Density of SMI-32 Immunopositive Neurons in Eye-Specific Layers of Lateral Geniculate Nuclei in Kittens Reared with Monocular Deprivation and Unilateral Convergent Squint †
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawson, S.N.; Waddell, P.J. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J. Physiol. 1991, 435, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Tsang, Y.M.; Chiong, F.; Kuznetsov, D.; Kasarskis, E.; Geula, C. Motor neurons are rich in non-phosphorylated neurofilaments: Cross-species comparison and alterations in ALS. Brain Res. 2000, 861, 45–58. [Google Scholar] [CrossRef]
- Kirkcaldie, M.T.; Dickson, T.C.; King, C.E.; Grasby, D.; Riederer, B.M.; Vickers, J.C. Neurofilament triplet proteins are restricted to a subset of neurons in the rat neocortex. J. Chem. Neuroanat. 2002, 24, 163–171. [Google Scholar] [CrossRef]
- Rowa, M.H.; Stone, J. Conduction velocity groupings among axons of cat retinal ganglion cells, and their relationship to retinal topography. Exp. Brain Res. 1976, 25, 339–357. [Google Scholar] [CrossRef]
- Hsiao, C.F.; Watanabe, M.; Fukuda, Y. The relation between axon diameter and axonal conduction velocity of Y, X and W cells in the cat retina. Brain Res. 1984, 309, 357–361. [Google Scholar] [CrossRef]
- Zeck, G.; Lambacher, A.; Fromherz, P. Axonal transmission in the retina introduces a small dispersion of relative timing in the ganglion cell population response. PLoS ONE 2011, 6, e20810. [Google Scholar] [CrossRef]
- Sherman, S.M.; Spear, P.D. Organization of visual pathways in normal and visually deprived cats. Physiol. Rev. 1982, 62, 738–855. [Google Scholar] [CrossRef]
