Identification of the Risk Factors Associated with Low Bone Density in Peri- and Early Postmenopausal Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Demographic Characteristics of Participants
3.2. Body Composition Factors of Participants and Bone Mineral Density
3.3. Physical Activity of Participants and Bone Mineral Density
3.4. Dietary Factors of Participants and Bone Mineral Density
3.5. Correlations between Total Calorie Intake, Nutrient Intake Status Related to Osteoporosis, and BMD
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daly, R.M.; Dalla Via, J.; Duckham, R.L.; Fraser, S.F.; Helge, E.W. Exercise for the prevention of osteoporosis in postmenopausal women: An evidence-based guide to the optimal prescription. Braz. J. Phys. Ther. 2019, 23, 170–180. [Google Scholar] [CrossRef]
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef]
- El Maghraoui, A.; Roux, C. DXA scanning in clinical practice. QJM Int. J. Med. 2008, 101, 605–617. [Google Scholar] [CrossRef]
- Cohen, A.; Shane, E. Evaluation and management of the premenopausal woman with low BMD. Curr. Osteoporos. Rep. 2013, 11, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.N.; Lie, J.D.; Wan, C.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. P T A Peer-Rev. J. Formul. Manag. 2018, 43, 92–104. [Google Scholar]
- Sale, J.E.M.; Gignac, M.A.; Hawker, G.; Frankel, L.; Beaton, D.; Bogoch, E.; Elliot-Gibson, V. Decision to take osteoporosis medication in patients who have had a fracture and are ‘high’ risk for future fracture: A qualitative study. BMC Musculoskelet. Disord. 2011, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Begic, Z.; Rizvanovic, M. Bone density and body fat distribution in postmenopausal women. Mater. Socio-Medica 2021, 33, 41–44. [Google Scholar] [CrossRef]
- Morin, S.; Leslie, W.D.; Manitoba Bone, D.P. High bone mineral density is associated with high body mass index. Osteoporos. Int. 2009, 20, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.R.; Franz, R.; Maturana, M.A.; Spritzer, P.M. Associations between body composition and lifestyle factors with bone mineral density according to time since menopause in women from southern brazil: A cross-sectional study. BMC Endocr. Disord. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Namwongprom, S.; Rojanasthien, S.; Wongboontan, C.; Mangklabruks, A. Contribution of android and gynoid adiposity to bone mineral density in healthy postmenopausal Thai women. J. Clin. Densitom. 2019, 22, 346–350. [Google Scholar] [CrossRef]
- Kuwahata, A.; Kawamura, Y.; Yonehara, Y.; Matsuo, T.; Iwamoto, I.; Douchi, T. Non-weight-bearing effect of trunk and peripheral fat mass on bone mineral density in pre- and post-menopausal women. Maturitas 2008, 60, 244–247. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Makizako, H.; Kiyama, R.; Tomioka, K.; Nakai, Y.; Kubozono, T.; Ohishi, M. The association between osteoporosis and grip strength and skeletal muscle mass in community-dwelling older women. Int. J. Environ. Res. Public Health 2019, 16, 1228. [Google Scholar] [CrossRef] [PubMed]
- Muir, J.M.; Ye, C.; Bhandari, M.; Adachi, J.D.; Thabane, L. The effect of regular physical activity on bone mineral density in post-menopausal women aged 75 and over: A retrospective analysis from the canadian multicentre osteoporosis study. BMC Musculoskelet. Disord. 2013, 14, 253. [Google Scholar] [CrossRef] [PubMed]
