptom Inventory–Head and Neck Module, a Patient-Reported Outcome Instrument, Accurately Predicts the Severity of Radiation-Induced Mucositis. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Isenring, E.; Yates, P. The Prevalence of Nutrition Impact Symptoms and Their Relationship to Quality of Life and Clinical Outcomes in Medical Oncology Patients. Support. Care Cancer 2009, 17, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, N.-C.; Handschel, J.; Holtmann, H.; Krüskemper, G. Oral Cancer Malnutrition Impacts Weight and Quality of Life. Nutrients 2015, 7, 2145–2160. [Google Scholar] [CrossRef]
- Knudsen, A.W.; Naver, A.; Bisgaard, K.; Nordgaard-Lassen, I.; Becker, U.; Krag, A.; Slinde, F. Nutrition Impact Symptoms, Handgrip Strength and Nutritional Risk in Hospitalized Patients with Gastroenterological and Liver Diseases. Scand. J. Gastroenterol. 2015, 50, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.E.; Dodd, M.J.; Aouizerat, B.E.; Jahan, T.; Miaskowski, C. A Review of the Prevalence and Impact of Multiple Symptoms in Oncology Patients. J. Pain Symptom Manag. 2009, 37, 715–736. [Google Scholar]
- de Pinho, N.B.; Martucci, R.B.; Rodrigues, V.D.; D’almeida, C.A.; Thuler, L.C.S.; Saunders, C.; Jager-Wittenaar, H.; Peres, W.A.F. High Prevalence of Malnutrition and Nutrition Impact Symptoms in Older Patients with Cancer: Results of a Brazilian Multicenter Study. Cancer 2020, 126, 156–164. [Google Scholar] [CrossRef]
- Bruera, E.; Moyano, J.R.; Sala, R.; Rico, M.A.; Bosnjak, S.; Bertolino, M.; Willey, J.; Strasser, F.; Palmer, J.L. Dexamethasone in Addition to Metoclopramide for Chronic Nausea in Patients with Advanced Cancer: A Randomized Controlled Trial. J. Pain Symptom Manag. 2004, 28, 381–388. [Google Scholar] [CrossRef]
- Hutton, J.L.; Baracos, V.E.; Wismer, W.V. Chemosensory Dysfunction Is a Primary Factor in the Evolution of Declining Nutritional Status and Quality of Life in Patients with Advanced Cancer. J. Pain Symptom Manag. 2007, 33, 156–165. [Google Scholar] [CrossRef]
- Pud, D.; Ben Ami, S.; Cooper, B.A.; Aouizerat, B.E.; Cohen, D.; Radiano, R.; Naveh, P.; Nikkhou-Abeles, R.; Hagbi, V.; Kachta, O.; et al. The Symptom Experience of Oncology Outpatients Has a Different Impact on Quality-of-Life Outcomes. J. Pain Symptom Manag. 2008, 35, 162–170. [Google Scholar] [CrossRef]
- Miaskowski, C.; Cooper, B.A.; Melisko, M.; Chen, L.; Mastick, J.; West, C.; Paul, S.M.; Dunn, L.B.; Schmidt, B.L.; Hammer, M.; et al. Disease and Treatment Characteristics Do Not Predict Symptom Occurrence Profiles in Oncology Outpatients Receiving Chemotherapy. Cancer 2014, 120, 2371–2378. [Google Scholar] [CrossRef]
- Kubrak, C.; Olson, K.; Jha, N.; Jensen, L.; McCargar, L.; Seikaly, H.; Harris, J.; Scrimger, R.; Parliament, M.; Baracos, V.E. Nutrition Impact Symptoms: Key Determinants of Reduced Dietary Intake, Weight Loss, and Reduced Functional Capacity of Patients with Head and Neck Cancer before Treatment. Head Neck 2010, 32, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, K.L. Cancer Symptom Cluster Management. Semin. Oncol. Nurs. 2016, 32, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Pt-Global. Patient Generated Subjective Global Assesesment (Pg-Sga). Available online: http://pt-global.org/?page_id=13 (accessed on 8 May 2020).
