Nutraceuticals for Knee Osteoarthritis Pain Relief. Results from a Preliminary Randomised Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
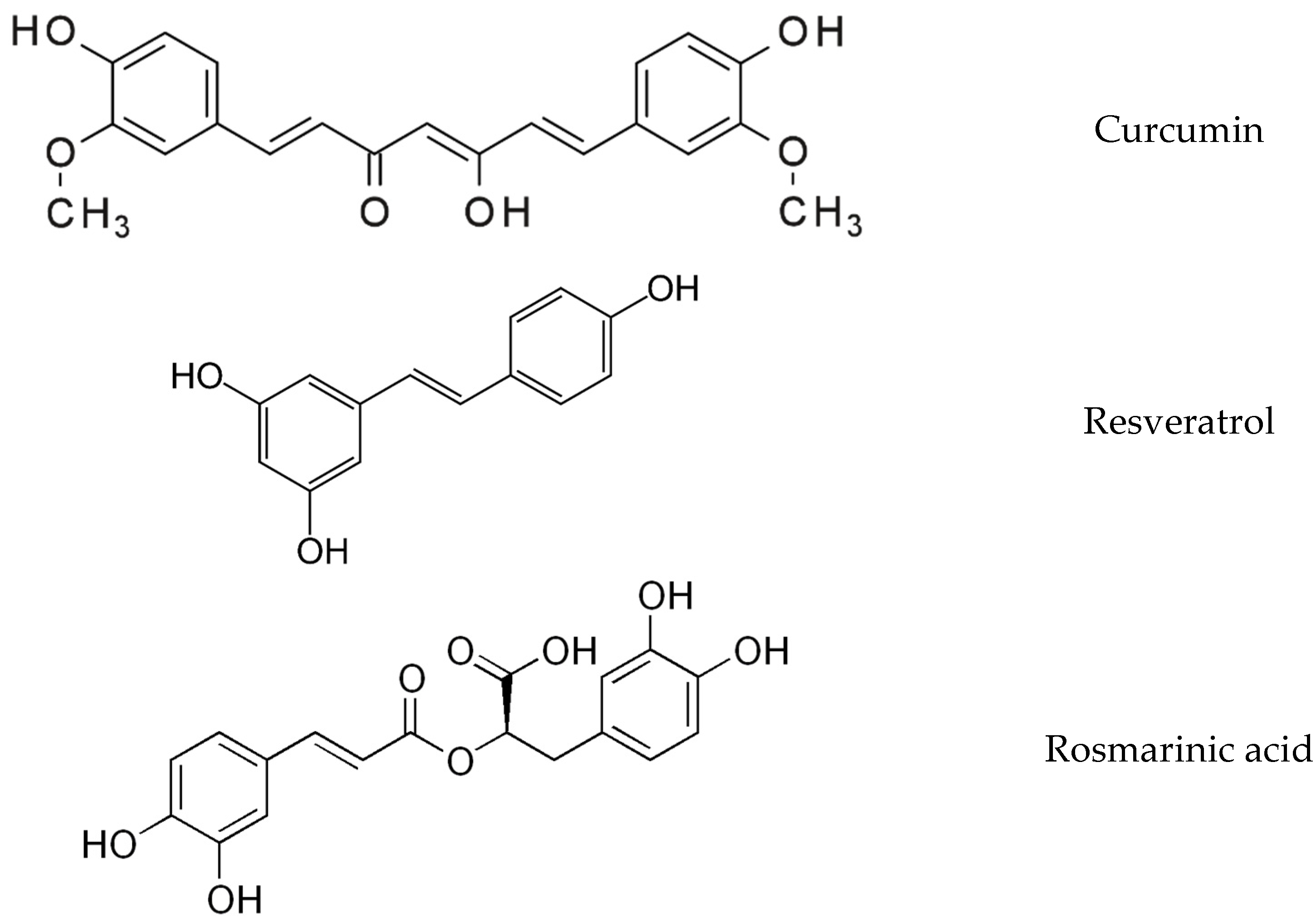
2.1. Supplements
2.2. Ethics
2.3. Study Design
2.4. Baseline Assessment & Outcome Measures
2.5. Statistical Analysis
3. Results
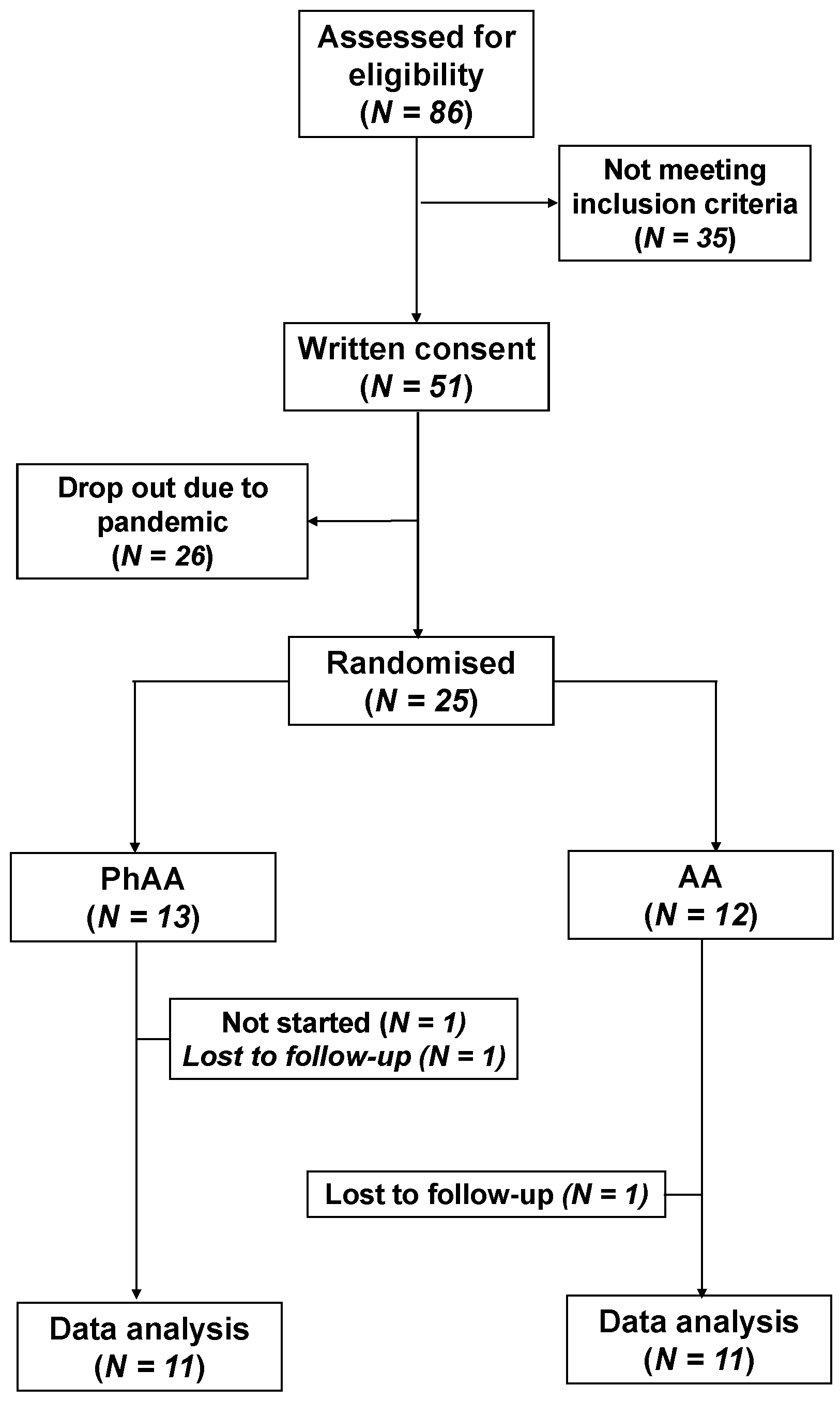
3.1. Allocation & Demographics
3.2. Adverse Events and Use of Rescue Medication
3.3. WOMAC
3.4. VAS
3.5. Sample Size
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | osteoarthritis |
| RCT | randomized controlled trial |
| VAS | visual analog scale |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis |
| AA | ascorbic acid |
| PhAA | polyphenols +AA |
| TENS | transcutaneous electrical nerve stimulation |
| K&L | Kellgren & Lawrence |
| WHR | waist-hip ratio |
| BIA | bioelectrical impedance analysis |
| BMI | body mass index |
| COX | cyclooxygenase |
| NF-κB | nuclear factor kappa-B |
| PGE2 | prostaglandin E2 |
References
- Safiri, S.; Kolahi, A.A.; Smith, Ε.; Hill, C.; Bettampadi, D.; Mansournia, Μ.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A.; et al. Global, regional and national burden of osteoarthritis 1990-2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef]
- Hou, A.; Chen, P.; Tang, H.; Meng, H.; Cheng, X.; Wang, Y.; Zhang, Y.; Peng, J. Cellular senescence in osteoarthritis and anti-aging strategies. Mech. Ageing Dev. 2018, 175, 83–87. [Google Scholar] [CrossRef]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- Berenbaum, F.; Eymard, F.; Houard, X. Osteoarthritis, inflammation and obesity. Curr. Opin. Rheumatol. 2013, 25, 114–118. [Google Scholar] [CrossRef]
- Courties, A.; Sellam, J.; Berenbaum, F. Metabolic syndrome-associated osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Woodell-May, J.E.; Sommerfeld, S.D. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J. Orthop. Res. 2020, 38, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Honvo, G.; Reginster, J.Y.; Rabenda, V.; Geerinck, A.; Mkinsi, O.; Charles, A.; Rizzoli, R.; Cooper, C.; Avouac, B.; Bruyère, O. Safety of Symptomatic Slow-Acting Drugs for Osteoarthritis: Outcomes of a Systematic Review and Meta-Analysis. Drugs Aging 2019, 36, 65–99. [Google Scholar] [CrossRef] [Green Version]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragni, E.; Mangiavini, L.; Viganò, M.; Brini, A.T.; Peretti, G.M.; Banfi, G.; de Girolamo, L. Management of Osteoarthritis during the COVID-19 Pandemic. Clin. Pharmacol. Ther. 2020, 108, 719–729. