2. Other
2.1. Does Cherry Angioma Have Association with Kidney Cancer? The Preliminary Report of an Observational Study
Selcuk Erdem 1, Yasemin Erdem 2, Ahmet Alperen Çevik 2, Yasin Ates 3, Oner Sanli 1 and Faruk Ozcan 1
- 1
Division of Urologic Oncology, Department of Urology, Istanbul University Istanbul Faculty of Medicine, Istanbul, Turkey
- 2
Department of Dermatology and Venereology, Istanbul University Istanbul Faculty of Medicine, Istanbul, Turkey
- 3
Department of Urology, Istanbul University Istanbul Faculty of Medicine, Istanbul, Turkey
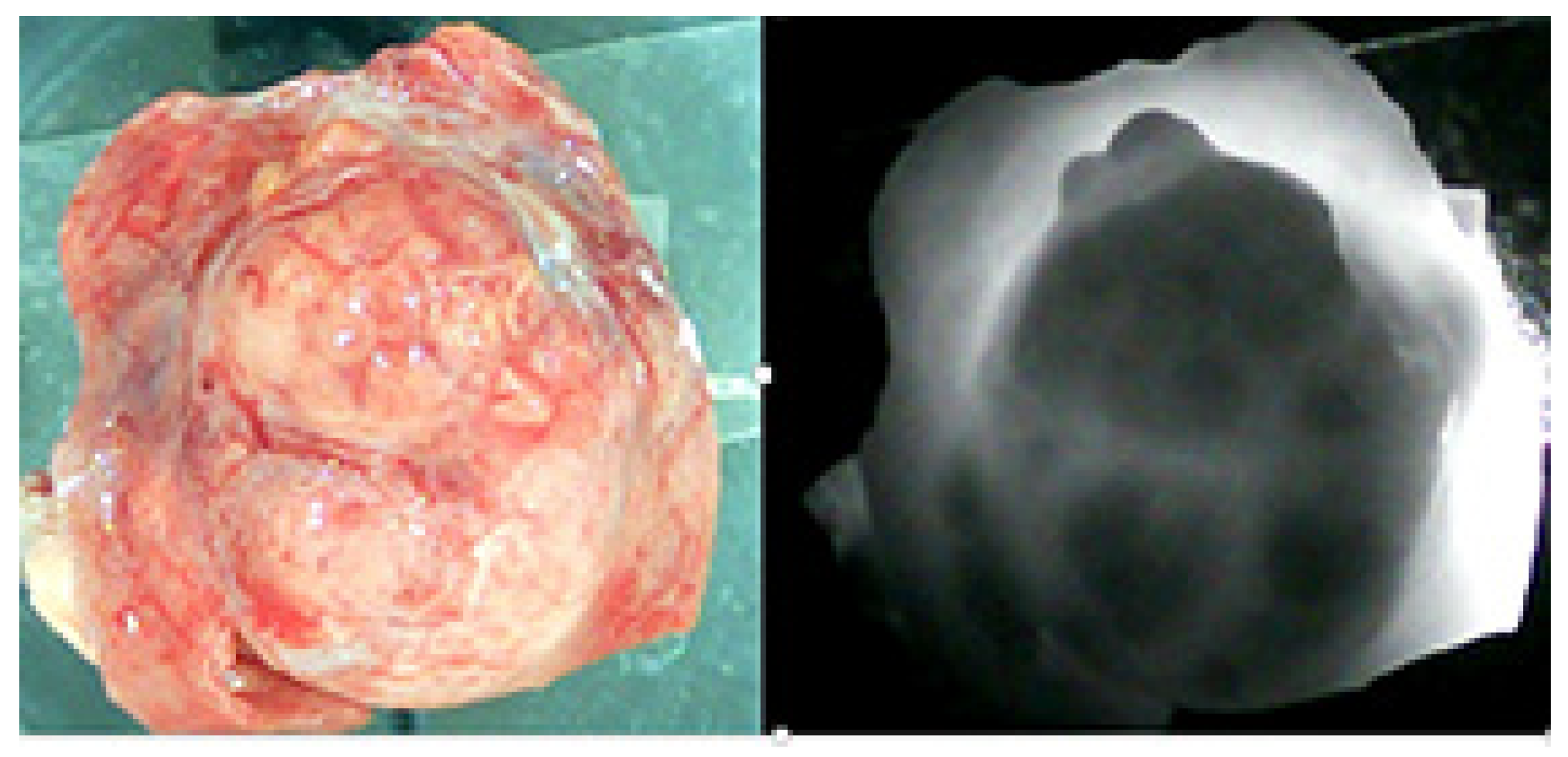
Introduction and Objective: Cherry angioma (CA) is the most common benign vascular proliferation of the skin, particularly in the elderly population. On the basis of clinical observation, we hypothesized that the number of CAs may increase in renal tumors. Accordingly, the aim of this study is to investigate the relationship between CAs and renal tumors.
Methods: Between September 2022 and October 2024, a total of 167 patients underwent partial or radical nephrectomy for localized suspected renal mass in a tertiary institution. Among them, 98 patients underwent a detailed whole-body skin examination, including the scalp and genital areas, by a dermatologist using a digital handheld dermatoscope one day before surgery. The counted number of CAs was prospectively registered for either the total body or for each body region. The overall cohort was assessed by descriptive statistics, and the clear cell renal cell carcinoma (ccRCC) subgroup was separately investigated by comparative statistics. For the comparison of the ccRCC subgroup, the patients were stratified into two groups according to the counts of CAs ≥ 1 mm in the ipsilateral operative side: Group 1 (the number of CAs < 2, less ipsilateral CAs) and Group 2 (the number of CAs ≥ 2, more ipsilateral CAs). The descriptive statistics and comparative analyses were performed by using prospectively recorded demographical, clinical, and histopathological parameters.
Results: In the overall cohort, the median age was 60 (IQRs: 28–86) years and 64 (65.3%) patients were male. The median pathological tumor size was 4.6 (IQRs:1.2–11.0) cm. Fifty-eight (59.2%) patients had at least one CA lesion with a median number of 3 (IQRs: 1–14) in the ipsilateral operative side, and 91 (92.9%) patients with a median number of 24 (IQRs: 2–38) in the total body. Eighty-five (86.7%) nephrectomy pathologies were malignant while 13 (13.3%) were benign. In the comparative analyses of 62 ccRCC patients, the median age was 59 (IQRs: 51–68) years, and it was not different between the two groups (p: 0.159). Body mass index was noted to be higher in Group 1 (28.5 vs. 25.7 kg/m2, p: 0.009). Both clinical (4.7 cm vs. 8 cm, p: 0.01) and pathological tumor size (4.0 cm vs. 5.5 cm, p: 0.021) were significantly higher in Group 2, while the pT stage did not differ significantly between the two groups. The nuclear grade was significantly different between the two groups. (Grade 1–2: 83.3% vs. 53.1%; Grade 3–4: 16.7% vs. 46.9%, p: 0.011). In addition, the percentage of CAs in the ipsilateral operative side to CAs in the total body was significantly higher in Group 2. (0% vs. 13.2%, p < 0.001) Lack of a control group and metastatic patient group are accepted as limitations in this study.
Conclusions: The preliminary report of this observational study suggested that cherry angioma might be associated with renal tumors. The association of CA between pathological tumor size and nuclear grade in clear cell RCC might be an area for future research.
2.2. Convergent Mixed Methods Evaluation of a Prototype Health Data Management and Sharing App: A Study with Kidney Cancer Support Charity Members
Alessandro Riccombeni 1 and Mat Rawsthorne 1
- 1
Axia Medicine, London, UK
Background: Health data is a critical asset for individuals and the broader healthcare ecosystem. However, common industry practices rely on harvesting from fragmented sources, often without meaningful patient control, participation, or reward. This ‘data abandonment’ model contributes to gaps in care, inefficiencies in research, and ethical concerns around data use. By designing systems that prioritize user agency and transparency, it is possible to improve data quality, facilitate responsible sharing, and ensure that patients—not just industry stakeholders—derive value from their own health information.
Methods: At the end of February 2025, 100 members (patients and family) of a kidney cancer support charity were invited to test the Smartomics personal health data management application over a two-week period. Activity logs were collected to analyze feature utilization and engagement. Participants completed in-app surveys to explore attitudes toward active data sharing, including perceptions of incentive mechanisms. A roundtable discussion was conducted with purposely sampled participants to explore user experiences, ethical concerns, and feedback issues raised.
Results: Although engagement with the study was limited (n = 7), a diverse group of testers found the proof-of-concept application had good–excellent user-friendliness, with a high Net Promoter Score (75% and 80% for the envisioned finished product). More than half the users explored donating a proportion of their compensation to a Patient Association and using the app to manage their health data. Survey results revealed reasons for sharing health data ranged from coordinating care with family and clinicians to helping clinical trials and receiving compensation. Confidence for sharing was related to security safeguards and knowing who was using the data and why. The roundtable also uncovered concerns regarding privacy and sustainability and unmet needs in condition self-management and carer wellbeing.
Conclusions: There is evidence of a potential gap in services for a patient- and carer-centric data sharing and reward application.