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer Qlq-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. JNCI J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Body Mass Index-BMI. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 21 January 2020).
- Moreau, J.; Ordan, M.-A.; Barbe, C.; Mazza, C.; Perrier, M.; Botsen, D.; Brasseur, M.; Portefaix, C.; Renard, Y.; Talliere, B.; et al. Correlation between Muscle Mass and Handgrip Strength in Digestive Cancer Patients Undergoing Chemotherapy. Cancer Med. 2019, 8, 3677–3684. [Google Scholar] [CrossRef]
- Paek, J.; Choi, Y.J. Association between Hand Grip Strength and Impaired Health-Related Quality of Life in Korean Cancer Survivors: A Cross-Sectional Study. BMJ Open 2019, 9, e030938. [Google Scholar] [CrossRef]
- Tein, J.-Y.; Coxe, S.; Cham, H. Statistical Power to Detect the Correct Number of Classes in Latent Profile Analysis. Struct. Equ. Model. A Multidiscip. J. 2013, 20, 640–657. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X. Structural Equation Modeling: Applications Using Mplus; Wiley: Chichester, UK, 2012. [Google Scholar]
- Muthén, L.K.; Muthén, B.O. Mplus User’s Guide, 6th ed.; Muthén & Muthén: Los Angeles, CA, USA, 2010. [Google Scholar]
- Yuan, K.-H.; Bentler, P.M. Three Likelihood-Based Methods for Mean and Covariance Structure Analysis with Nonnormal Missing Data. Sociol. Methodol. 2000, 30, 165–200. [Google Scholar] [CrossRef]
- Akaike, H. Factor Analysis and Aic. Psychometrika 1987, 52, 317–332. [Google Scholar] [CrossRef]
- Sclove, S.L. Application of Model-Selection Criteria to Some Problems in Multivariate Analysis. Psychometrika 1987, 52, 333–343. [Google Scholar] [CrossRef]
- Lo, Y.; Mendell, N.R.; Rubin, D.B. Testing the Number of Components in a Normal Mixture. Biometrika 2001, 88, 767–778. [Google Scholar] [CrossRef]
- Nylund, K.L.; Asparouhov, T.; Muthén, B.O. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Struct. Equ. Model. A Multidiscip. J. 2007, 14, 535–569. [Google Scholar] [CrossRef]