- Segev, D.; Hellerstein, D.; Dunsky, A. Physical Activity-does it Really Increase Bone Density in Postmenopausal Women? A Review of Articles Published Between 2001–2016. Curr. Aging Sci. 2018, 11, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Shojaa, M.; Von Stengel, S.; Schoene, D.; Kohl, M.; Barone, G.; Bragonzoni, L.; Kemmler, W. Effect of exercise training on bone mineral density in post-menopausal women: A systematic review and meta-analysis of intervention studies. Front. Physiol. 2020, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.A.; Mota, J.; Machado, L.; Sousa, F.; Coelho, M.; Moreira, P.; Carvalho, J. Multicomponent training program with weight-bearing exercises elicits favorable bone density, muscle strength, and balance adaptations in older women. Calcif. Tissue Int. 2011, 88, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.D.; Modlesky, C.M. Nutrition, physical activity, and bone health in women. Int. J. Sport Nutr. 1998, 8, 250–284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rizzoli, R.; Bischoff-Ferrari, H.; Dawson-Hughes, B.; Weaver, C. Nutrition and bone health in women after the menopause. Womens Health 2014, 10, 599–608. [Google Scholar] [CrossRef]
- Park, S.; Joo, S.; Min, H.; Park, J.K.; Kim, Y.; Kim, S.S.; Ahn, Y. Dietary patterns and osteoporosis risk in postmenopausal korean women. Osong Public Health Res. Perspect. 2012, 3, 199–205. [Google Scholar] [CrossRef]
- Ilesanmi-Oyelere, B.; Coad, J.; Roy, N.C.; Kruger, M.C. Dietary patterns, body composition, and bone health in new zealand postmenopausal women. Front. Nutr. 2020, 7, 563689. [Google Scholar] [CrossRef]
- Al-Khammash, A.; Tayyem, R.F.; Ajeen, R. Assessment of Nutrients Associated with the Risk of Osteoporosis in Postmenopausal Women: A Case-Control Study. Curr. Res. Nutr. Food Sci. 2021, 10. [Google Scholar] [CrossRef]
- Özpak Akkuş, Ö.; Atalay, B. Post-menopausal osteoporosis: Do body composition, nutritional habits, and physical activity affect bone mineral density? [Osteoporosis posmenopáusica: ¿afectan a la densidad mineral ósea la composición corporal, los hábitos nutricionales y la actividad física?]. Nutr. Hosp. 2020, 37, 977–983. [Google Scholar] [CrossRef]
- Macdonald, H.M.; New, S.A.; Golden, M.H.N.; Campbell, M.K.; Reid, D.M. Nutritional associations with bone loss during the menopausal transition: Evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am. J. Clin. Nutr. 2004, 79, 155–165. [Google Scholar] [CrossRef]
- Borba-Pinheiro, C.J.; Drigo, A.J.; de Alencar Carvalho, M.C.G.; da Silva, N.S.L.; Dantas, E.H.M. Factors that contribute to low bone density in postmenopausal women in different amazonian communities. Ther. Adv. Musculoskelet. Dis. 2011, 3, 81–90. [Google Scholar] [CrossRef]
- Nosal, B.M.; Sakaki, J.R.; Macdonald, Z.; Mahoney, K.; Kim, K.; Madore, M.; Chun, O.K. Blackcurrants Reduce the Risk of Postmenopausal Osteoporosis: A Pilot Double-Blind, Randomized, Placebo-Controlled Clinical Trial. Nutrients 2022, 14, 4971. [Google Scholar] [CrossRef]
- Jetté, M.; Sidney, K.; Blümchen, G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef]
- American Bone Health. Understanding Bone Density Results. Available online: https://americanbonehealth.org/bone-density/understanding-the-bone-density-t-score-and-z-score/ (accessed on 22 December 2023).
- Added Sugars on the Nutrition Facts Label. 2023. Available online: https://www.fda.gov/food/nutrition-facts-label/added-sugars-nutrition-facts-label (accessed on 22 December 2023).
- Potassium Fact Sheet for Health Professionals. 2022. Available online: https://ods.od.nih.gov/factsheets/Potassium-HealthProfessional/ (accessed on 22 December 2023).
- Vitamin E Fact Sheet for Health Professionals. 2021. Available online: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ (accessed on 22 December 2023).