2.3. ROB’N’SAFE—Robot-Assisted Radical Nephrectomy as SAFE Same-Day Surgery Now and in the Future
Anne-Sofie Eisum 1, Theresa Junker 2, Clara Bender 3, Lone Jørgensen 4, Hayder Al-Husseinawi 5, Pia Danstrup-Dinesen 6 and Tommy Kjærgaard Nielsen 1
- 1
Department of Urology, Aalborg University Hospital, Aalborg, Denmark
- 2
Department of Urology, Odense University Hospital
- 3
Department of Health Science and Technology, Aalborg University
- 4
Clinical Nursing Unit, Aalborg University Hospital and Department of Clinical Medicine, Aalborg University
- 5
Department of Urology, Gødstrup Hospital
- 6
Department of Anesthesia and Intensive Care, Aalborg University Hospital
Background: Robot-assisted radical nephrectomy (RARN) is a well-established treatment for localized kidney cancer. However, identifying frail patients at an increased risk of postoperative complications remains a key challenge to future same-day RARN. The project aims to identify key predictors to refine the preoperative frailty assessment by identifying key predictors of postoperative complications to refine patient selection and develop targeted pre- and postoperative care strategies.
Methods: The following three studies will be conducted.
Study I: A prospective observational cohort study assessing preoperative predictors of frailty and their impact on same-day discharge feasibility. Variables include Clinical Frailty Scale, handgrip strength, chair stand test, CT-derived body composition, and wearable-derived biometric data. Primary outcome: 24 h readmission rate. Secondary outcomes: 30-day complications and readmission, health literacy, and patient reported quality of life.
Study II: An observational cohort study evaluating postoperative recovery in relation to frailty using validated questionnaires and pain management assessments. Primary outcome: Patient-reported quality of recovery. Secondary outcomes: Opioid consumption, functional recovery metrics, and frailty progression.
Study III: A qualitative study exploring the role of relatives in supporting patients postoperatively, focusing on preparedness, satisfaction, and health literacy in home-based care.
Results and Conclusion: Our expected outcomes include improved patient selection criteria for same-day RARN through the integration of wearable technology, CT-based body composition analysis, and AI-driven predictive models, ultimately optimizing surgical safety. Furthermore, insights into the role of relatives in postoperative support may strengthen home-based care while increasing confidence and reassurance among healthcare professionals and patients.
2.4. A Phase 1, Multiple-Dose Study to Evaluate the Safety and Tolerability of First-in-Class XmAb819 (ENPP3 x CD3) in Subjects with Relapsed or Refractory Clear Cell Renal Cell Carcinoma (ccRCC)
Sumanta Pal 1, Benjamin Garmezy 2, Ritesh R Kotecha 3, Walter M Stadler 4, Mehmet Bilen 5, Christopher Hoimes 6, Yuanquan Yang 7, Joseph J Maly 8, Robert A Franklin 9, Claud M Grigg 10 and Chet Bohac 11
- 1
City of Hope Comprehensive Cancer Center, Duarte, California, USA
- 2
Sarah Cannon Research Institute, Nashville, TN, USA
- 3
Memorial Sloan Kettering Cancer Center, New York, NY, USA Karie Runcie, Columbia University Medical Center, New York, NY, USA
- 4
The University of Chicago, Chicago, IL, USA
- 5
Winship Cancer Institute at Emory University, Atlanta, GA, USA Scott S. Tykodi, University of Washington and Fred Hutchinson Cancer Center, Seattle, WA, USA
- 6
Duke University Medical Center, Durham, NC, USA
- 7
The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA
- 8
Norton Cancer Center, Louisville, KY, USA
- 9
University of Cincinnati, Cincinnati, OH, USA
- 10
Atrium Health Levine Cancer Institute, Charlotte, NC, USA
- 11
Xencor, Inc., Pasadena, CA, USA
Background: Despite advances in the treatment of metastatic ccRCC, few patients are cured. Therapies exploiting novel targets are needed. Antigen screening identified ENPP3 (ectonucleotide pyrophosphatase/phosphodiesterase family member 3) as having consistent high expression in ccRCC and low expression in normal tissue. ENPP3 is a transmembrane ectoenzyme involved in the hydrolysis of extracellular nucleotides. XmAb819 is a 2 + 1 (high-avidity bivalent ENPP3 binding with low-affinity monovalent CD3 binding) bispecific antibody. XmAb819 is engineered for the preferential engagement of high ENPP3-expressing cancer cells to induce T-cell-mediated cytolysis of cancer cells.
Methods: This is a multicenter, open-label, dose-escalation/expansion study enrolling up to 190 participants with advanced ccRCC. The primary objective is safety and tolerability; the secondary objective is preliminary anti-tumor activity. Part A, dose escalation, establishes a priming dose, step-up dose(s), a cohort-limit dose, and the dosing schedule for both intravenous (IV) and subcutaneous (SC) administration. Part B, dose expansion, evaluates the safety and efficacy of the recommended dose established in Part A. All subjects will have disease progression on standard-of-care therapies. XmAb819 will be administered weekly; cohort-limit doses will be administered in 21-day cycles until disease progression or unacceptable toxicity. Adverse events are graded using CTCAE v5.0; CRS using ASTCT Consensus Grading [
1]. Efficacy is assessed per investigator using RECIST v1.1.
Enrollment has been initiated, and dose escalation continues in both the IV and SC cohorts. Clinical trial information: NCT05433142.
3. Tumor Biomarkers and Pathology
3.1. Predictors of Cabozantinib Response in Patients with Advanced Renal Cell Carcinoma
Pablo Alvarez Ballesteros 1, Jorge Esteban Villarrubia 1, Marta Dueñas 2, Juan Manuel Funes Quesada 2, Jose Ignacio Dominguez Dominguez 2, Enrique Perez Navarro 2, Juan Burgos 2, Jesús Paramio 2 and Daniel Castellano 3
- 1
Hospital Universitario 12 de Octubre, Medical Oncology Department, Madrid, Spain
- 2
Fundación para la Investigación Biomédica—Hospital 12 de Octubre, Centro de Oncología Experimental, Madrid, Spain
- 3
Hospital Universitario 12 de Octubre, Medical Oncology Department, Madrid, Spain. Enrique González Billalabeitia, Hospital Universitario 12 de Octubre, Medical Oncology Department, Madrid, Spain. Guillermo Hospital Universitario 12 de Octubre, Medical Oncology Department, Madrid, Spain, De Velasco
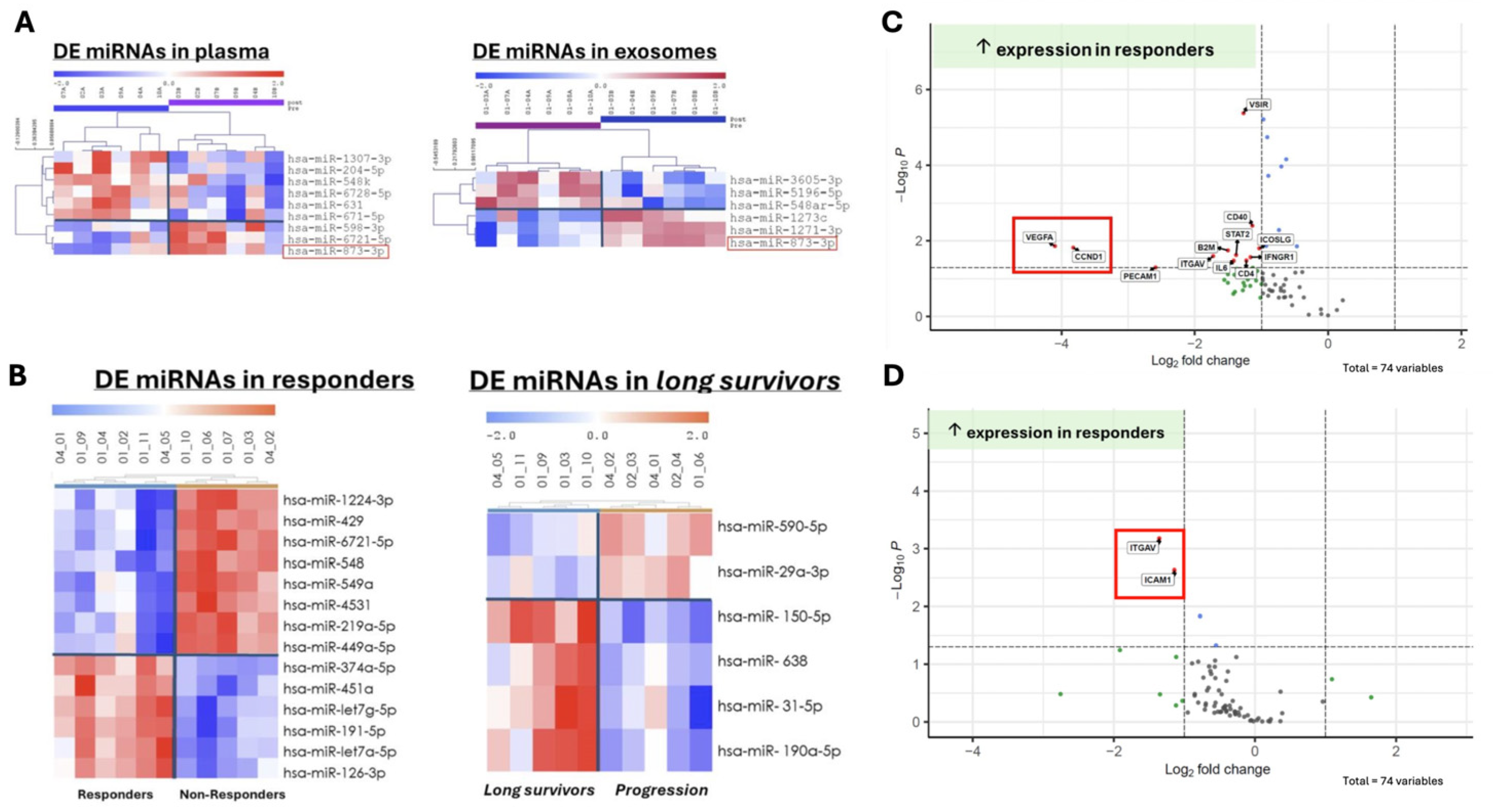
Introduction: Paired samples from the CABOPRE trial were used to determine the differential expression of miRNAs and key genes associated with responses to neoadjuvant cabozantinib treatment in patients with metastatic renal cell carcinoma. This analysis aimed to identify potential biomarkers that could predict treatment efficacy and provide insights into the molecular mechanisms underlying cabozantinib’s therapeutic effects.