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Guermazi, A.; Guehring, H.; Aydemir, A.; Wax, S.; Fleuranceau-Morel, P.; Bihlet, A.R.; Byrjalsen, I.; Andersen, J.R.; Eckstein, F. Effect of Intra-Articular Sprifermin vs Placebo on Femorotibial Joint Cartilage Thickness in Patients with Osteoarthritis: The Forward Randomized Clinical Trial. JAMA 2019, 322, 1360–1370. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Bowes, M.A.; Kingsbury, S.R.; Brett, A.; Guillard, G.; Rizoska, B.; Sjögren, N.; Graham, P.; Jansson, Å.; Wadell, C. Disease-Modifying Effects of a Novel Cathepsin K Inhibitor in Osteoarthritis: A Randomized Controlled Trial. Ann. Intern. Med. 2020, 172, 86–95. [Google Scholar] [CrossRef]
- McClurg, O.; Tinson, R.; Troeberg, L. Targeting Cartilage Degradation in Osteoarthritis. Pharmaceuticals 2021, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.M.; Sanga, P.; Zaki, N.; Wang, S.; Haeussler, J.; Louie, J.; Thipphawong, J. Safety and efficacy of fulranumab in osteoarthritis of the hip and knee: Results from four early terminated phase III randomized studies. Curr. Med. Res. Opin. 2019, 35, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F.; Blanco, F.J.; Guermazi, A.; Miki, K.; Yamabe, T.; Viktrup, L.; Junor, R.; Carey, W.; Brown, M.T.; West, C.R.; et al. Subcutaneous tanezumab for osteoarthritis of the hip or knee: Efficacy and safety results from a 24-week randomised phase III study with a 24-week follow-up period. Ann. Rheum. Dis. 2020, 79, 800–810. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Cooper, C.; Oreffo, R.O.C.; Price, A.J.; Kaux, J.F.; Maheu, E.; Cutolo, M.; Honvo, G.; Conaghan, P.G.; Berenbaum, F.; et al. Alternative and complementary therapies in osteoarthritis and cartilage repair. Aging Clin. Exp. Res. 2020, 32, 547–560. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Lane, N.E.; Hunter, D.J.; Wei, J.; Choi, H.K.; McAlindon, T.E.; Li, H.; Lu, N.; Lei, G.; Zhang, Y. Intra-articular corticosteroids and the risk of knee osteoarthritis progression: Results from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2019, 27, 855–862. [Google Scholar] [CrossRef]
- Aghamohammadi, D.; Dolatkhah, N.; Bakhtiari, F.; Eslamian, F.; Hashemian, M. Nutraceutical supplements in management of pain and disability in osteoarthritis: A systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 20892. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food. Argic. 2018, 98, 1653–1659. [Google Scholar] [CrossRef]