- Wu, S.; Du, X. Body mass index may positively correlate with bone mineral density of lumbar vertebra and femoral neck in postmenopausal females. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 145–151. [Google Scholar] [CrossRef]
- Ho-Pham, L.; Nguyen, U.D.T.; Nguyen, T.V. Association Between Lean Mass, Fat Mass, and Bone Mineral Density: A Meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Hosseini, S.R.; Ghadimi, R.; Noreddini, H.; Bijani, A. Association between body fat and bone mineral density in non-obese post-menopausal women over 60 years old. Casp. J. Intern. Med. 2021, 12, 200–206. [Google Scholar] [CrossRef]
- Fan, J.; Jiang, Y.; Qiang, J.; Han, B.; Zhang, Q. Associations of Fat Mass and Fat Distribution With Bone Mineral Density in Non-Obese Postmenopausal Chinese Women Over 60 Years Old. Front. Endocrinol. 2022, 13, 829867. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.; Fan, C.H.; Lin, Z.; Hsu, R.W. Effect of basal metabolic rate on the bone mineral density in middle to old age women in Taiwan. Maturitas 2013, 76, 70–74. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Shimomura, I.; Nakamura, T.; Keno, Y.; Kotani, K.; Tokunaga, K. Pathophysiology and pathogenesis of visceral fat obesity. Obes. Res. 1995, 3, 187s–194s. [Google Scholar] [CrossRef] [PubMed]