Results: Paired and sequential samples from 15 patients were used to evaluate treatment responses. Fourteen differentially expressed miRNAs were identified in pretreatment between responders and non-responders, including miR-549a, with the majority associated with the upregulation of VEGFA and CCND1 in responders. Additionally, nine miRNAs were differentially expressed (six downregulated and three upregulated) following cabozantinib treatment compared to the baseline, which could serve as predictive biomarkers for cabozantinib response.
Furthermore, the comparison of miRNA levels in plasma and exosomes revealed that only miR-873-3p exhibited concordant expression changes in both compartments, suggesting a potential role for this specific miRNA in mediating the effects of cabozantinib. The analysis of long-term responders (PFS > 12 months) exhibited a distinct miRNA signature characterized by the downregulation of two miRNAs and upregulation of four miRNAs compared to the baseline (
Figure 2A,B).
Digital spatial profiling was performed on tissue samples from eight nephrectomized patients categorized as responders (n = 3) and non-responders (n = 5). The analysis revealed pretreatment increased the expression of 10 genes in the tumor, including VEGFA and CCND1 in the responders to cabozantinib. Conversely, CCND1 expression displayed an increase in non-responders posttreatment, suggesting a potential resistance pathway (
Figure 2C).
Finally, cabozantinib treatment in responders was associated with the upregulation of adhesion molecules ITGAV and ICAM1, which may explain neutrophil infiltration into the tumor microenvironment (
Figure 2D). Additional MPO staining on the tissue samples showed increased neutrophil expression after treatment in 66% of responders compared to 40% of non-responders.
Conclusion: The biomarker analysis reveals that increased VEGFBA and CCND1 expression, along with differential miRNA expression and neutrophil infiltration, can predict the cabozantinib response in RCC patients.
3.2. A Biomarker Analysis of Tide-A, a Phase 2 Study of First-Line Avelumab Plus Intermittent Axitinib: Circulating Kidney Injury Molecule-1 (KIM-1) in mRCC
Romina Rose Pedone 1, Antonio Agostini 1, Martina Panebianco 2, Giulia Claire Giudice 3, Matteo Perrino 4, Marco Maruzzo 5, Emanuela Fantinel 6, Elena Verzoni 7, Caterina Accettura 8, Lucia Bonomi 9, Consuelo Buttigliero 10, Giuseppe Fornarini 11, Roberto Sabbatini 12, Geny Piro 1, Sebastiano Buti 3, Paolo Andrea Zucali 4, Giampaolo Tortora 1, Carmine Carbone 1, Roberto Iacovelli 1 and Chiara Ciccarese 1
- 1
Fondazione Policlinico Universitario A. Gemelli IRCCS, Department of Medical Oncology, Rome, Italy
- 2
Ospedale San Paolo, Dept. of Medical Oncology, Civitavecchia, Italy
- 3
University of Parma, Dept. of Medical Oncology, Parma, Italy
- 4
Humanitas Research Hospital, Dept. of Medical Oncology, Rozzano, Italy
- 5
Istituto Oncologico Veneto, Dept. of Medical Oncology, Padua, Italy
- 6
University of Verona, Dept. of Medical Oncology, Verona, Italy
- 7
Fondazione IRCCS Istituto Nazionale dei Tumori, Dept. of Medical Oncology, Milan, Italy
- 8
Vito Fazzi Hospital, Dept. of Medical Oncology, Lecce, Italy
- 9
ASST Papa Giovanni XXIII Hospital, Dept. of Medical Oncology, Bergamo, Italy
- 10
San Luigi Gonzaga Hospital, Dept. of Medical Oncology, Orbassano, Italy
- 11
Ospedale Policlinico San Martino, Dept. of Medical Oncology, Genova, Italy
- 12
Azienda Ospedaliero—Universitaria Policlinico di Modena, Dept. of Medical Oncology, Modena, Italy
KIM-1 is a plasma biomarker of microscopical residual disease, disease recurrence after nephrectomy, and potential benefit from adjuvant immunotherapy (IO). No data is available about its role in mRCC. We explored KIM-1 in the prospective cohort of the Tide-A study: an intermittent strategy of axitinib plus avelumab in treatment-naïve mRCC. We performed a proteomics analysis with the aptamer-based technology SomaScan 7K (Somalogic, Boulder, CO, USA). KIM-1 levels were analyzed in baseline samples and normalized by Adaptive Normalization by Maximum Likelihood to combine samples and correct for batch effects. The KIM-1 cut-off (10.456 relative fluorescence units) was determined by Maximally Selected Rank Statistics using the maxstat R package (version 0.7-26) with the Van der Waerden test and log-rank score. KIM-1 levels were evaluated at baseline in the overall population and adjusted for IMDC and for DOR with avelumab maintenance. Data for the KIM-1 analysis at baseline were available for 69 pts; 13% were KIM-1-high and 87% KIM-1-low. KIM-1-high was related with shorter mOS (24.2 months [95%CI 21.1–NR] in KIM-1-high vs. not reached (NR) in KIM-1-low; p = 0.0019). The 2-yOS rate was 90% in KIM-1-low vs. 56% in KIM-1-high (p = 0.002). KIM-1 levels were correlated with OS when adjusted for IMDC. No correlation was observed between KIM-1 levels and mPFS (22.9 vs. 29.9 months in KIM-1-high and low, respectively; p = 0.4). KIM-1 data were available for 28/29 pts that discontinued axitinib; the mDOR of ave-maintenance was 15.9 wks in 25 pts with KIM-1-low and NR in 3 pts with KIM-1-high, (p = 0.19). A high baseline plasma KIM-1 level is an independent negative prognostic factor; regardless, the IMDC in mRCC treated with VEGFR-TKI + IO. KIM-1 seems to not have a pivotal role in selecting the TKI-intermittent strategy.
3.3. How Different Are the Expression Levels of Immune Checkpoint Molecules and Their Roles on the Prognosis in Surgically Treated Non-Metastatic Clear Cell, Papillary, and Chromophobe Subtypes of Renal Cell Carcinoma?
Serdar Turan 1, Selcuk Erdem 2, Ozge Hurdogan 3, Yasemin Ozluk 3, Isin Kilicaslan 3, Rifat Ergul 1, Oner Sanli 2 and Faruk Ozcan 2
- 1
Department of Urology, Istanbul University Istanbul Faculty of Medicine, Istanbul, Turkey
- 2
Division of Urologic Oncology, Department of Urology, Istanbul University Istanbul Faculty of Medicine, Istanbul, Turkey
- 3
Department of Pathology, Istanbul University Istanbul Faculty of Medicine, Istanbul, Turkey
Background and Objectives: The immune checkpoint inhibitors (ICIs), either as adjuvant treatment in intermediate–high-risk non-metastatic disease or as first-line treatment in metastatic disease, are approved as standards of care in clear cell renal cell carcinoma (ccRCC). However, there is limited evidence suggesting there are ICIs in the management of non-clear cell subtypes. This study aims to compare the expression levels of immune checkpoint molecules in tumor cells and tumor microenvironment and their role in prognosis among surgically treated non-metastatic three RCC subtypes including ccRCC, papillary (papRCC), and chromophobe (chRCC).
Methods: This study included 103 non-metastatic RCC patients undergoing partial or radical nephrectomy between 2015 and 2022 at a tertiary institution. Immunohistochemical methods were used to assess the expression of PD-L1 (Tumor Proportion Score-TPS, Combined Positivity Score-CPS, and Immune Cell-IC), PD-1, and CTLA-4 in the paraffin-embedded tissue samples of patients. The threshold was defined as % 1 for immunohistochemical positivity. The present study seeks answers to two questions of the co-primary outcome: (1) whether the expression levels of ICI molecules differ between subtypes and (2) whether the positivity of ICI molecules predict RFS after curative surgery in each subtype. The secondary outcomes were defined as whether the positivity of ICI molecules predict CSS and OS.