- Schell, J.; Scofield, R.H.; Barrett, J.R.; Kurien, B.T.; Betts, N.; Lyons, T.J.; Zhao, Y.D.; Basu, A. Strawberries Improve Pain and Inflammation in Obese Adults with Radiographic Evidence of Knee Osteoarthritis. Nutrients 2017, 9, 949. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Smith, A.; Avalos, M.; South, S.; Crabtree, K.; Wang, W.; Kwon, Y.H.; Vijayagopal, P.; Juma, S. Blueberries Improve Pain, Gait Performance, and Inflammation in Individuals with Symptomatic Knee Osteoarthritis. Nutrients 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, H.R.; Pullman-Mooar, S.; Gupta, S.R.; Dinella, J.E.; Kim, R.; McHugh, M.P. Randomized double-blind crossover study of the efficacy of a tart cherry juice blend in treatment of osteoarthritis (OA) of the knee. Osteoarthr. Cartil. 2013, 21, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Connelly, A.E.; Tucker, A.J.; Tulk, H.; Catapang, M.; Chapman, L.; Sheikh, N.; Yurchenko, S.; Fletcher, R.; Kott, L.S.; Duncan, A.M.; et al. High-rosmarinic acid spearmint tea in the management of knee osteoarthritis symptoms. J. Med. Food 2014, 17, 1361–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, C.; Boutron, I.; Baron, G.; Coudeyre, E.; Berenbaum, F.; Poiraudeau, S.; Rannou, F. Evolution of pain at 3 months by oral resveratrol in knee osteoarthritis (ARTHROL): Protocol for a multicentre randomised double-blind placebo-controlled trial. BMJ Open 2017, 7, e017652. [Google Scholar] [CrossRef]
- Panahi, Y.; Rahimnia, A.R.; Sharafi, M.; Alishiri, G.; Saburi, A.; Sahebkar, A. Curcuminoid treatment for knee osteoarthritis: A randomized double-blind placebo-controlled trial. Phytother. Res. 2014, 28, 1625–1631. [Google Scholar] [CrossRef]
- Heidary-Beni, M.; Moravejolahkami, A.R.; Gorgian, P.; Askari, G.; Tarrahi, M.J.; Bahreini-Esfahani, N. Herbal formulation “turmeric extract, black pepper, and ginger” versus Naproxen for chronic knee osteoarthritis: A randomized, double-blind, controlled clinical trial. Phytother. Res. 2020, 34, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Farid, R.; Mirfeizi, Z.; Mirheidari, M.; Rezaieyazdi, Z.; Mansouri, H.; Esmaelli, H.; Zibadi, S.; Rohdewald, P.; Watchon, R.R. Pycnogenol supplementation reduces pain and stiffness and improves physical function in adults with knee arthritis. Nutr. Res. 2007, 27, 692–697. [Google Scholar] [CrossRef]
- Panahi, Y.; Alishiri, G.H.; Bayat, N.; Hosseini, S.M.; Sahebkar, A. Efficacy of Elaeagnus Angustifolia extract in the treatment of knee osteoarthritis: A randomized controlled trial. EXCLI J. 2016, 15, 203–210. [Google Scholar] [PubMed]