- Kodoth, V.; Scaccia, S.; Aggarwal, B. Adverse Changes in Body Composition During the Menopausal Transition and Relation to Cardiovascular Risk: A Contemporary Review. Women’s Health Rep. 2022, 3, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, L.; Shao, X. Association Analysis of Insulin Resistance and Osteoporosis Risk in Chinese Patients with T2DM. Ther. Clin. Risk Manag. 2021, 17, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Mandal, C.C. High Cholesterol Deteriorates Bone Health: New Insights into Molecular Mechanisms. Front. Endocrinol. 2015, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, G.F.; Lunz, T.M.; de Jesus, T.R.; Costa, M.B.; Vidigal, C.V.; Albergaria, B.; Guandalini, V.R. Influence of the appendicular skeletal muscle mass index on the bone mineral density of postmenopausal women. BMC Musculoskelet. Disord. 2021, 22, 861. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Hou, Y.; Li, Z.; Zhou, Y.; Xue, H.; Wen, T.; Ma, T. Association between physical activity and bone mineral density in postmenopausal women: A cross-sectional study from the NHANES 2007–2018. J. Orthop. Surg. Res. 2023, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Hong, J.; Han, K.; Lee, J.C.; Shin, B.; Choi, S.; Bang, C. Relationship between bone mineral density and alcohol intake: A nationwide health survey analysis of postmenopausal women. PLoS ONE 2017, 12, e0180132. [Google Scholar] [CrossRef]