- Mower, G.D.; Burchfiel, J.L.; Duffy, F.H. Animal models of strabismic amblyopia: Physiological studies of visual cortex and the lateral geniculate nucleus. Brain Res. 1982, 281, 311–327. [Google Scholar] [CrossRef]
- Mangel, S.C.; Wilson, J.R.; Sherman, S.M. Development of neuronal response properties in the cat dorsal lateral geniculate nucleus during monocular deprivation. J. Neurophysiol. 1983, 50, 240–264. [Google Scholar] [CrossRef]
- Bickford, M.E.; Guido, W.; Godwin, D.W. Neurofilament proteins in Y-cells of the cat lateral geniculate nucleus: Normal expression and alteration with visual deprivation. J. Neurosci. 1998, 18, 6549–6557. [Google Scholar] [CrossRef]
- Kutcher, M.R.; Duffy, K.R. Cytoskeleton alteration correlates with gross structural plasticity in the cat lateral geniculate nucleus. Vis. Neurosci. 2007, 24, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Fennell, H.M. Strabismus-Induced Cytoskeletal Change in the Feline Lateral Geniculate Nucleus. Master’s Thesis, Dalhousie University, Halifax, NS, Canada, 2007. [Google Scholar]
- O’Leary, T.P.; Kutcher, M.R.; Mitchell, D.E.; Duffy, K.R. Recovery of neurofilament following early monocular deprivation. Front. Syst. Neurosci. 2012, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Timney, B. Development of binocular depth perception in kittens. Investig. Ophthalmol. Vis. Sci. 1981, 21, 493–496. [Google Scholar]
- Pettigrew, J.D. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J. Physiol. 1974, 237, 49–74. [Google Scholar] [CrossRef]
- Hubel, D.H.; Wiesel, T.N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. 1970, 206, 419–436. [Google Scholar] [CrossRef]
