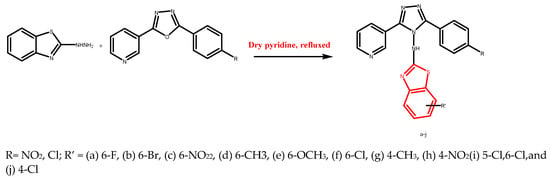
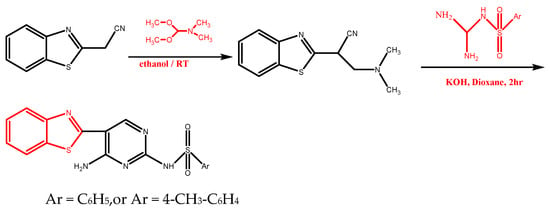
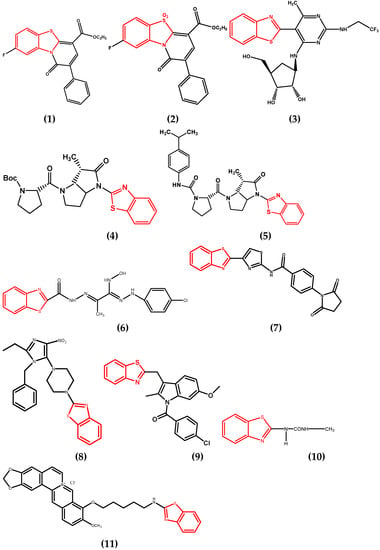
Abstract
A virus is a microorganism that uses the machinery of the host to multiply. At present, there are various species of viruses known to us that are dangerous for the health of human beings. One of such viruses has destroyed many lives nowadays and that is a coronavirus. Such other viruses like Human Immunodeficiency Virus, Poliovirus, etc., destroy one’s capability to survive normally. As science progresses, we invent many antiviral drugs as per the type of virus. There are many antiviral drugs available to treat viral infections. From them, benzothiazole derivatives are potent antiviral agents. Researchers continuously work on benzothiazole moiety to get more effective benzothiazole derivatives that can be used as antiviral agents. This review article gives information about various benzothiazole derivatives that are invented during 1980 to 2021, that act against the various viruses as antiviral agents, the structure–activity relationship of benzothiazole as an antiviral agent, various schemes to synthesize benzothiazole derivatives as an antiviral agent as well as includes various methods to evaluate the antiviral activity of novel synthetic compounds against specific viruses.
1. Introduction
Avirus is very tiny infectious agent that replicate only inside the living cells of an organism. Viruses infect all types of plants, animals and microorganisms also, like bacteria, etc. [1]. Viral infections are considered to be one of the major threats to the health of human beings. Virus infections take place due to globalization and unexpected climate change [2]. We are informed about just about 260 varieties of viruses, but the unknown varieties of viruses are responsible for 99.9% of total infection cases. These viruses come to the picture when they show some symptoms to the host [3]. Despite of the development of many molecules as antiviral, they are unable to satisfy the requirement criteria to treat the viral infection and drug resistance of current viruses. That’s why there is still a need for newer vaccines, diagnostic agents and antiviral molecules [4]. Because, benzothiazole is such a versatile moiety, it shows many biological activities including antiviral effect against various species of viruses [5]. Due to feasible physical and chemical properties of benzothiazole moiety, many researchers have tried to synthesize various benzothiazole derivatives that shows potent antiviral effects against various strain of viruses [6].
Structurally, benzothiazole is a fusion of two aryl rings; benzene and thiazole. As thiazole bears nitrogen and sulfur moiety, benzothiazole derivatives successfully binds to viruses and gives antiviral activity. Many articles are available for reference to develop newer antiviral agents, but this review article includes various novel synthesized antiviral compounds bearing benzothiazole moiety, the pathway to synthesize benzothiazole based antiviral agents, the structure–activity relationship of benzothiazole as antiviral agents and in vitro and in vivo methods to evaluate antiviral activity of novel synthetic compounds. That is why it is a unique article containing all the information to guide a researcher for synthesis of benzothiazole based antiviral agents.