- Feskanich, D.; Korrick, S.A.; Greenspan, S.L.; Rosen, H.N.; Colditz, G.A. Moderate alcohol consumption and bone density among postmenopausal women. J. Women’s Health 1999, 8, 65–73. [Google Scholar] [CrossRef]
- Mazidi, M.; Ferns, G.A. Plasma trans fatty acids concentrations are inversely related to bone mineral density and this appears to be independent of inflammation. J. Am. Nutr. Assoc. 2023, 42, 427–434. [Google Scholar] [CrossRef]
- Park, Y. Conjugated linoleic acid (CLA): Good or bad trans fat? J. Food Compos. Anal. 2009, 22, S4–S12. [Google Scholar] [CrossRef]
- Ahn, H.; Park, Y.K. Sugar-sweetened beverage consumption and bone health: A systematic review and meta-analysis. Nutr. J. 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Yu, X. Fat, Sugar, and Bone Health: A Complex Relationship. Nutrients 2017, 9, 506. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.S.; Corey, P.N.; Cheung, A.M. Effects of vitamin E on bone turnover markers among US postmenopausal women. J. Bone Miner. Res. 2012, 27, 1368–1380. [Google Scholar] [CrossRef]
- Ha, J.; Kim, S.; Lim, K.; Shin, S. The association of potassium intake with bone mineral density and the prevalence of osteoporosis among older Korean adults. Nutr. Res. Pract. 2020, 14, 55–61. [Google Scholar] [CrossRef]

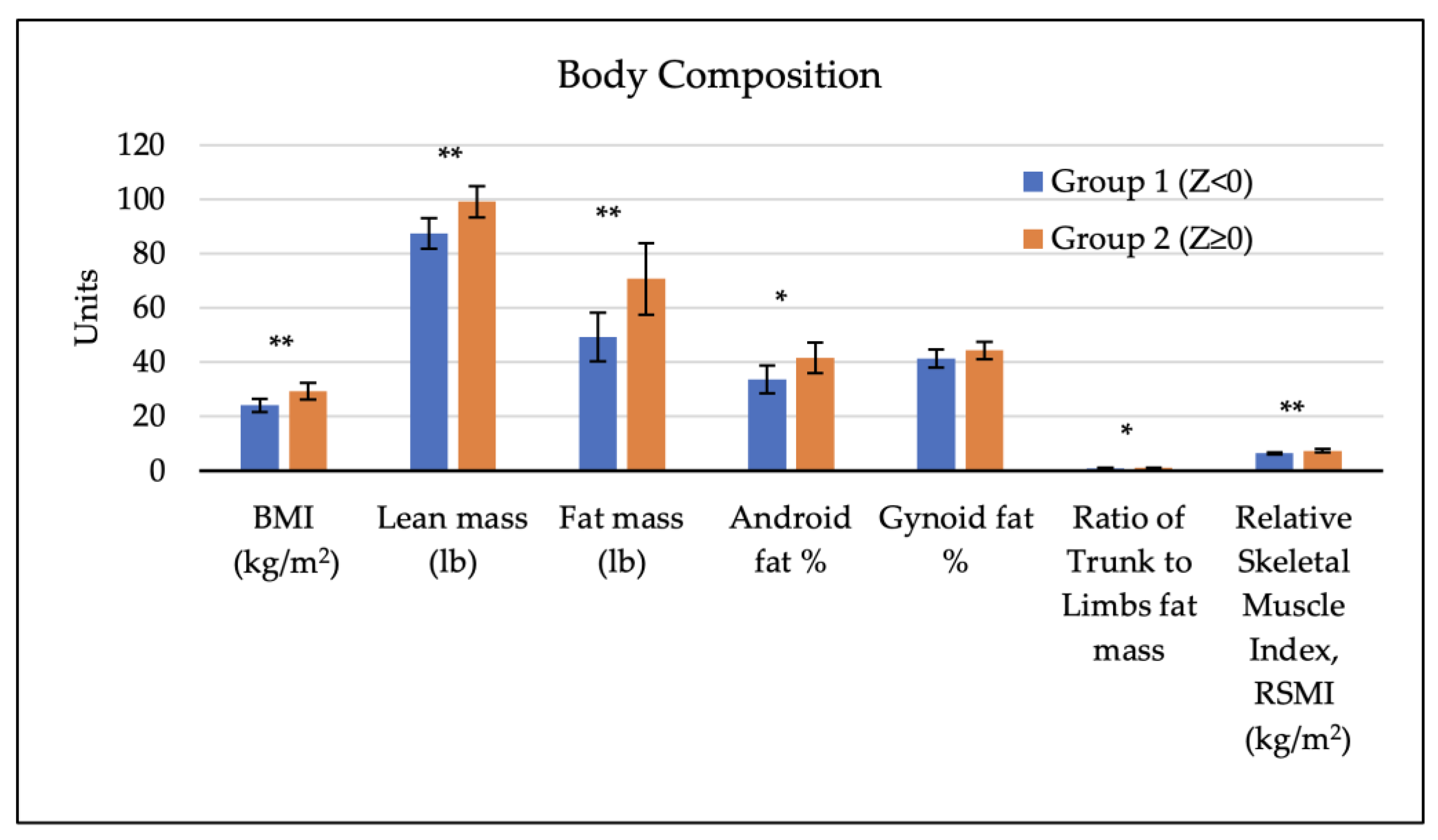


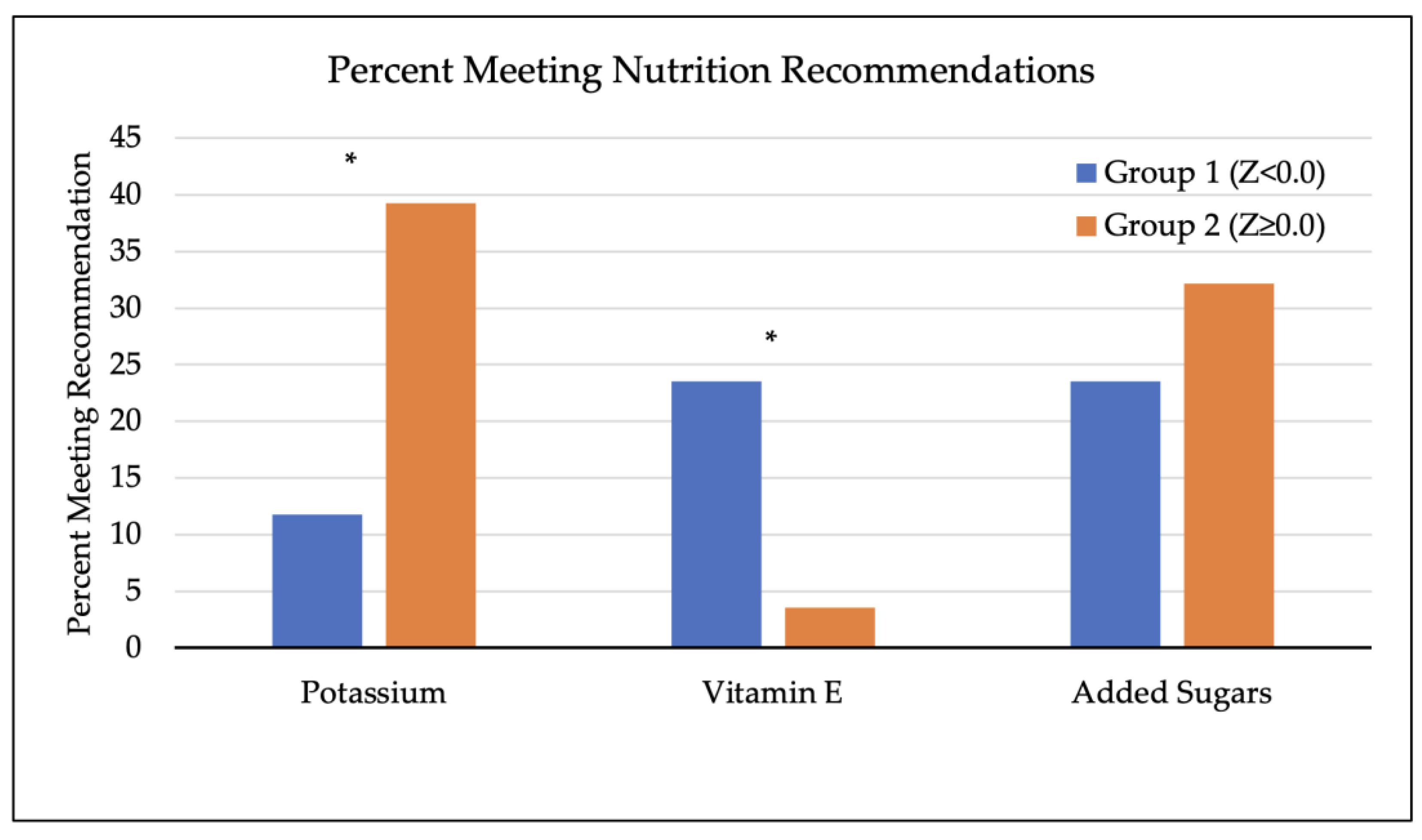
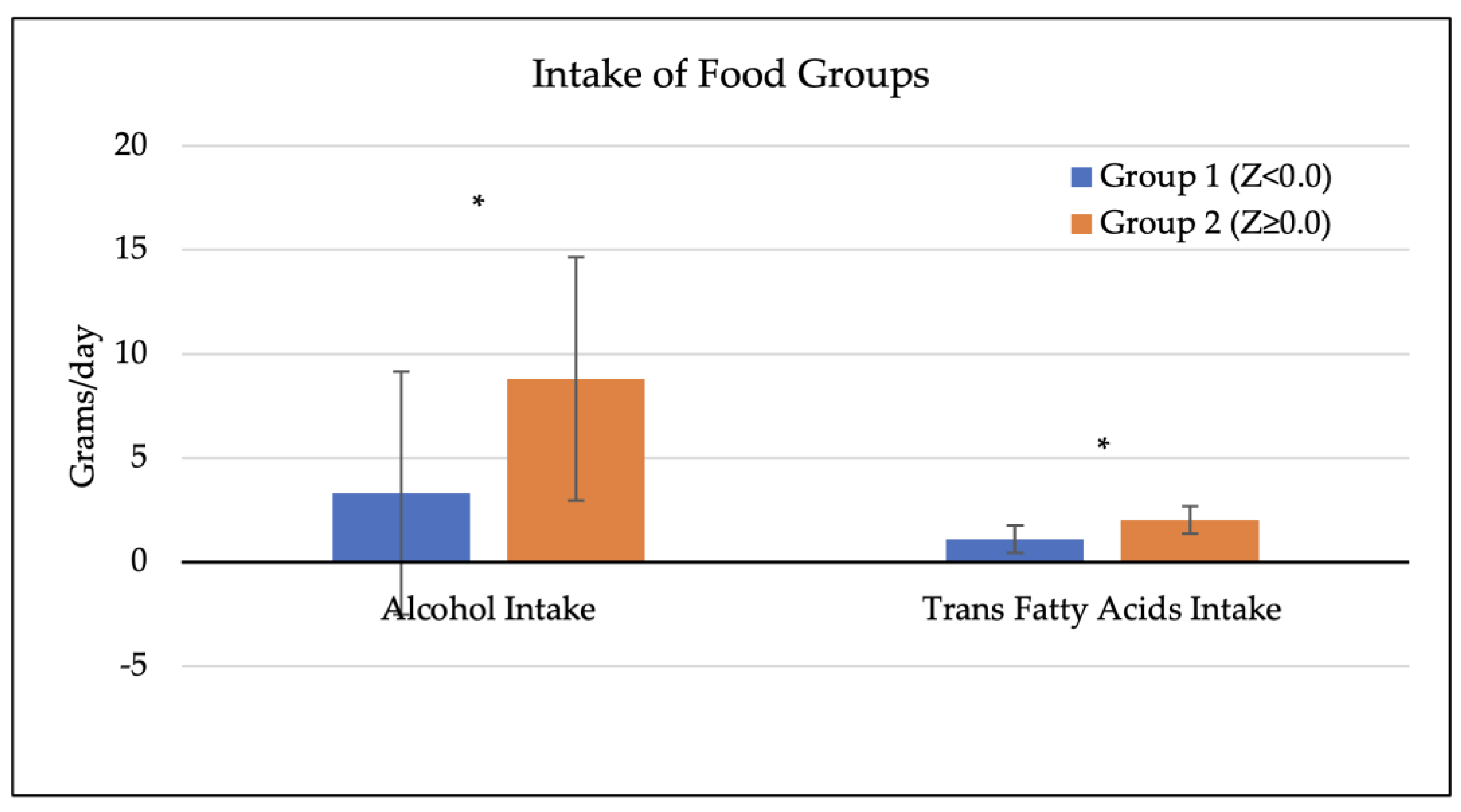
| Factor | All Participants (n = 45) | Group 1, Z < 0.0 (n = 17) | Group 2, Z ≥ 0.0 (n = 28) | p-Value (t-Test) a |