Results: PD-L1 (IC) (p = 0.047) and PD-1 (p = 0.006) positivity were significantly higher in ccRCC when compared to papRCC and chRCC. PD-L1 (TPS), PD-L1 (CPS), and CTLA-4 positivity were similar between the three subtypes. The median follow-up was 49 months, and 10 (9.7%) patients recurred during this follow-up. Kaplan–Meier survival analyses found that PD-1 positivity significantly predicted worse RFS (p = 0.008), CSS (p = 0.009), and OS (p = 0.008) in papRCC and worse RFS (p = 0.014) and OS (p = 0.029) in the overall cohort. CTLA-4 positivity predicted worse OS (p = 0.002) in papRCC, while PD-L1 (CPS) positivity predicted better CSS (p = 0.036) in ccRCC. PD-L1 (TPS) positivity did not predict any survival outcome either in the overall cohort or in each subtype.
Conclusions: These findings suggest that the expression levels of immune checkpoint molecules in the tumor and its microenvironment differ among RCC subtypes, and PD-1 is a promising target for further research on ICIs in papillary RCC, as it significantly predicts worse prognosis.
3.4. BAP Loss in Renal Cancer Predisposes in Spinal Cord Metastatic Early Presentation: Two Cases Reported and Review of Relevant Literature
Nektarios Alevizopoulos 1, Michael Pavlakis 2, Vaios Oreopoulos 3, Ioannis Xoxakos 4 and Ioannis Eythymiou 4
- 1
Evaggelismow General Hospital
- 2
Biocliniki Oncology Department
- 3
Evaggelismos General Hospital
- 4
Evaggelismos Hospital Urology Department
Background: Mutations drive renal cell carcinoma biology and tumor growth. The BRCA1-associated protein-1 (BAP1) gene is frequently mutated in clear cell renal cell carcinoma (ccRCC) and has been focused on as a potential worse prognostic and predictive biomarker. We review two cases and discuss the role of BAP1 as a signature event of a subtype of RCC underlying aggressiveness in presentation and possibly a minor response to immunotherapy, hinting at the resistance of an unknown mechanism.
Purpose: To present two cases of patients with extensive metastatic disease in thoracic vertebra associated with confirmed clear renal cell cancer which documented BAP1 immunostaining loss and a demonstrated worse prognosis and outcome.
Patients and methods: Two patients were assigned with an established clear cell renal cancer diagnosis and a simultaneous metastatic disease histologically confirmed in thoracic vertebra.
Results: Both of them showed the negative expression of BAP1. They presented with disease in the Spinal area at the onset of their first symptom complaint. They did not easily respond to the local treatment of palliative radiotherapy and to subsequent immunotherapy plus axitinib treatment offered
They both experienced adverse events of colitis and thyroid dysfunction; while easily manipulated, they did not even have a 3-month response to the first-line treatment. Subsequent molecular and chromosomal analysis was demonstrated, which will be disposed.
Conclusions: The molecular detection of BAP loss may be associated with both poor survival and an increased rate of worse outcome in patients with clear cell renal cancer; this approach may make physicians aware to detect metastatic cascade in renal cancer patients early and offer them novel approaches with promising anticancer benefits.
4. Real-World Evidence
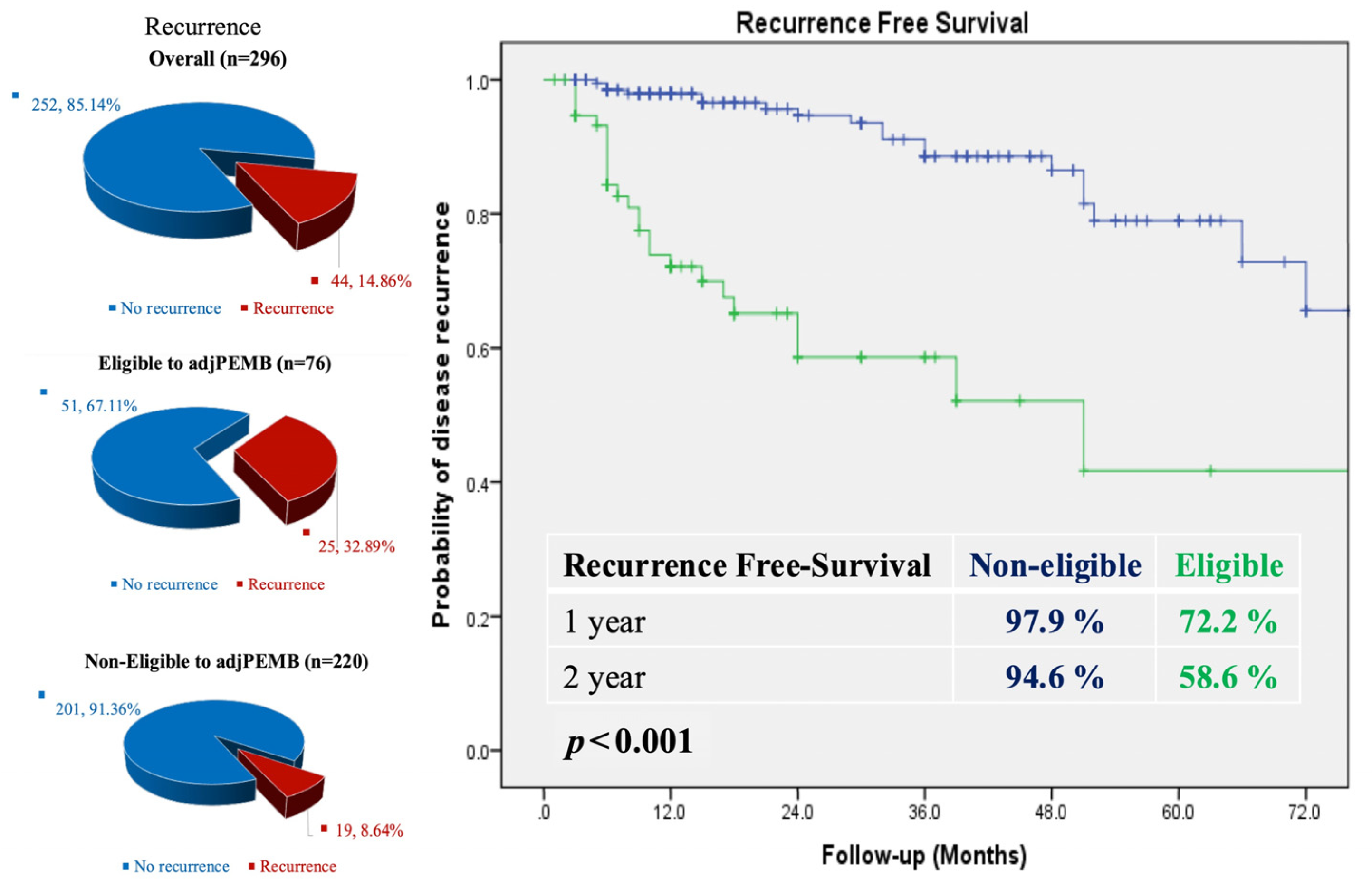
4.1. Adjuvant Pebrolizumab in Renal Cancer Patients: Real Data of a Single Greek Centre
Nektarios Alevizopoulos 1, Ioannis Xoxakos 2, Michael Pavlakis 3, Areti Dimitriadou 1, Dimitrios Ioannou 1, Ioannis Euthimiou 2 and Vaios Oreopoulos 1
- 1
Evaggelismos General Hospital Oncology Department
- 2
Evaggelismos General Hospital Uloroly Department
- 3
Biocliniki Hospital Oncology Department Athens
Introduction and Objectives: Pebrolizumab is the only adjuvant approved treatment promising improvement in disease-free and overall survival in clear renal cell cancer patients post-surgical procedure.
Herein, we report our real-world data experience, since its approval in 2022, to reconfirm the Keynote trial’s conclusions.
Materials and Methods: All Medical electronic data of renal cancer patient candidates for adjuvant therapy were reviewed between October 2022 to the current date. Data demographics, pathology features, multidisciplinary discussion referrals, and all stated reasons for co-decisions for treatment or no treatment and noticed toxicity will be presented.
Results: Data from 48 patients, eligible for adjuvant pebrolizumab treatment based on pathology criteria as they are identified in the Keynote 564 trial, have been recorded from a multi-disciplinary team discussion. All referred patients had intermediate or high-risk resected disease. Only five patients had Sarcomatoid features and nobody had microscopically resected tumoral deposits. Fifteen of the discussed patients by the multi-disciplinary team were excluded due to comorbidities, hinting at a high risk of revealing immune-related events. So, 32 had been offered pebrolizumab adjuvant treatment. A total of 25 patients completed 1 year of adjuvant treatment successfully. The rest of the seven patients are still on ongoing adjuvant treatment. One patient, post-adjuvant completion therapy, presented metastatic brain disease, hinting at coexistence underneath with no clinical signs. The rest of the 24 patients are still being monitored and are still free of any metastatic disease. Immune-related adverse events of any grade recorded were as follows: fatigue, pneumonitis, and thyroid dysfunction, presented in five patients. No grade III/IV-related events were noticed. Two patients with pneumonitis were offered steroid treatment; their recovery was easy, with no hospitalization required. The rest of the adverse events were easily resolved.