- Ferreira, K.A.; Kimura, M.; Teixeira, M.J.; Mendoza, T.R.; da Nóbrega, J.C.M.; Graziani, S.R.; Takagaki, T.Y. Impact of Cancer-Related Symptom Synergisms on Health-Related Quality of Life and Performance Status. J. Pain Symptom Manag. 2008, 35, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Reese, J.B.; Blackford, A.; Sussman, J.; Okuyama, T.; Akechi, T.; Bainbridge, D.; Howell, D.; Snyder, C.F. Cancer Patients’ Function, Symptoms and Supportive Care Needs: A Latent Class Analysis across Cultures. Qual. Life Res. 2015, 24, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Miaskowski, C.; Dunn, L.; Ritchie, C.; Paul, S.M.; Cooper, B.; Aouizerat, B.E.; Alexander, K.; Skerman, H.; Yates, P. Latent Class Analysis Reveals Distinct Subgroups of Patients Based on Symptom Occurrence and Demographic and Clinical Characteristics. J. Pain Symptom Manag. 2015, 50, 28–37. [Google Scholar] [CrossRef]
- Dodd, M.J.; Cho, M.H.; Cooper, B.A.; Petersen, J.; Bank, K.A.; Lee, K.A.; Miaskowski, C. Identification of Latent Classes in Patients Who Are Receiving Biotherapy Based on Symptom Experience and Its Effect on Functional Status and Quality of Life. Oncol. Nurs. Forum 2011, 38, 33–42. [Google Scholar] [CrossRef]
- Illi, J.C.; Miaskowski, B.; Cooper, J.D.; Levine, L.; Dunn, C.; West, M.; Dodd, A.; Dhruva, S.M.; Paul, C.; Baggott, J.; et al. Association between Pro- and Anti-Inflammatory Cytokine Genes and a Symptom Cluster of Pain, Fatigue, Sleep Disturbance, and Depression. Cytokine 2012, 58, 437–447. [Google Scholar] [CrossRef]
- Miaskowski, C.; Cooper, B.A.; Paul, S.M.; Dodd, M.; Lee, K.; Aouizerat, B.E.; West, C.; Cho, M.; Bank, A. Subgroups of Patients with Cancer with Different Symptom Experiences and Quality-of-Life Outcomes: A Cluster Analysis. Oncol. Nurs. Forum 2006, 33, E79–E89. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Osoba, D.; Wu, A.W.; Wyrwich, K.W.; Norman, G.R. Methods to Explain the Clinical Significance of Health Status Measures. Mayo Clin. Proc. 2002, 77, 371–383. [Google Scholar] [CrossRef]