|---|---|---|---|---|
| Whole-body BMD (Z) | 0.35 ± 1.06 | −0.74 ± 0.56 | 1.03 ± 0.64 | <0.05 |
| Household annual income b | ||||
| <USD 90,000 | 10 (23.26) | 2 (11.76) | 8 (30.77) | 0.607 |
| USD 90,000–USD 125,000 | 13 (30.23) | 7 (41.18) | 6 (23.08) | |
| >USD 125,000 | 20 (46.51) | 8 (47.06) | 12 (46.15) | |
| Education b | ||||
| Has college degree | 34 (79.07) | 14 (82.35) | 20 (76.92) | 0.669 |
| No college degree | 9 (20.93) | 3 (17.65) | 6 (23.08) | |
| Race/ethnicity b | ||||
| White | 39 (90.70) | 15 (88.24) | 24 (92.31) | 0.130 |
| Hispanic | 1 (2.33) | 0 (0) | 1 (3.85) | |
| Asian | 2 (4.65) | 1 (5.88) | 1 (3.85) | |
| Other | 1 (2.33) | 1 (5.88) | 0 (0) |
| Whole-Body BMD | Total Calories | Added Sugars | Vitamin E | Potassium | Alcohol | Trans Fatty Acids | Calcium | Vitamin D | |
|---|---|---|---|---|---|---|---|---|---|
| Whole-Body BMD | 1.0 | ||||||||
| Total calories | 0.20579 (0.1802) | 1.0 | |||||||
| Added sugars | 0.30032 (0.0476) * | 0.37379 (0.0124) * | 1.0 | ||||||
| Vitamin E | −0.02822 (0.8557) | 0.45351 (0.0020) * | 0.03309 (0.8312) | 1.0 | |||||
| Potassium | 0.09037 (0.5596) | 0.50223 (0.0005) * | 0.00111 (0.9943) | 0.67198 (<0.0001) * | 1.0 | ||||
| Alcohol | 0.13251 (0.3912) | 0.50247 (0.0005) * | −0.10197 (0.5101) | 0.03184 (0.8374) | 0.12593 (0.4153) | 1.0 | |||
| Trans fatty acids | 0.16705 (0.2784) | 0.49853 (0.0006) * | 0.06899 (0.6563) | −0.09928 (0.5214) | 0.04174 (0.7879) | 0.56243 (<0.0001) * | 1.0 | ||