Conclusions: Our real data demonstrate that the majority of patients identified as eligible for adjuvant pebrolizumab when discussed by a multi-disciplinary team are prone to immune-related adverse events and experience clinical benefits of the existing therapeutic adjuvant renal cancer algorithm. Close monitoring of adverse events protects from worse outcomes and secures the prompt identification of low grade immune-related toxicities.
The presented data of a few reported patient series confirm the endpoints of the Keynote 564 trial and underline the need for multi-disciplinary team discussion to guarantee the success of the subgroup of renal cancer patient candidates for adjuvant treatment, while also being capable of manipulating possible related adverse events.
4.2. Real-World Evidence on Adjuvant Pembrolizumab for Renal Cell Carcinoma (ARON-1)
Matteo Santoni 1, Ray Manneh Kopp 2, Enrique Grande 3, Yüksel Ürün 4, Javier Molina-Cerrillo 5, Ondrej Fiala 6, Sebastiano Buti 7, Fernando Sabino Marques Monteiro 8, Tomas Buchler 9, Jindrich Kopecky 10, Andrey Soares 11, Alessandro Rizzo 12, Thomas Büttner 13, Mimma Rizzo 14, Zin W Myint 15, Umberto Basso 16, Haoran Li 17, Renate Pichler 18 and Mehmet Asim Bilen 19
- 1
Medical Oncology Unit, Macerata Hospital, Macerata, Italy Francesco Massari, Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- 2
Clinical Oncology, Sociedad de Oncología y Hematología del Cesar, Valledupar, Colombia
- 3
Department of Medical Oncology, MD Anderson Cancer Center Madrid, Madrid, Spain
- 4
Department of Medical Oncology, Ankara University Faculty of Medicine, 06620 Ankara, Türkiye
- 5
Department of Medical Oncology, Hospital Ramón y Cajal, Madrid, Spain
- 6
Department of Oncology and Radiotherapeutics, Faculty of Medicine and University Hospital in Pilsen, Charles University, Pilsen, Czech Republic
- 7
Medical Oncology Unit, University Hospital of Parma, Parma, Italy
- 8
Oncology and Hematology Department, Hospital Sírio Libanês, Brasília, Brazil
- 9
Department of Oncology, Second Faculty of Medicine, Charles University and University Hospital Motol, Prague, Czech Republic
- 10
Department of Clinical Oncology and Radiotherapy, University Hospital Hradec Kralove, Hradec Kralove, Czechia
- 11
Hospital Israelita Albert Einstein, São Paulo, SP, Brazil
- 12
S.S.D. C.O.r.O. Bed Management Presa in Carico, TDM, IRCCS Istituto Tumori “Giovanni Paolo II”, Viale Orazio Flacco 65, 70124, Bari, Italy
- 13
Department of Urology, University Hospital Bonn (UKB), Bonn, Germany
- 14
Medical Oncology Unit, Azienda Ospedaliera Universitaria Consorziale Policlinico di Bari, Bari, Italy
- 15
Division of Medical Oncology, Department of Internal Medicine, Markey Cancer Center, University of Kentucky, Lexington, KY 40506
- 16
Medical Oncology 1 Unit, Department of Oncology, Istituto Oncologico Veneto IOV IRCCS, 35128, Padova, Italy
- 17
Division of Medical Oncology, Department of Internal Medicine, University of Kansas Cancer Center, Westwood, KS, USA
- 18
Department of Urology, Medical University of Innsbruck, Innsbruck, Austria
- 19
Winship Cancer Institute of Emory University, Atlanta, GA, US
Background: Pembrolizumab as an adjuvant treatment strategy after radical surgery has been demonstrated to improve disease-free survival (DFS) and overall survival (OS) when compared with placebo in patients with clear cell RCC at a higher risk of relapse. In this study from the ARON-1 dataset, we analyzed real-world data on the use of adjuvant pembrolizumab on ccRCC patients.
Methods: We retrospectively collected data from ccRCC patients who received adjuvant pembrolizumab at 56 hospitals from 12 countries. Patients were assessed for DFS, OS, and severe adverse events (SAEs). The statistical analysis encompassed the Kaplan–Meier methodology, the log-rank test, as well as univariable and multivariable Cox proportional hazard regression models.
Results: 311 patients were included from the ARON-1 dataset. The median age was 61y (range 25–85 y); 257 (83%) presented T3 stage at diagnosis, with 17% of cases reporting sarcomatoid differentiation. The median follow-up was 15.4 months (95%CI 11.2–18.8). The median OS and DFS were not reached (NR), with a 95% 2y-OS rate and a 69% 2y-DFS rate. Sixty-one patients (20%) recurred. Lungs (11%) and bones (5%) were the most common distant sites of recurrence. The time to local recurrence was 5.4 months (95%CI 3.0–31.0), while the time to distant recurrence was 6.7 months (95%CI 5.2–31.0). The median DFS was impaired in patients aged <65 y (NR vs. NR, HR 2.14, 95%CI 1.26–3.63, and p = 0.005), in the N1 subgroup (HR 5.42, 95%CI 1.72–17.1, and p = 0.004), and in subjects with sarcomatoid de-differentiation (21.5 months, 95%CI 17.1–25.8 vs. NR, HR 2.54 1.29–4.98, and p = 0.007). the discontinuation rate due to SAEs was 19%; the most common SAEs were colitis (4%), hepatopathy (4%), and nephritis (3%).
Conclusions: In this large real-world study, pembrolizumab was demonstrated to be an effective and tolerable treatment for the adjuvant setting of ccRCC at a higher risk of relapse.
4.3. CABONEXT: Advanced Renal Cell Carcinoma Treatment Following Cabozantinib: A Retrospective Analysis of Sequencing Therapies
Simon Nannini 1, Fabien Moinars-Butot 1, Sylvain Ladoire 2, Stéphane Oudard 3, Dorian Bochaton 3, Pierre Bigot 4, Marine Gross-Goupil 5, Hakim Mahammedi 6, Fabien Calcagno 7, Jean-Baptiste Barbe-Richaud 8, Sabrina Falkowski 9, Karim Amrane 10, Pierre Cornillon 11 and Philippe Barthélémy 1
- 1
Department of Medical Oncology, Institut de Cancérologie Strasbourg Europe, 17 Rue Albert Calmette, 67033 Strasbourg, France
- 2
Department of Medical Oncology, Centre Georges-François Leclerc, 21000 Dijon, France
- 3
Department of Medical Oncology, Hôpital européen Georges Pompidou, Assistance Publique-Hôpitaux de Paris—Paris Centre, F-75015 Paris, France; Université Paris Cité, 75006 Paris, France
- 4
Department of Urology, University of Angers, Rennes, France
- 5
Department of Medical Oncology, Bordeaux University Hospital, Hôpital Saint-André, Bordeaux, France
- 6
Department of Medical Oncology, Centre Jean Perrin, 63011 Clermont-Ferrand, France
- 7
Department of Medical Oncology, CHRU Jean Minjoz, Besançon, France
- 8
Department of Medical Oncology, Institut de Cancérologie Strasbourg Europe, 17 Rue Albert Calmette, 67033 Strasbourg, France. Luca Medical Oncology, Pitié Salpêtrière Hospital, Sorbonne Université, 75013 Paris, France, Campedel
- 9
Department of Medical Oncology, polyclinique de Limoges, site Chénieux, 18, rue du Général-Catroux, 87000 Limoges, France
- 10
Department of Oncology, Regional Hospital of Morlaix, Morlaix, France
- 11
Department of Medical Oncology, North Hospital, University Hospital of Saint-Etienne, Saint-Etienne, France
Introduction: Cabozantinib combined with nivolumab is considered to be a standard of care in first-line treatment for metastatic renal cell carcinoma (mRCC). Cabozantinib is a TKI targeting VEGFR, MET, and AXL and showed efficacy as a monotherapy or combined with checkpoint inhibitors (CPI). Little is known about subsequent systemic therapy following cabozantinib. This study aimed to report clinical outcomes from subsequent lines following a first- or second-line treatment in mRCC pts treated with cabozantinib.
Methods: We performed a multicentric retrospective study at 12 French centers. All included pts that received at least one treatment after first or second line, based on cabozantinib for mRCC, from August 2018 to December 2024. The primary endpoint was time to treatment failure (TTF). Secondary endpoints included the objective response rate (ORR), disease control rate (DCR), and safety.