- Winfield, D.A.; Headon, M.P.; Powell, T.P. Postnatal development of the synaptic organisation of the lateral geniculate nucleus in the kitten with unilateral eyelid closure. Nature 1976, 263, 591–594. [Google Scholar] [CrossRef]
- Olson, C.R.; Freeman, R.D. Profile of the sensitive period for monocular deprivation in kittens. Exp. Brain Res. 1980, 39, 17–21. [Google Scholar] [CrossRef]
- Sireteanu, R.; Singer, W.; Fronius, M.; Greuel, J.M.; Best, J.; Fiorentini, A.; Bisti, S.; Schiavi, C.; Campos, E. Eye alignment and cortical binocularity in strabismic kittens: A comparison between tenotomy and recession. Vis. Neurosci. 1993, 10, 541–549. [Google Scholar] [CrossRef]
- Aarts, E.; Verhage, M.; Veenvliet, J.V.; Dolan, C.V.; van der Sluis, S. A solution to dependency: Using multilevel analysis to accommodate nested data. Nat. Neurosci. 2014, 17, 491–496. [Google Scholar] [CrossRef]
- Schmidt, K.E.; Stephan, M.; Singer, W.; Löwel, S. Spatial analysis of ocular dominance patterns in monocularly deprived cats. Cereb. Cortex 2002, 12, 783–796. [Google Scholar] [CrossRef][Green Version]
- Crair, M.C.; Horton, J.C.; Antonini, A.; Stryker, M.P. Emergence of ocular dominance columns in cat visual cortex by 2 weeks of age. J. Comp. Neurol. 2001, 430, 235–249. [Google Scholar] [CrossRef]
- Blakemore, C.; Van Sluyters, R.C. Innate and environmental factors in the development of the kitten’s visual cortex. J. Physiol. 1975, 248, 663–716. [Google Scholar] [CrossRef] [PubMed]
- Burnat, K. Are visual peripheries forever young? Neural Plast. 2015, 2015, 307929. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Plant, G.T.; Tremain, K.E. Nasal field loss in kittens reared with convergent squint: Neurophysiological and morphological studies of the lateral geniculate nucleus. J. Physiol. 1977, 270, 345–366. [Google Scholar] [CrossRef]