- Farid, R.; Rezaieyazdi, Z.; Mirfeizi, Z.; Hatef, M.R.; Mirheidari, M.; Mansouri, H.; Esmaelli, H.; Bentley, G.; Lu, Y.; Foo, Y.; et al. Oral intake of purple passion fruit peel extract reduces pain and stiffness and improves physical function in adult patients with knee osteoarthritis. Nutr. Res. 2010, 30, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.M.; Saikovsky, R.; Shmidt, E.; Khokhlov, A.; Burnett, B.P. Flavocoxid is as effective as naproxen for managing the signs and symptoms of osteoarthritis of the knee in humans: A short-term randomized, double-blind pilot study. Nutr. Res. 2009, 29, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Karlapudi, V.; Prasad Mungara, A.V.V.; Sengupta, K.; Davis, B.A.; Raychaudhuri, S.P. A Placebo-Controlled Double-Blind Study Demonstrates the Clinical Efficacy of a Novel Herbal Formulation for Relieving Joint Discomfort in Human Subjects with Osteoarthritis of Knee. J. Med. Food 2018, 21, 511–520. [Google Scholar] [CrossRef]
- Marlouf, B.H.; Hussain, S.A.; Ali, Z.S.; Ahmmad, R.S. Resveratrol Supplementation Reduces Pain and Inflammation in Knee Osteoarthritis Patients Treated with Meloxicam: A Randomized Placebo-Controlled Study. J. Med. Food 2018, 21, 12. [Google Scholar]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar]
- Dieppe, P.A.; Lohmander, L.S. Pathogenesis and management of pain in osteoarthritis. Lancet 2005, 365, 965–973. [Google Scholar] [CrossRef]
- O’Neill, T.W.; Felson, D.T. Mechanisms of Osteoarthritis (OA) Pain. Curr. Osteoporos. Rep. 2018, 16, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Schaible, H.G. Osteoarthritis pain. Recent advances and controversies. Curr. Opin. Support. Palliat. Care 2018, 12, 148–153. [Google Scholar] [CrossRef]
- Dimitroulas, T.; Duarte, R.V.; Behura, A.; Kitas, G.D.; Raphael, J.H. Neuropathic pain in osteoarthritis: A review of pathophysiological mechanisms and implications for treatment. Semin. Arthr. Rheum. 2014, 44, 145–154. [Google Scholar] [CrossRef]
- Carlesso, L.C.; Segal, N.A.; Frey-Law, L.; Zhang, Y.; Na, L.; Nevitt, M.; Lewis, C.E.; Neogi, T. Pain Susceptibility Phenotypes in Those Free of Knee Pain with or at Risk of Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthr. Rheumatol. 2019, 71, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.J.; Choudhury, M.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; Sun, H.B. Nutraceuticals: Potential for Chondroprotection and Molecular Targeting of Osteoarthritis. Int. J. Mol. Sci. 2013, 14, 23063–23085. [Google Scholar] [CrossRef] [Green Version]