- Osoba, D. Interpreting the Meaningfulness of Changes in Health-Related Quality of Life Scores: Lessons from Studies in Adults. Int. J. Cancer 1999, 83, 132–137. [Google Scholar] [CrossRef]
- Crossnohere, N.L.; Richardson, D.R.; Reinhart, C.; O’donoghue, B.; Love, S.M.; Smith, B.D.; Bridges, J.F.P. Side effects from acute myeloid leukemia treatment: Results from a national survey. Curr. Med. Res. Opin. 2019, 35, 1965–1970. [Google Scholar] [CrossRef]
- Frick, M.A.; Vachani, C.C.; Hampshire, M.K.; Bach, C.; Arnold-Korzeniowski, K.; Metz, J.M.; Hill-Kayser, C.E. Survivorship after lower gastrointestinal cancer: Patient-reported outcomes and planning for care. Cancer 2017, 123, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.; O’Donovan, B.; Drummond, F.; Donnelly, C. The Unmet Needs of Cancer Survivors in Ireland: A Scoping Review; National Cancer Registry: Cork, Ireland, 2019.
- Yeo, T.P.; Cannaday, S. Cancer-related fatigue: Impact on patient quality of life and management approaches. Nurs. Res. Rev. 2015, 5, 65–76. [Google Scholar] [CrossRef]
- Liang, Y.; Hao, G.; Wu, M.; Hou, L. Social isolation in adults with cancer: An evolutionary concept analysis. Front. Psychol. 2022, 13, 973640. [Google Scholar] [CrossRef] [PubMed]
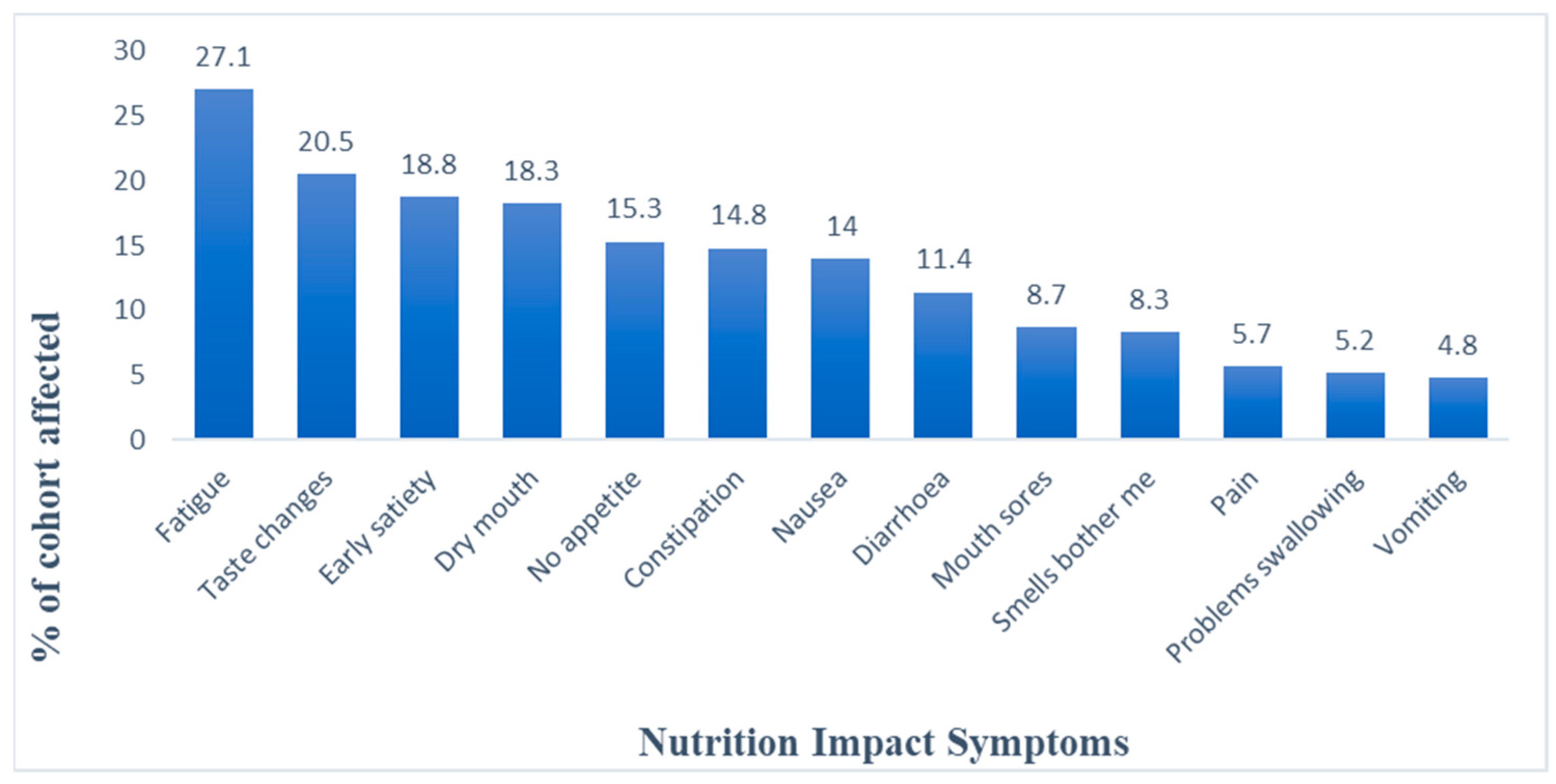

| Classes | LL | Par | AIC | BIC | ssaBIC | PB-LRT | p |
|---|---|---|---|---|---|---|---|
| 1 | −1111.921 | 13 | 2249.842 | 2294.480 | 2253.279 | ||
| 2 | −890.959 | 27 | 1835.918 | 1928.628 | 1843.056 | 441.924 | 0.000 |
| 3 | −860.126 | 41 | 1802.251 | 1943.034 | 1813.090 | 61.666 | 0.000 |
| 4 | −843.937 | 55 | 1797.874 | 1986.729 | 1812.414 | 32.377 | 0.200 |
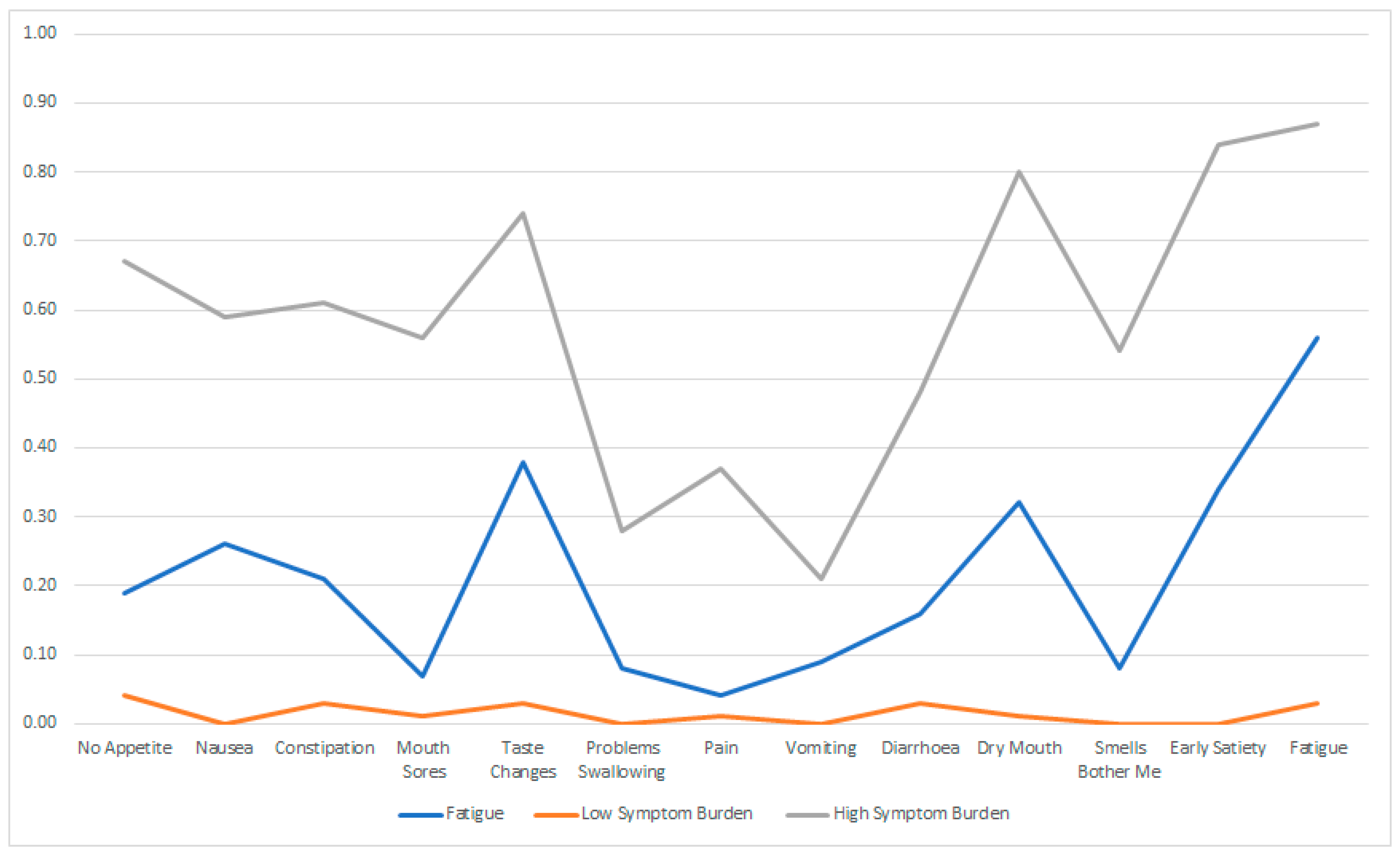
| Fatigue | Low Symptom Burden | High Symptom Burden | |
|---|---|---|---|
| 1 | 2 | 3 | |