Results: Sixty-five pts were included, and 80% were males with a median age of 61 years. Thirteen pts (20%) received cabozantinib with nivolumab as the first-line therapy (group A) and 80% in second line were pretreated with dual CPI in 35% and CPI with TKI in 45% (group B). In group A, 63% received axitinib, 27% received a first generation TKI, and one pt received lenvatinib. The median TTF was 4.98 months with an ORR of 44.4% and a DCR of 88.9%. Six pts (55%) had a subsequent line, three pts were still on treatment, and two died before starting a new line. In group B, 29% received axitinib, 25% everolimus, 18% lenvatinib, and 12% CPI, including two in combination with tivozanib and one with belzutifan. The median TTF was 3.21 months with an ORR of 9.3% and a DCR of 46.5%. Nine pts (18%) were still on treatment, 24 pts (47%) had a subsequent line, and 21 (41%) died before. Additional data will be added until May 2025.
Conclusion: In our cohort, TKI demonstrated meaningful activity after cabozantinib and nivolumab. TKI after second-line cabozantinib had modest activity and either the treatment targeted needs to acquire resistance or new antitumoral pathways are needed.
4.4. Impact of Cabozantinib Monotherapy Starting Dose on Survival in Metastatic Renal Cell Carcinoma: A Real-World Cohort Study
Sakari Kosola 1, Kalle E Mattila 2, Laura Ahtiainen 3, Julia Rockberg 4, Ann-Charlotte Mårdby 3, Judith Miedema 3, Tommi Kauko 5 and Lotta Tapana 5
- 1
Department of Oncology and Radiotherapy, University of Turku and Turku University Hospital and FICAN West Cancer Centre
- 2
Helsinki University Hospital. Comprehensive Cancer Center, Helsinki; Department of Oncology and Radiotherapy, University of Turku and Turku University Hospital and FICAN West Cancer Centre; InFLAMES Research Flagship Center, University of Turku
- 3
Ipsen, Kista, Sweden
- 4
Ipsen, Kista, Sweden; AUWEAI, Stockholm, Sweden
- 5
The wellbeing services county of Southwest Finland, Auria Clinical Informatics, Finland
Cabozantinib is a tyrosine kinase inhibitor used for the treatment of metastatic renal cell carcinoma. This study describes patient characteristics in 1L and 2L+ and evaluates the effect of the starting dose, 60 mg vs. 40 mg daily on survival.
Adult patients diagnosed between 1996 and 2021 and treated with cabozantinib monotherapy between 2018 and 2021 at the Hospital District of Southwest Finland were included. Patient characteristics, treatment patterns, and survival were analyzed and stratified by the treatment line and starting dose.
In total, 34 (48%) patients received cabozantinib in 1L (60 mg n = 14, 40 mg n = 20) and 37 (52%) in 2L+ (60 mg n = 10, 40 mg n = 27). Patients in 1L were older (median age 69 vs. 65 years) and had poorer prognosis (IMDC favorable 7% vs. 32%, intermediate 54% vs. 59%, and poor risk 39% vs. 9%) compared to 2L+. Patients had undergone prior nephrectomy 14 (41%) in 1L and 24 (65%) in 2L+.
The median duration of treatment in 1L was 9 months (IQR 4.8–11.8) for the 60 mg starting dose and 13 months (IQR 4.3–19.5) for the 40 mg starting dose, and in 2L+, it was 6 (IQR 3.0–8.8) and 4 (3.0–11.0), respectively. Dose reductions were more common with the 60 mg vs. 40 mg starting dose (64% vs. 5% in 1L and 40% vs. 11% in 2L+). The median OS was 22 months (95%CI 20–NA) vs. 28 (95%CI 15−NA) with the 60 mg vs. 40 mg starting dose in 1L and 12 months (95%CI 5.3–NA) vs. 9.6 months (95%CI 5.9–NA), respectively, in 2L+.
Patients with 1L treatment had poorer prognostic characteristics vs. patients with 2L+ treatment. Patients with a 60 mg starting dose also had poorer characteristics compared to patients starting with 40 mg. There were no significant differences in the overall survival between patients with a 60 mg vs. 40mg starting dose in 1L or 2L. Dose reductions were required to manage side effects.
4.5. Sunitinib for Metastatic Renal Cell Carcinoma: A Real-World Evidence Study in Denmark
Mohammad Haman 1, Johanne Ahrenfeldt 2 and Niels Fristrup 3
- 1
Aarhus University Hospital Department of oncology, Denmark
- 2
Institute of Clinical Medicine—Department of Molecular Medicine (MOMA), Aarhus, Denmark
- 3
Assoc. Professor, Department of Clinical medicine and Clinical Oncology, Aarhus University Hospital, Denmark
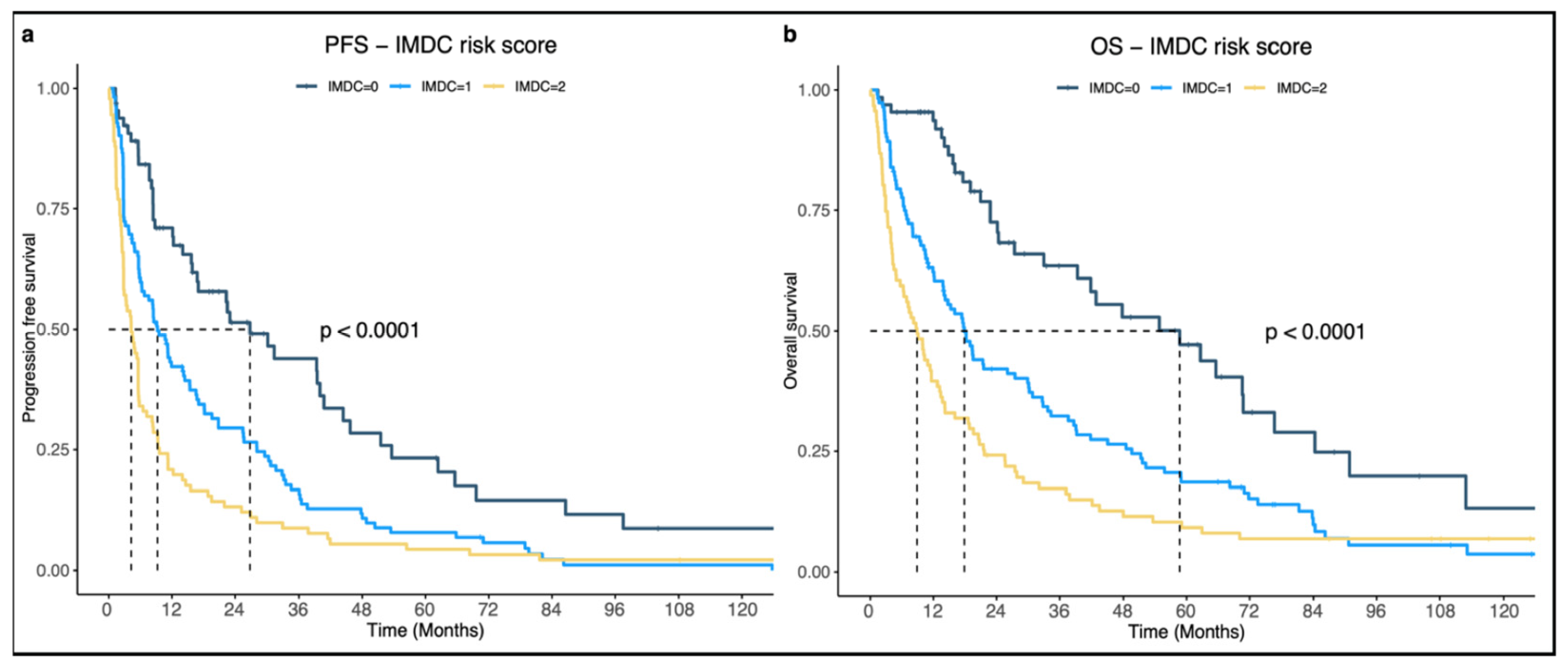
Background: Sunitinib, a multitarget TKI, has been a cornerstone in mRCC treatment since 2007. Until 2019, it was the preferred first-line treatment, but dual-immunotherapy has since shifted treatment guidelines, limiting sunitinib use primarily to patients in the IMDC-favorable prognosis group. While multiple RWE studies have assessed the effectiveness of sunitinib, this study represents the first Danish RWE study, providing data on treatment outcomes.
Objectives: The aim of this thesis is to characterize mRCC patients treated with sunitinib at Aarhus University Hospital (AUH) and evaluate their PFS and OS. PFS and OS will be compared with other RWEs and RCTs, and the impact of characteristics such as the ECOG-PS and IMDC score on PFS and OS will be analyzed.
Methods: Lists of patients treated with sunitinib for mRCC from 1 January 2010, to 1 September 2024, were extracted from a sunitinib registry at the Department of Oncology, AUH. Patient data were collected through medical record review in electronic patient-journals and the pathology database. The data were then organized and entered into RedCap. Analyses were conducted and presented as forest plots and KM-curves.
Results: The median PFS was 8.43 months (95% CI: 6.47–11.2) and mOS was 18.6 months (95% CI: 14.73–22.8). In the favorable risk group (IMDC = 0), mPFS reached 26.26 months (95%CI: 16.52–43.67) and mOS 57.73 months (95%CI: 38.62–75.31). For ECOG-PS 0, mPFS was 11.05 months (95%CI: 8.05–16.46) and mOS 23.77 months (95% CI: 19.05–36.92) (
Figure 3a,b).