- Cisár, P.; Jány, R.; Waczulíková, I.; Sumegová, K.; Muchová, J.; Vojtassák, J.; Duraćková, Z.; Lisý, M.; Rohdewald, P. Effect of pine bark extract (Pycnogenol) on symptoms of knee osteoarthritis. Phytother. Res. 2008, 22, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Errichi, S.; Zulli, C.; Errichi, B.M.; Vinciguerra, G.; Ledda, A.; Di Renzo, A.; Stuard, S.; Duggal, M.; et al. Variations in C-reactive protein, plasma free radicals and fibrinogen values in patients with osteoarthritis treated with Pycnogenol. Redox Rep. 2008, 13, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Grimm, T.; Chovanová, Z.; Muchová, J.; Sumegová, K.; Liptáková, A.; Duracková, Z.; Högger, P. Inhibition of NF-κB activation and MMP-9 secretion by plasma of human volunteers after ingestion of maritime pine bark extract (Pycnogenol®). J. Inflamm. 2006, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, A.; Chovanova, Z.; Muchova, J.; Sumegová, K.; Liptáková, A.; Duracková, Z.; Högger, P. Inhibition of COX-1 and COX-2 activity by plasma of human volunteers after ingestion of French maritime pine bark extract (Pycnogenol®). Biomed. Pharmacother. 2006, 60, 5–9. [Google Scholar] [CrossRef]
- Levy, R.M.; Khokhlov, A.; Kopenkin, S.; Bart, B.; Ermolova, T.; Kantemirova, R.; Mazurov, V.; Bell, M.; Caldron, P.; Pillai, L.; et al. Efficacy and Safety of Flavocoxid, a Novel Therapeutic, Compared with Naproxen: A Randomized Multicenter Controlled Trial in Subjects with Osteoarthritis of the Knee. Adv. Ther. 2010, 27, 731–742. [Google Scholar] [CrossRef]
- Levy, R.; Saikovsky, R.; Shmidt, E.; Khokhlov, A. Safety and efficacy of flavocoxid (Limbrel) compared with naproxen in subjects with osteoarthritis of the knee: A pilot study. Osteoarthr. Cartil. 2007, 15, B91–B94. [Google Scholar] [CrossRef] [Green Version]
- Burnett, B.P.; Jia, Q.; Zhao, Y.; Levy, R.M. A medicinal extract of Scutellaria baicalensis and Acacia catechu acts as a dual inhibitor of cyclooxygenase and 5-lipoxygenase to reduce inflammation. J. Med. Food 2007, 10, 442–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.G.; Rohrbaugh, A.L.; Otterness, I.; Kraus, V.B. The effects of ascorbic acid on cartilage metabolism in guinea pig articular cartilage explants. Matrix. Biol. 2002, 2, 175–184. [Google Scholar] [CrossRef]
- Sandell, L.J.; Daniel, J.C. Effects of ascorbic acid on collagen mRNA levels in short term chondrocyte cultures. Connect. Tissue Res. 1988, 17, 11–22. [Google Scholar] [CrossRef]
