Incidence of Oral Mucositis in Patients Undergoing Head and Neck Cancer Treatment: Systematic Review and Meta-Analysis †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
Conflicts of Interest
References
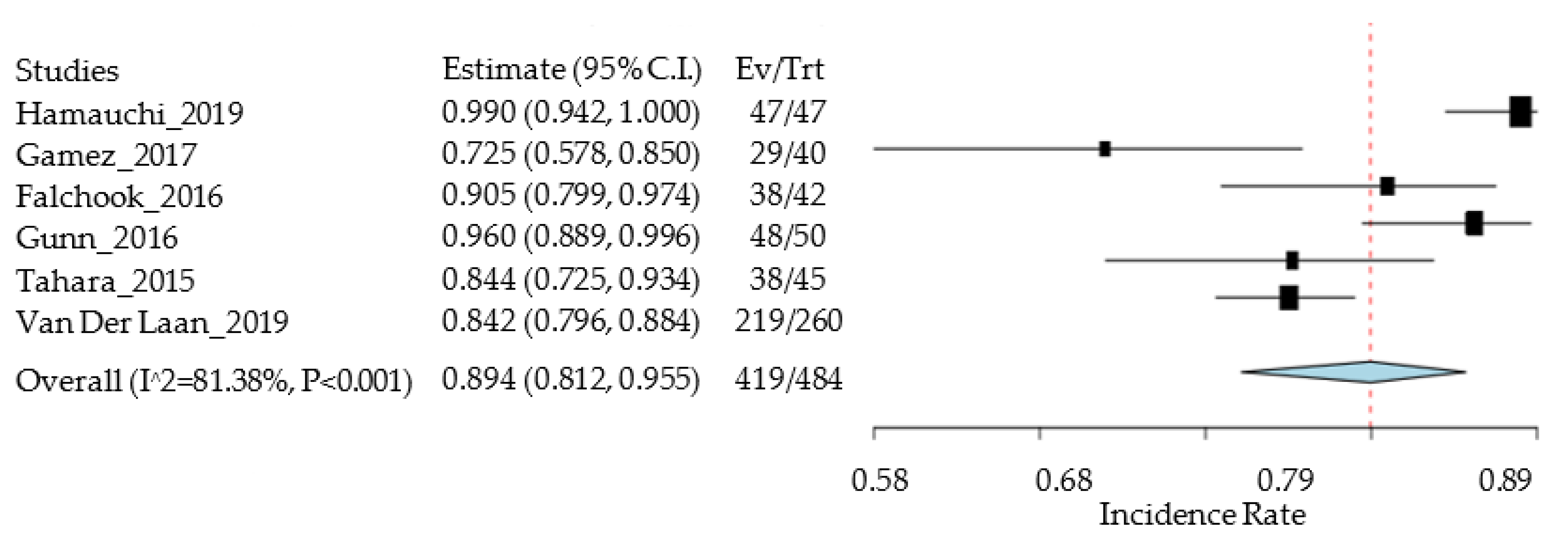
- Hamauchi, S.; Yokota, T.; Mizumachi, T.; Onozawa, Y.; Ogawa, H.; Onoe, T.; Kamijo, T.; Iida, Y.; Nishimura, T.; Onitsuka, T.; et al. Safety and efficacy of concurrent carboplatin or cetuximab plus radiotherapy for locally advanced head and neck cancer patients ineligible for treatment with cisplatin. Int. J. Clin. Oncol. 2019, 24, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Gamez, M.E.; Halyard, M.Y.; Hinni, M.L.; Hayden, R.E.; Nagel, T.H.; Vargas, C.E.; Wong, W.W.; Curtis, K.K.; Zarka, M.A.; Ma, D.; et al. Mucosal Sparing Radiation Therapy in Resected Oropharyngeal Cancer. Ann. Otol. Rhinol. Laryngol. 2017, 126, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Falchook, A.D.; Green, R.; Knowles, M.E.; Amdur, R.J.; Mendenhall, W.; Hayes, D.N.; Grilley-Olson, J.E.; Weiss, J.; Reeve, B.B.; Mitchell, S.A.; et al. Comparison of patient- and practitioner-reported toxic effects associated with chemoradiotherapy for head and neck cancer. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunn, G.B.; Blanchard, P.; Garden, A.S.; Zhu, X.R.; Fuller, C.D.; Mohamed, A.S.; Morrison, W.H.; Phan, J.; Beadle, B.M.; Skinner, H.D.; et al. Clinical Outcomes and Patterns of Disease Recurrence after Intensity Modulated Proton Therapy for Oropharyngeal Squamous Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 360–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahara, M.; Kiyota, N.; Mizusawa, J.; Nakamura, K.; Hayashi, R.; Akimoto, T.; Hasegawa, Y.; Iwae, S.; Monden, N.; Matsuura, K.; et al. Phase II trial of chemoradiotherapy with S-1 plus cisplatin for unresectable locally advanced head and neck cancer (JCOG0706). Cancer Sci. 2015, 106, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Van Der Laan, B.F.A.M.; Van Der Laan, H.P.; Bijl, H.P.; Steenbakkers, R.J.H.M.; Van Der Schaaf, A.; Chouvalova, O.; Vemer-Van Den Hoek, J.G.M.; Gawryszuk, A.; Oosting, S.F.; Roodenburg, J.L.N.; et al. Acute symptoms during the course of head and neck radiotherapy or chemoradiation are strong predictors of late dysphagia. Radiat. Oncol. 2015, 115, 56–62. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, R.; Cavacas, M.A.; Mascarenhas, P.; Oliveira, P.; Zagalo, C. Incidence of Oral Mucositis in Patients Undergoing Head and Neck Cancer Treatment: Systematic Review and Meta-Analysis. Med. Sci. Forum 2021, 5, 23. https://doi.org/10.3390/msf2021005023
Pacheco R, Cavacas MA, Mascarenhas P, Oliveira P, Zagalo C. Incidence of Oral Mucositis in Patients Undergoing Head and Neck Cancer Treatment: Systematic Review and Meta-Analysis. Medical Sciences Forum. 2021; 5(1):23. https://doi.org/10.3390/msf2021005023
Chicago/Turabian StylePacheco, Raquel, Maria Alzira Cavacas, Paulo Mascarenhas, Pedro Oliveira, and Carlos Zagalo. 2021. "Incidence of Oral Mucositis in Patients Undergoing Head and Neck Cancer Treatment: Systematic Review and Meta-Analysis" Medical Sciences Forum 5, no. 1: 23. https://doi.org/10.3390/msf2021005023
APA StylePacheco, R., Cavacas, M. A., Mascarenhas, P., Oliveira, P., & Zagalo, C. (2021). Incidence of Oral Mucositis in Patients Undergoing Head and Neck Cancer Treatment: Systematic Review and Meta-Analysis. Medical Sciences Forum, 5(1), 23. https://doi.org/10.3390/msf2021005023