Author Contributions
Schemes for synthesis—S.S., Remaining—K.B.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data has been taken from the sources or articles available on internet.
Acknowledgments
We are very thankful to Subhash Technical Campus staff for contribution in this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar]
- Carroll, D.; Watson, B.; Togami, E.; Daszak, P.; Mazet, J.A.; Chrisman, C.J.; Rubin, E.M.; Wolfe, N.; Morel, C.M.; Gao, G.F.; et al. Building a global atlas of zoonotic viruses. Bull. World Health Organ. 2018, 96, 292. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 2016, 123, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Bhagdev, K.; Sarkar, S. Benzothiazole: As an Antidiabetic Agent. Ann. Rom. Soc. Cell Biol. 2021, 10, 20269–20285. [Google Scholar]
- Agarwal, S.; Gandhi, D.; Kalal, P. Benzothiazole: A versatile and multitargeted pharmacophore in the field of medicinal chemistry. Lett. Org. Chem. 2017, 14, 729–742. [Google Scholar] [CrossRef]
- Patel, N.B.; Khan, I.H.; Pannecouque, C.; De Clercq, E. Anti-HIV, antimycobacterial and antimicrobial studies of newly synthesized 1, 2, 4-triazole clubbed benzothiazoles. Med. Chem. Res. 2013, 22, 1320–1329. [Google Scholar] [CrossRef]
- Montalvão, S.; Leino, T.O.; Kiuru, P.S.; Lillsunde, K.E.; Yli-Kauhaluoma, J.; Tammela, P. Synthesis and biological evaluation of 2-aminobenzothiazole and benzimidazole analogs based on the clathrodin structure. Archiv. Pharm. 2016, 349, 137–149. [Google Scholar] [CrossRef]
- Manfroni, G.; Meschini, F.; Barreca, M.L.; Leyssen, P.; Samuele, A.; Iraci, N.; Sabatini, S.; Massari, S.; Maga, G.; Neyts, J.; et al. Pyridobenzothiazole derivatives as new chemotype targeting the HCV NS5B polymerase. Bioorg. Med. Chem. 2012, 20, 866–876. [Google Scholar] [CrossRef]
- Girijavallabhan, V.M.; Alvarez, C.; Bennett, F.; Chen, L.; Gavalas, S.; Huang, Y.; Kim, S.H.; Kosinski, A.; Pinto, P.; Rizvi, R.; et al. Synthesis and SAR of pyridothiazole substituted pyrimidine derived HCV replication inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 5652–5657. [Google Scholar] [CrossRef]
- Landolfo, S.; Gariglio, M.; Gribaudo, G.; Lembo, D. The human cytomegalovirus. Pharmacol. Ther. 2003, 98, 269–297. [Google Scholar] [CrossRef]
- Borthwick, A.D.; Davies, D.E.; Ertl, P.F.; Exall, A.M.; Haley, T.M.; Hart, G.J.; Jackson, D.L.; Parry, N.R.; Patikis, A.; Trivedi, N.; et al. Design and synthesis of pyrrolidine-5, 5′-trans-lactams (5-oxo-hexahydropyrrolo [3, 2-b] pyrroles) as novel mechanism-based inhibitors of human cytomegalovirus protease. 4. Antiviral activity and plasma stability. J. Med. Chem. 2003, 46, 4428–4449. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziza, H.A.; Abdel-Wahab, B.F.; Badria, F.A. Stereoselective Synthesis and Antiviral Activity of (1E, 2Z, 3E)-1-(Piperidin-1-yl)-1-(arylhydrazono)-2-[(benzoyl/benzothiazol-2-oyl) hydrazono]-4-(aryl1) but-3-enes. Archiv Pharmazie Int. J. Pharm. Med. Chem. 2010, 343, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Martina, B.E.; Koraka, P.; Osterhaus, A.D. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef] [Green Version]