- Ripani, U.; Manzarbeitia-Arroba, P.; Guijarro-Leo, S.; Urrutia-Graña, J.; De Masi-De Luca, A. Vitamin C May Help to Reduce the Knee’s Arthritic Symptoms. Outcomes Assessment of Nutriceutical Therapy. Med. Arch. 2019, 73, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nevittt, M.; Niu, J.; Lewis, C.; Torner, J.; Guermazi, A.; Roemer, F.; McCulloch, C.; Felson, D.T. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthr. Rheum. 2011, 63, 691–699. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hodge, A.M.; Wluka, A.E.; English, D.R.; Giles, G.G.; O’Sullivan, R.; Forbes, A.; Cicuttini, F.M. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: A cross-sectional study. Arthr. Res. Ther. 2007, 9, R66. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; McCall, C. The role of vitamin C in the treatment of pain: New insights. J. Transl. Med. 2017, 15, 77. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zeng, C.; Wei, J.; Yang, T.; Gao, S.G.; Li, Y.S.; Lei, G.H. Associations between dietary antioxidants intake and radiographic knee osteoarthritis. Clin. Rheumatol. 2016, 35, 1585–1592. [Google Scholar] [CrossRef]
- Chaganti, R.K.; Tolstykh, I.; Javaid, M.K.; Neogi, T.; Torner, J.; Curtis, J.; Jacques, P.; Felson, D.; Lane, N.E.; Nevitt, M.C. High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 190–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanuka Katz, I.; Eran Nagar, E.; Okun, Z.; Shpigelman, A. The Link between Polyphenol Structure, Antioxidant Capacity and Shelf-Life Stability in the Presence of Fructose and Ascorbic Acid. Molecules 2020, 25, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, O.A.K.; De Faria Oliveira, O.M.M.; Vellosa, J.C.R.; De Quadros, A.U.; Dalposso, L.M.; Karam, T.K.; Mainardes, R.M.; Khalil, N.M. Curcumin antifungal and antioxidant activities are increased in the presence of ascorbic acid. Food Chem. 2012, 133, 1001–1005. [Google Scholar] [CrossRef]
- Millum, J.; Grady, C. The Ethics of Placebo-controlled Trials: Methodological Justifications. Contemp. Clin. Trials 2013, 36, 510–514. [Google Scholar] [CrossRef] [Green Version]
- Adegbehingbe, O.O.; Adesanya, S.A.; Idowu, T.O.; Okimi, O.C.; Oyelami, O.A.; Iwalewa, E.O. Clinical effects of Garcinia kola in knee osteoarthritis. J. Orthop. Surg. Res. 2008, 3, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Treatment | p | ||
|---|---|---|---|
| AA N = 12 | PhAA N = 13 | ||
| N (%) | N (%) | ||
| Sex | |||
| Men | 4 (33.3) | 5 (38.5) | 1.000 ++ |
| Women | 8 (66.7) | 8 (61.5) | |
| Age (years), mean (SD) | 60.6 (11.9) | 60.4 (10.9) | 0.966 + |