| No appetite | 0.19 | 0.04 | 0.67 |
| Nausea | 0.26 | 0.00 | 0.59 |
| Constipation | 0.21 | 0.03 | 0.61 |
| Mouth sores | 0.07 | 0.01 | 0.56 |
| Taste changes | 0.38 | 0.03 | 0.74 |
| Problems swallowing | 0.08 | 0.00 | 0.28 |
| Pain | 0.04 | 0.01 | 0.37 |
| Vomiting | 0.09 | 0.00 | 0.21 |
| Diarrhoea | 0.16 | 0.03 | 0.48 |
| Dry mouth | 0.32 | 0.01 | 0.80 |
| Smells bother me | 0.08 | 0.00 | 0.54 |
| Early satiety | 0.34 | 0.00 | 0.84 |
| Fatigue | 0.56 | 0.03 | 0.87 |
| Percentage | 25% | 64% | 11% |
| N | 58 | 146 | 25 |
| Fatigue (1) n = 58 (25%) | Low Symptom Burden (2) n = 146 (64%) | High Symptom Burden (3) n = 25 (11%) | Significance | |
|---|---|---|---|---|
| Age (mean ± SD) | 63.66 (10.94) | 63.17 (12.45) | 63.54 (11.88) | NS (p = 0.69) |
| Age (years), n (%) | NS (p =0.98) | |||
| <65 | 30 (51.7) | 72 (50.3) | 13 (52.0) | |
| ≥65 | 28 (48.3) | 71 (49.7) | 12 (48.0) | |
| Gender, n (%) | X2 = 8.05 p = 0.05 1 < 3 2 < 3 | |||
Males | 20 (34.5) | 64 (44.8) | 4 (16.0) | |
| Females | 38 (65.5) | 79 (55.2) | 21 (84.0) | |
| Education, n (%) | NS (p = 0.08) | |||
| <Third level | 39 (68.4) | 108 (76.6) | 14 (56.0) | |
| ≥Third level | 18 (31.6) | 33 (23.4) | 11 (44.0) | |
| Employment, n (%) * | NS (p = 0.11) | |||
| Not working | 43 (75.4) | 87 (60.8) | 18 (72.0) | |
| Working | 14 (24.6) | 56 (39.2) | 7 (28.0) | |
| Living situation, n (%) | NS (p = 0.08) | |||
| Alone | 7 (12.3) | 38 (27.0) | 5 (20.0) | |
| Family/Others | 50 (87.7) | 103 (73.0) | 20 (80.0) | |
| BMI, median (IQR) | 25.41 (5.12) | 27.21 (6.53) | 25.97 (6.88) | NS (p = 0.16) |
| BMI category, n (%) | NS (p = 0.35) | |||
| <25 kg/m2 | 26 (44.8) | 66 (46.1) | 12 (48.0) | |
| 25–29.99 kg/m2 | 23 (39.7) | 40 (28.0) | 9 (36.0) | |
| ≥30 kg/m2 | 9 (15.5) | 37 (25.9) | 4 (16.0) | |
| Time since diagnosis, n (%) | X2 = 10.16 p = 0.01 1 < 3 2 < 3 1 > 2 | |||
| <2 years | 16 (27.6) | 73 (51.4) | 9 (36.0) | |
| ≥2 years | 42 (72.4) | 69 (48.6) | 16 (56.4) | |