| Calcium | 0.21885 (0.1535) | 0.52041 (0.0003) * | 0.39916 (0.0073) * | 0.46289 (0.0016) * | 0.41897 (0.0046) * | 0.19362 (0.2079) | 0.31577 (0.0368) * | 1.0 | |
| Vitamin D | 0.02204 (0.8871) | 0.74634 (<0.0001) * | 0.05598 (0.7182) | 0.57478 (<0.0001) * | 0.46271 (0.0016) * | 0.26600 (0.0809) | 0.33201 (0.0277) * | 0.47527 (0.0011) * | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, D.B.; Nosal, B.M.; Mofrad, M.D.; Chun, O.K. Identification of the Risk Factors Associated with Low Bone Density in Peri- and Early Postmenopausal Women. Dietetics 2024, 3, 75-86. https://doi.org/10.3390/dietetics3010007
Patel DB, Nosal BM, Mofrad MD, Chun OK. Identification of the Risk Factors Associated with Low Bone Density in Peri- and Early Postmenopausal Women. Dietetics. 2024; 3(1):75-86. https://doi.org/10.3390/dietetics3010007
Chicago/Turabian StylePatel, Dave B., Briana M. Nosal, Manije Darooghegi Mofrad, and Ock K. Chun. 2024. "Identification of the Risk Factors Associated with Low Bone Density in Peri- and Early Postmenopausal Women" Dietetics 3, no. 1: 75-86. https://doi.org/10.3390/dietetics3010007
APA StylePatel, D. B., Nosal, B. M., Mofrad, M. D., & Chun, O. K. (2024). Identification of the Risk Factors Associated with Low Bone Density in Peri- and Early Postmenopausal Women. Dietetics, 3(1), 75-86. https://doi.org/10.3390/dietetics3010007