| Marital status | |||
| Married, divorced | 11 (91.7) | 10 (76.9) | 0.593 ++ |
| Unmarried | 1 (8.3) | 3 (23.1) | |
| Education | |||
| 1–9 years | 4 (33.3) | 3 (23.1) | 0.358 ++ |
| 10–12 years | 3 (25.0) | 1 (7.7) | |
| >12 years | 5 (41.7) | 9 (69.2) | |
| Smoking | |||
| No | 7 (58.3) | 11 (84.6) | 0.202 ++ |
| Yes | 5 (41.7) | 2 (15.4) | |
| Fat %, mean (SD) | 38.2 (9.1) | 30.5 (10.5) | 0.096 + |
| BMI (kg/m2), mean (SD) | 32.2 (6.1) | 28.7 (4.7) | 0.119 + |
| WHR, mean (SD) | 0.91 (0.08) | 0.92 (0.06) | 0.731 + |
| K&L (disease severity) | |||
| 2 | 1 (8.3) | 4 (30.8) | 0.119 ++ |
| 3 | 5 (41.7) | 7 (53.8) | |
| 4 | 6 (50.0) | 2 (15.4) | |
| Treatment | Baseline | Follow-up | p 2 | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| WOMAC (pain) | |||||
| AA | 8.92 | 3.09 | 6.30 | 3.08 | 0.027 |
| PhAA | 7.31 | 3.84 | 3.60 | 3.09 | 0.001 |
| p 1 | 0.263 | 0.039 | |||
| WOMAC (stiffness) | |||||
| AA | 2.08 | 1.83 | 1.45 | 1.51 | 0.071 |
| PhAA | 1.54 | 1.66 | 1.30 | 1.34 | 0.278 |
| p 1 | 0.549 | 0.807 | |||
| WOMAC (physical function) | |||||
| AA | 27.08 | 13.72 | 21.18 | 9.21 | 0.335 |
| PhAA | 17.15 | 10.55 | 15.30 | 12.97 | 0.182 |
| p 1 | 0.395 | 0.242 | |||
| WOMAC (composite) | |||||
| AA | 39.66 | 17.83 | 29.35 | 12.83 | 0.100 |
| PhAA | 26.84 | 15.58 | 19.22 | 15.40 | 0.033 |
| p 1 | 0.283 | 0.109 | |||
| Treatment | VAS | p 2 | |||
|---|---|---|---|---|---|
| Baseline | Follow-up | ||||
| Mean | SD | Mean | SD | ||
| AA | 6.13 | 1.94 | 6.00 | 1.57 | >0.999 |
| PhAA | 7.04 | 2.02 | 3.92 | 2.24 | <0.001 |
| p 1 | 0.261 | 0.017 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valsamidou, E.; Amerikanou, C.; Tzavara, C.; Zoumpoulakis, P.; Mariolis-Sapsakos, T.D.; Skarpas, G.; Kaliora, A.C. Nutraceuticals for Knee Osteoarthritis Pain Relief. Results from a Preliminary Randomised Clinical Trial. Dietetics 2022, 1, 2-14. https://doi.org/10.3390/dietetics1010002
Valsamidou E, Amerikanou C, Tzavara C, Zoumpoulakis P, Mariolis-Sapsakos TD, Skarpas G, Kaliora AC. Nutraceuticals for Knee Osteoarthritis Pain Relief. Results from a Preliminary Randomised Clinical Trial. Dietetics. 2022; 1(1):2-14. https://doi.org/10.3390/dietetics1010002
Chicago/Turabian StyleValsamidou, Evdokia, Charalampia Amerikanou, Chara Tzavara, Panagiotis Zoumpoulakis, Theodoros D. Mariolis-Sapsakos, George Skarpas, and Andriana C. Kaliora. 2022. "Nutraceuticals for Knee Osteoarthritis Pain Relief. Results from a Preliminary Randomised Clinical Trial" Dietetics 1, no. 1: 2-14. https://doi.org/10.3390/dietetics1010002