| Currently receiving treatment, n (%) | X2 = 8.08 p = 0.02 1 > 2 2 > 3 | |||
| Yes | 49 (84.5) | 92 (64.3) | 18 (72.0) | |
| No | 9 (15.5) | 51 (35.7) | 7 (28.0) | |
| Experienced weight loss in last six months, n (%) | X2 = 8.56 p = 0.01 No significant contrasts | |||
| Yes | 28 (48.3) | 45 (31.5) | 14 (56.0) | |
| No | 30 (51.7) | 98 (68.5) | 11 (44.0) | |
| Food intake (last month), n (%) | X2 = 28.610 p = 0.00 1, 3 < 2, 3 > 1, 1, 2 > 3 1, 2 < 3 | |||
| Unchanged | 22 (37.9) | 102 (71.3) | 10 (41.7) | |
| Consuming more | 16 (27.6) | 17 (11.9) | 2 (8.3) | |
| Consuming less | 20 (34.5) | 24 (16.8) | 12 (50.0) | |
| Handgrip strength, median (IQR) | F(2) = 4.8, p = 0.01 1 < 2 NS 2 > 1 NS 3 < 2 p = 0.02 | |||
| 25.0 (8.4) | 28.2 (11.3) | 21.7 (8.1) |
| M | SD | DF | ||||
|---|---|---|---|---|---|---|
| Variables | F | Sig | ||||
| Global Health Status | Fatigue | 62.86 | 21.65 | 2 | 15.4 | 0.00 |
| Low Symptom Burden | 71.38 | 18.70 | 222 | |||
| High Symptom Burden | 48.66 | 21.87 | ||||
| Physical functioning | Fatigue | 75.66 | 21.29 | 2 | 16.5 | 0.00 |
| Low Symptom Burden | 83.81 | 17.58 | 221 | |||
| High Symptom Burden | 61.33 | 19.72 | ||||
| Role functioning | Fatigue | 68.42 | 30.16 | 2 | 10.8 | 0.00 |
| Low Symptom Burden | 81.47 | 26.84 | 222 | |||
| High Symptom Burden | 55.99 | 32.59 | ||||
| Emotional functioning | Fatigue | 79.87 | 21.43 | 2 | 11.0 | 0.00 |
| Low Symptom Burden | 84.63 | 20.29 | 218 | |||
| High Symptom Burden | 62.84 | 25.89 | ||||
| Cognitive functioning | Fatigue | 81.87 | 19.99 | 2 | 8.2 | 0.00 |
| Low Symptom Burden | 82.98 | 22.25 | 222 | |||
| High Symptom Burden | 63.33 | 28.87 | ||||
| Social functioning | Fatigue | 71.05 | 27.19 | 2 | 7.8 | 0.00 |
| Low Symptom Burden | 77.15 | 26.52 | 222 | |||
| High Symptom Burden | 53.99 | 33.08 |
| Variable | Class A | Class B | Mean Difference (A)-(B) | Sig |
|---|---|---|---|---|
| Global Health Status | Fatigue | Low Symptom Burden | −8.5 * | 0.02 |