Conclusions: When compared to other RWEs, our mPFS and mOS fell within the spectrum of previously published results. ECOG-PS and IMDC were identified as the most significant factors in both PFS and OS. Adjusted for IMDC-0, the PFS and OS in our study were among the highest reported in existing RWE’s.
4.6. Retrospective Observational Real-Life Study on Metastatic Renal Cell Carcinoma Treated in First Line with Axitinib + Pembrolizumab: A Project of the Campania Oncology Network
Marilena Di Napoli 1, Sabrina Rossetti 2, Carmine D’Aniello 3, Luigi Formisano 4, Andrea Muto 5, Davide Bosso 6, Dario Franzese 7, Roberto Contieri 7, Carmela Pisano 1, Rosa Tambaro 1, Sabrina Chiara Cecere 1, Anna Passarelli 1, Jole Ventriglia 1, Elisabetta Coppola 1, Lorenzo Lobianco 1, Gabriele Calvanese 8 and Sandro Pignata 1
- 1
Uro-Gynecological Department, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy
- 2
Uro-Gynecological Department, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy Carlo Buonerba, Oncology Research Assistance, Salerno, Italy
- 3
Division of Medical Oncology, AORN dei Colli-Monaldi Hospital, Naples, Italy Sarah Scagliarini, Department of Medical Oncology, AORN “A. Cardarelli”, Naples—Italy
- 4
Department of Clinical Medicine and Surgery, University of Naples “Federico II”, Napoli, Italy Francesco Sabbatino, Oncology Department, University Hospital “San Giovanni di Dio e Ruggi d’Aragona”, Salerno, Italy
- 5
Division of Medical Oncology, “San Giuseppe Moscati” Hospital, Avellino, Italy
- 6
Oncology Unit, Ospedale del Mare, 80147 Naples, Italy
- 7
Urology Department, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy
- 8
Uro-Gynecological Department, Istituto Nazionale Tumori di Napoli, IRCCS “G. Pascale”, Naples, Italy. Department of Oncology and Hemato-Oncology, Università degli Studi di Milano, Milan, Italy
Background: Several combination therapies are used as first-line treatments for metastatic renal cell carcinoma (mRCC). However, clinical trial populations often differ from the general population, resulting in varied outcomes and toxicities in real-world settings. Oncology Networks are key in generating Real-World Data (RWD) and supporting clinical decision-making through Real-World Evidence (RWE). We retrospectively analyzed mRCC patients treated with first-line pembrolizumab plus axitinib in the Campania Oncology Network (ROC).
Methods: This multicentre retrospective study included untreated mRCC patients receiving pembrolizumab plus axitinib at eight ROC centers. Primary endpoints were progression-free survival (PFS) and overall survival (OS), while secondary endpoints were the objective response rate (ORR) and safety.
Results: From January 2021 to November 2023, 117 mRCC patients were treated with pembrolizumab plus axitinib at eight ROC centers. International Metastatic RCC Database Consortium (IMDC) risk was favourable in 19.6% of patients, intermediate/poor in 65%, and unknown in 15.4%. The median age at diagnosis was 59 years, with 53.8% having an ECOG Performance Status (PS-ECOG) ≥ 1. Clear cell histology was most common (87.2%), and the main metastatic sites included the brain (36%), lungs (35%), bones (30%), and lymph nodes (29%). After a median follow-up of 12.8 months, the median PFS was 15.1 months, ORR was 27.3%, and the median OS was not reached. PD caused 49% of treatment discontinuations, and adverse events (AEs) were responsible for 6%. Most adverse events (AEs) were G1–2, including diarrhea (23.9%), asthenia (18%), hypothyroidism (12.8%), and hypertension (9.4%). An indirect comparison with Keynote 426 at first Interim Analysis (2019) showed minimal differences (
Table 1).
Conclusions: Our study supports the applicability of pembrolizumab plus axitinib in a real-world setting. As no front-line regimen has proven to be superior, RWD comparisons may help personalize treatment strategies in clinical practice.
4.7. A Two-Center Real-World Observational Study of Ipilimumab + Nivolumab in Advanced Renal Cell Carcinoma (aRCC)
Ben Crosby 1, Jose Tapia 1, Niall Moon 2, John McGrane 2 and Ricky Frazer 1
- 1
Velindre Cancer Centre, Cardiff
- 2
Royal Cornwall Hospitals NHS Trust
Introduction: We aim to report outcomes from patients with aRCC who received first line ipilimumab and nivolumab (I + N) at two tertiary cancer centres across England and Wales between June 2019 and July 2023.
Methods: Retrospective study. We included aRCC patients with a minimum follow-up of 18 months, intermediate/poor IMDC status, clear cell aRCC, and an age ≥18 years.
Results: 117 patients were included, with 44.9% patients > 65 years old and 68.4% males. Most patients had an intermediate IMDC risk status (71.8%) and completed four cycles of I + N (73.4%). Approximately 41% of patients had undergone prior nephrectomy. The median follow-up was 40 months. Overall, the median overall survival (mOS) and median progression-free survival (mPFS) were 27.9 and 15.3 months, respectively. Eighteen-month OS was 62.4%. Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were 11.1%, 48.7%, 58.8%, 22.2%, and 14.5, respectively. mOS and mPFS in those with an objective response (CR + PR) were 45 and 25.5 months, respectively. mOS in CR, PR, SD, and PD were not reached at 40.8, 21.7, and 7.3 months, respectively. A significantly greater mOS was associated with prior nephrectomy (38.3 months vs. 18.7 months, HR: 0.54 [95% CI 0.32–0.89], and p = 0.015), intermediate IMDC status (35.8 months vs. 11.8 months, HR: 0.51 [95% CI 0.31–0.84], and p = 0.007), and completing four cycles of I + N (39.3 months vs. 10.6 months, HR: 0.28 [95% CI 0.16–0.51], and p < 0.001).
Discussion: In a real-world setting, the vast majority of patients achieved a durable response. Those who received four cycles of I + N, had a prior nephrectomy, and those who had an intermediate IMDC risk status had significantly greater survival.
4.8. Metastatic Renal Cell Carcinoma (mRCC) Primary Refractory Patients to First-Line IO-TKI and IO-IO Combinations (Meet-URO 33 Analysis)
Davide Bimbatti 1, Davide Bimbatti 1, Alessio Signori 2, Sebastiano Buti 3, Federico Paolieri 4, Cinzia Ortega 5, Lucia Fratino 6, Emilia Cocorocchio 7, Cristian Lolli 8, Laura Lombardo 9, Martina Buffoni 10, Diego Zara 11, Valeria Sardaro 12, Debora D’Ausilio 13, Stefania Di Girolamo 14, Claudia Mosillo 15, Letizia Galeasso 16, Anna Tortorella 17, Carlo Messina 18 and Sara Elena Rebuzzi 19
- 1
Oncology Unit 1, Istituto Oncologico Veneto, IOV—IRCCS, Padua, Italy
- 2
Department of Health Sciences (DISSAL), Section of Biostatistics, University of Genova, Genova, Italy
- 3
Medical Oncology Unit, University Hospital of Parma, Parma, Italy; Department of Medicine and Surgery, University of Parma, Parma, Italy Francesca Vignani, Division of Medical Oncology, Ordine Mauriziano Hospital, Torino, Italy
- 4
Department of Oncology, Hospital of Prato, Azienda USL Toscana Centro, Prato, Italy
- 5
Department of Medical Oncology, Michele e Pietro Ferrero Hospital, Verduno-Azienda Sanitaria Locale CN2, Alba-Bra, Cuneo, Italy
- 6
Department of Medical Oncology, Centro di Riferimento Oncologico di Aviano (CRO), IRCCS, Aviano, Italy
- 7
Division of Medical Oncology, Humanitas Gavazzeni, Bergamo, Italy
- 8
Department of Medical Oncology, IRCCS Istituto Romagnolo per lo Studio dei Tumori Dino Amadori, Meldola, Italy
- 9
Oncology Unit, AUSL Modena, Ramazzini Hospital, Carpi, Italy
- 10
Unit of Medical Oncology, Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, ASST Spedali Civili di Brescia, University of Brescia, Brescia, Italy
- 11
Department of Oncology, Azienda Sanitaria Universitaria Friuli Centrale Santa Maria Della Misericordia, Udine, Italy
- 12
Medical Oncology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
- 13
Department of Urology and Gynecology, Istituto Nazionale Tumori IRCCS Fondazione G. Pascale, Naples, Italy
- 14
Medical Oncology Unit, AUSL-IRCCS of Reggio Emilia, Reggio Emilia, Italy
- 15
Oncologia Medica e Traslazionale, Azienda Ospedaliera Santa Maria di Terni, Terni, Italy
- 16
Department of Oncology, University of Turin, Torino, Italy
- 17
Medical Oncology Unit, IRCCS Policlinico San Matteo, Pavia, Italy
- 18
Ospedale Arnas Civico, Clinical Oncology, Palermo, Italy
- 19
Medical Oncology Unit, Ospedale San Paolo, Savona, Italy
Background: Immune combinations are the standard first-line of mRCC patients but direct comparisons are lacking and few real-world studies are available. Little evidence is gathered on primary refractory patients (disease progression as best response) who have poor prognosis and lower treatment possibilities.