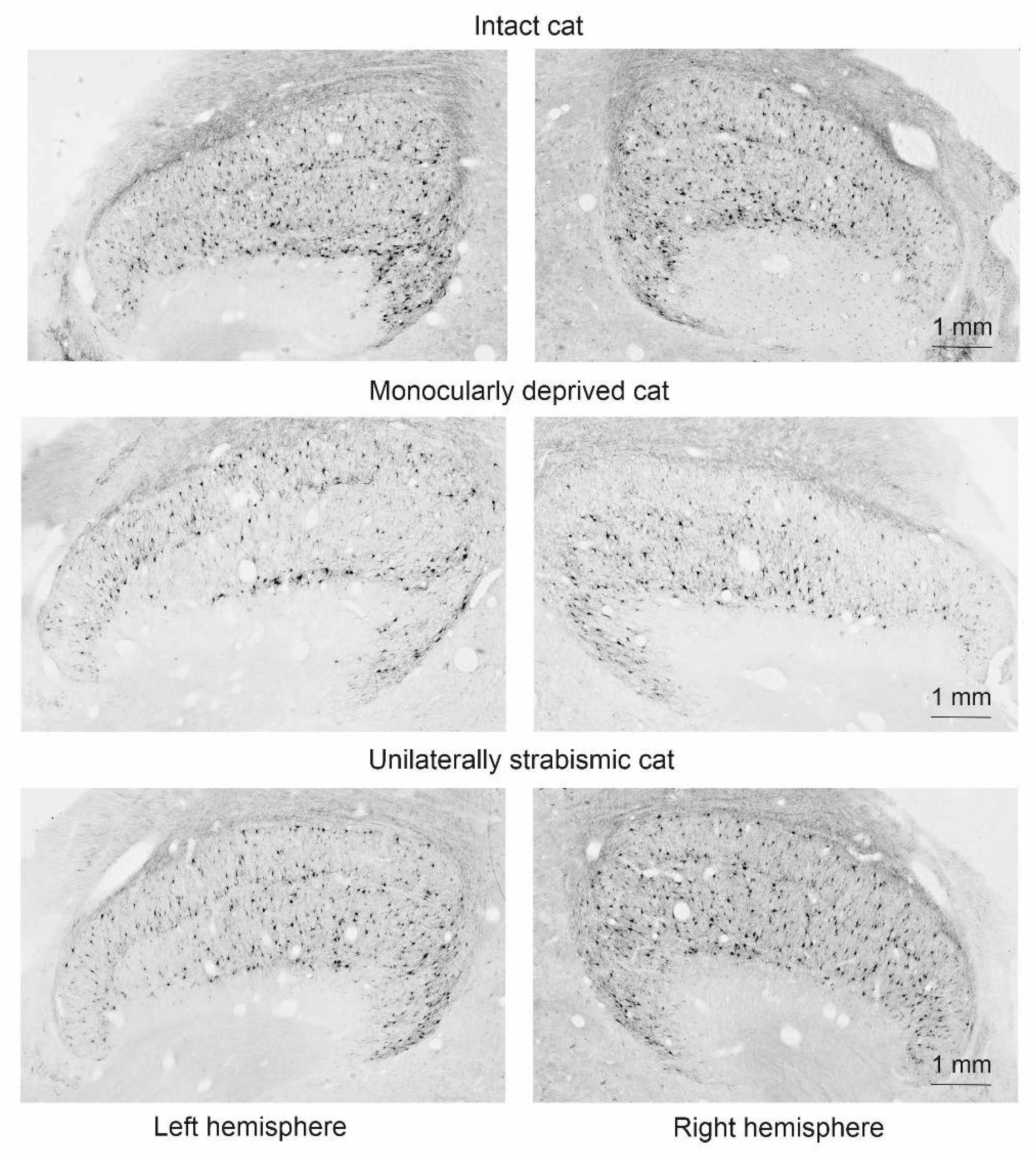

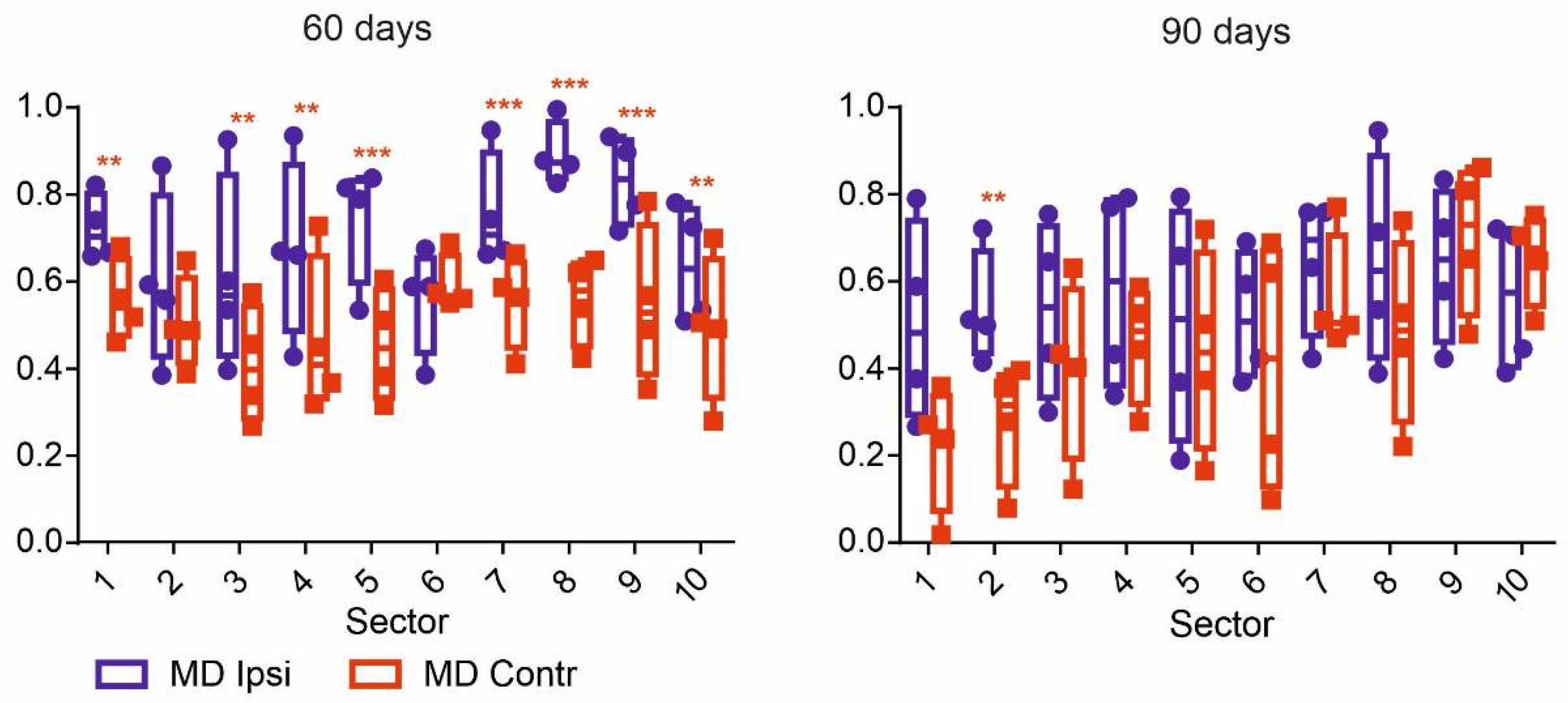
| Group Name | Age, Days | Number of Animals |
|---|---|---|
| Norm-60 | 60 | 4 |
| Norm-90 | 90 | 2 |
| MD-60 | 60 | 4 |
| MD-90 | 90 | 4 |
| Strab-60 | 60 | 4 |
| Strab-90 | 90 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkorbatova, P.Y.; Lyakhovetskii, V.A.; Alexeenko, S.V. Density of SMI-32 Immunopositive Neurons in Eye-Specific Layers of Lateral Geniculate Nuclei in Kittens Reared with Monocular Deprivation and Unilateral Convergent Squint. Med. Sci. Forum 2022, 8, 3. https://doi.org/10.3390/IECBS2021-10666
Shkorbatova PY, Lyakhovetskii VA, Alexeenko SV. Density of SMI-32 Immunopositive Neurons in Eye-Specific Layers of Lateral Geniculate Nuclei in Kittens Reared with Monocular Deprivation and Unilateral Convergent Squint. Medical Sciences Forum. 2022; 8(1):3. https://doi.org/10.3390/IECBS2021-10666
Chicago/Turabian StyleShkorbatova, Polina Y., Vsevolod A. Lyakhovetskii, and Svetlana V. Alexeenko. 2022. "Density of SMI-32 Immunopositive Neurons in Eye-Specific Layers of Lateral Geniculate Nuclei in Kittens Reared with Monocular Deprivation and Unilateral Convergent Squint" Medical Sciences Forum 8, no. 1: 3. https://doi.org/10.3390/IECBS2021-10666
APA StyleShkorbatova, P. Y., Lyakhovetskii, V. A., & Alexeenko, S. V. (2022). Density of SMI-32 Immunopositive Neurons in Eye-Specific Layers of Lateral Geniculate Nuclei in Kittens Reared with Monocular Deprivation and Unilateral Convergent Squint. Medical Sciences Forum, 8(1), 3. https://doi.org/10.3390/IECBS2021-10666