| High Symptom Burden | 14.2 * | 0.01 | ||
| Low Symptom Burden | Fatigue | 8.5 * | 0.02 | |
| High Symptom Burden | 22.7 * | 0.00 | ||
| Physical functioning | Fatigue | Low Symptom Burden | −8.1 * | 0.02 |
| High Symptom Burden | 14.3 * | 0.00 | ||
| Low Symptom Burden | Fatigue | 8.2 * | 0.02 | |
| High Symptom Burden | 22.5 * | 0.00 | ||
| Role functioning | Fatigue | Low Symptom Burden | −13.1 * | 0.01 |
| High Symptom Burden | 12.4 | 0.16 | ||
| Low Symptom Burden | Fatigue | 13.1 * | 0.01 | |
| High Symptom Burden | 25.5 * | 0.00 | ||
| Emotional functioning | Fatigue | Low Symptom Burden | −4.8 | 0.33 |
| High Symptom Burden | 17.0 * | 0.00 | ||
| Low Symptom Burden | Fatigue | 4.8 | 0.33 | |
| High Symptom Burden | 21.8 * | 0.00 | ||
| Cognitive functioning | Fatigue | Low Symptom Burden | −1.1 | 0.95 |
| High Symptom Burden | 18.5 * | 0.00 | ||
| Low Symptom Burden | Fatigue | 1.1 | 0.95 | |
| High Symptom Burden | 19.7 * | 0.00 | ||
| Social functioning | Fatigue | Low Symptom Burden | −6.1 | 0.33 |
| High Symptom Burden | 17.1 * | 0.03 | ||
| Low Symptom Burden | Fatigue | 6.1 | 0.33 | |
| High Symptom Burden | 23.2 * | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keaver, L.; McLaughlin, C. A Latent Class Analysis of Nutrition Impact Symptoms in Cancer Survivors. Dietetics 2024, 3, 423-434. https://doi.org/10.3390/dietetics3040031
Keaver L, McLaughlin C. A Latent Class Analysis of Nutrition Impact Symptoms in Cancer Survivors. Dietetics. 2024; 3(4):423-434. https://doi.org/10.3390/dietetics3040031
Chicago/Turabian StyleKeaver, Laura, and Christopher McLaughlin. 2024. "A Latent Class Analysis of Nutrition Impact Symptoms in Cancer Survivors" Dietetics 3, no. 4: 423-434. https://doi.org/10.3390/dietetics3040031
APA StyleKeaver, L., & McLaughlin, C. (2024). A Latent Class Analysis of Nutrition Impact Symptoms in Cancer Survivors. Dietetics, 3(4), 423-434. https://doi.org/10.3390/dietetics3040031