- Low, J.G.; Ooi, E.E.; Vasudevan, S.G. Current status of dengue therapeutics research and development. J. Infect. Dis. 2017, 215 (Suppl. S2), S96–S102. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, K.D. Essentials of Medical Pharmacology; JP Medical Ltd.: New Delhi, India, 2013. [Google Scholar]
- Al-Soud, Y.A.; Al-Sa’doni, H.; Amajaour, H.A.; Al-Masoudib, N.A. Nitroimidazoles, Part 3. Synthesis and anti-HIV activity of new N-alkyl-4-nitroimidazoles bearing benzothiazole and benzoxazole backbones. Z. Nat. B 2007, 62, 523–528. [Google Scholar]
- Al-Masoudi, N.A.; Jafar, N.N.; Abbas, L.J.; Baqir, S.J.; Pannecouque, C. Synthesis and anti-HIV activity of new benzimidazole, benzothiazole and carbohyrazide derivatives of the anti-inflammatory drug indomethacin. Z. Nat. B 2011, 66, 953–960. [Google Scholar]
- Kumar, M.; Chung, S.M.; Enkhtaivan, G.; Patel, R.V.; Shin, H.S.; Mistry, B.M. Molecular Docking Studies and Biological Evaluation of Berberine–Benzothiazole Derivatives as an Anti-Influenza Agent via Blocking of Neuraminidase. Int. J. Mol. Sci. 2021, 22, 2368. [Google Scholar] [CrossRef]
- De Clercq, E.; Descamps, J.; Verhelst, G.; Walker, R.T.; Jones, A.S.; Torrence, P.F.; Shugar, D. Comparative efficacy of antiherpes drugs against different strains of herpes simplex virus. J. Infect. Dis. 1980, 141, 563–574. [Google Scholar] [CrossRef]
- Field, A.K.; Davies, M.E.; De Witt, C.M.; Perry, H.C.; Schofield, T.L.; Karkas, J.D.; Germershausen, J.; Wagner, A.F.; Cantone, C.L.; MacCoss, M.; et al. Efficacy of 2′-nor-cyclicGMP in treatment of experimental herpes virus infections. Antivir. Res. 1986, 6, 329–341. [Google Scholar] [CrossRef]
- Boyd, M.R.; Bacon, T.H.; Sutton, D.A.; Cole, M.A. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxy-methylbut-1-yl) guanine (BRL 39123) in cell culture. Antimicrob. Agents Chemother. 1987, 31, 1238–1242. [Google Scholar] [CrossRef] [Green Version]
- Amtmann, E.; Müller-Decker, K.; Hoss, A.; Schalasta, G.; Doppler, C.; Sauer, G. Synergistic antiviral effect of xanthates and ionic detergents. Biochem. Pharmacol. 1987, 36, 1545–1549. [Google Scholar] [CrossRef]
- Collins, P.; Bauer, D.J. Relative potencies of anti-herpes compounds. Ann. N. Y. Acad. Sci. 1977, 284, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Färber, I.; Klinger, C.; Wutzler, P.; Thiel, K.D.; Reefschläger, J.; Herrmann, G. Effect of (E)-5-(2-bromovinyl)-and 5-vinyl-1-beta-D-arabinofuranosyluracil on Epstein-Barr virus antigen expression in P3HR-1 cells: Comparison with acyclovir. Acta Virol. 1987, 31, 13–18. [Google Scholar] [PubMed]
- Hutt-Fletcher, L.M.; Balachandran, N.; LeBlanc, P.A. Modification of Epstein-Barr virus replication by tunicamycin. J. Virol. 1986, 57, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsuya, H.; Broder, S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2′,3′-dideoxynucleosides. Proc. Natl. Acad. Sci. USA 1986, 83, 1911–1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.C.; DeClercq, E.; Pagano, J.S. Novel acyclic adenosine analogs inhibit Epstein-Barr virus replication. Antimicrob. Agents Chemother. 1987, 31, 1431–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).