Methods: The Meet-URO 33 is an Italian ambispective study in a first-line setting of mRCC patients (CESC IOV 2023-78, PMID: 38914928) to answer unmet clinical questions. This analysis focused on assessing baseline characteristics and survival/response outcomes of primary refractory mRCC patients to first-line immune combinations.
Results: Among 892 patients from 40 centres, 166 (13%) were primary refractory: 37 (31.9%) received IO-IO, 68 (58.6%) IO-TKI, and 11 (9.5%) TKI. Compared to other patients, primary refractory patients had a worse IMDC score (favorable risk: 7.1% vs. 23.8%, poor-risk: 36.3% vs. 18.6%, and p < 0.001), Meet-URO score (group 1: 7.5% vs. 19.5%, group 5: 12.9% vs. 6.4%, and p < 0.001), and performance status (ECOG PS 2–4: 19.8% vs. 7.9, p < 0.001); higher pre-therapy steroid use (11.2% vs. 6.2%, p = 0.046); lower percentage of nephrectomy (50% vs. 65.3%, p = 0.001), clear-cell histology (75% vs. 83%, p = 0.037), and sarcomatoid features (51.7% vs. 58.3%, p = 0.066); higher percentage of lung (69% vs. 56.1%, p = 0.09), lymph-node (55.2% vs. 43.4%, p = 0.018), and bone metastases (38.8% vs. 27.8%, p = 0.016); and a lower percentage of pancreatic metastases (4.3% vs. 9.3%, p = 0.076). Primary refractory patients received a higher percentage of IO-IO (31.9% vs. 19.3%) and a lower percentage of TKI (9.5% vs. 20.6%) (p < 0.001). After a mFUP of 6.9 months, the mPFS was 2.8 months and mOS 8.2 months, with no differences between IO-TKI vs. IO-IO (HR for PFS: 0.75, p = 0.17; HR for OS: 1.06, p = 0.81).
Conclusions: These preliminary analyses showed distinct baseline characteristics of primary refractory mRCC patients to first-line immune combinations. No survival differences were recorded according to the type of immune combination (IO-TKI vs. IO-IO). Further analyses are planned with a longer follow-up.
4.9. Real-Life, First-Line Treatment with Cabozantinib and Nivolumab in Advanced/Metastatic Renal Cell Carcinoma (aRCC): Interim Analysis of the Non-Interventional CaboCare Study
Philipp Zimmer 1, Hartmut Kirchner 2, Harald Müller-Huesmann 3, Andreas Neisius 4, Jan Janssen 5, Arne Strauß 6, Laura Müller 7, Sara Hamdan 8, Andrea Raspel 8, Sybill Hessler 8, Christian Bach 8 and Heiko Wunderlich 9
- 1
TU Dortmund University, Institute for Sport and Sports Science, Dortmund, Germany
- 2
MVZ Onko Medical GmbH Hannover, Hannover, Germany
- 3
Brüderkrankenhaus St. Josef Paderborn, Department of Hematology and Oncology, Paderborn, Germany
- 4
Krankenhaus der Barmherzigen Brüder Trier, Department of Urology and Pediatric Urology, Trier, Germany
- 5
Medizinische Studiengesellschaft Nord-West GmbH, Westerstede, Germany
- 6
Universitätsmedizin Göttingen, Georg-August-Universität, Department for Urology, Göttingen, Germany
- 7
Universitätsklinikum Marburg, Department of Urology, Marburg, Germany
- 8
Ipsen Pharma GmbH, Munich, Germany
- 9
St Georg Klinikum Eisenach, Department of Urology and Pediatric Urology, Eisenach, Germany
In this prospective, non-interventional study, aRCC patients from Germany and Austria treated with first-line cabozantinib and nivolumab (CaboNivo) or cabozantinib monotherapy were observed in routine clinical practice. The study aims to assess the number of dose reductions, therapy interruptions, and discontinuations due to adverse events (AEs), serious or not. Efficacy outcomes, e.g., objective response rate (ORR) and disease control rate (DCR), were investigated. Physical activity was monitored (Actigraph® GT9X device) at the start of therapy and every three months to explore possible correlations between physical activity levels and outcome parameters. Here we present the first interim analysis results of patients treated with CaboNivo. At the time of interim analysis, the first 61 patients treated with CaboNivo were included, who had either completed the study by attending their last scheduled study visit (~12 months) or discontinued prematurely for any reason. Mean ± standard deviation age of patients was 67.2 ± 11.7 years, with 57.4% being ≥65 years. Male patients comprised 67.2% of the cohort, and 53.6% of patients had an ECOG performance status of zero. Most patients (59.0%) were grouped into the intermediate-risk group. Higher baseline physical activity levels were more frequently reported by patients in the favorable-risk group (83.3%) compared to those in the intermediate-risk (50.0%) and poor-risk (28.6%) groups. Overall, 51.2% of patients reported physical activity levels above this threshold. The median daily cabozantinib dose was 29.9 mg, with dose reductions due to AEs occurring in 37.7% of patients. The DCR was 83.7%, and the ORR was 59.2%. Treatment-emergent AEs were reported by 96.7% of patients.
These interim data on real-world, first-line CaboNivo treatment are broadly consistent with phase 3 trial findings, further supporting its efficacy and safety in the management of aRCC.
4.10. Integration of First-Line Nivolumab–Cabozantinib Combination with Radiotherapy: Achieving Complete Response in Metastatic Fumarate Hydratase-Deficient Renal-Cell Carcinoma, a Case Series
Chiara Ligato 1, Valeria Sardaro 1, Stefano Lepori 2, Luca Tagliaferri 3, Maria Antonietta Gambacorta 3, Pierpaolo Dragonetti 3, Iolanda Bisogno 4, Ylenia Antonicelli 4, Susanna Yedro 4, Chiara Codella 4, Daniela Arduini 1, Fortuna Migliaccio 1, Denis Occhipinti 1, Romina Rose Pedone 1, Gloria Messina 1, Luigi Roca 1, Rachele Belletto 1, Davide Di Leo 1, Alessio Neri 1, Chiara Ciccarese 1 and Roberto Iacovelli 1
- 1
Oncology Unit, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Rome, Italy
- 2
Ospedale Armando Businco ARNAS Brotzu, Cagliari, Italy
- 3
Dipartimento di Diagnostica per Immagini e Radioterapia Oncologica, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Rome, Italy, Università Cattolica del Sacro Cuore, 00168, Rome, Italy
- 4
UOC Oncologia Medica, Unità Coordinamento Trials, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Rome, Italy
Introduction: Fumarate hydratase-deficient renal-cell carcinoma (FH-d RCC) is an aggressive disease characterized by poor prognosis and often presenting as metastatic with an earlier age of onset than clear-cell RCC (ccRCC). To date, there is no clear evidence as to what the best therapeutic algorithm is, borrowed from ccRCC.
Case Presentations: In June 2023, a 33-year-old woman affected by Hereditary Leiomyomatosis and renal-cell cancer (HLRCC) started first-line therapy with nivolumab and cabozantinib for mediastinal and abdominal lymph node recurrence (short axis of 29, 21, and 21 mm) of a previously radically resected FH-d RCC. After one year of treatment, almost all the lymphadenopathies achieved a radiologic partial response, which was then consolidated with radiotherapy. Four months later, the most recent CT scan showed complete response, and the treatment is still ongoing.
A second case of FHd-RCC in a young male patient came to the attention of our department in July 2023. Following radical nephrectomy, a post-operative CT scan showed the presence of two pathological abdominal lymph nodes (short axis of 11 mm). Hence, the patient started first-line nivolumab–cabozantinib. Radiotherapy was performed as a consolidative treatment six months after the start of systemic therapy, with a CT scan showing a radiologically complete response. Fourteen months from the start of treatment, the patient still has no evidence of disease and continues nivolumab–cabozantinib therapy.
Conclusion: These cases provide the opportunity to appreciate the outstanding efficacy of an integrated multimodal therapeutic approach that combines systemic therapy with Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitor and Immune Checkpoint Inhibitor combinations associated with radiotherapy for the management of metastatic FH-d RCC. Enrollment of patients with rare RCC histologies in clinical trials is widely advocated to improve evidence in daily clinical practice.
4.11. Genomic Profiling of Non-Clear Cell Renal Cell Carcinoma: Insights for Prognosis and Treatment
Mimma Rizzo 1, Gaetano Pezzicoli 2, Camilla Porta 3, Massimiliano Povero 3, Lorenzo Pradelli 3, Emilia Sicari 4, Valentina Barbiero 4 and Camillo Porta 2
- 1
Medical Oncology Unit, Azienda Ospedaliera Universitaria Consorziale Policlinico di Bari, Bari, Italy
- 2
Interdisciplinary Department of Medicine, University of Bari ‘Aldo Moro’, Bari, Italy
- 3
AdRes Health Economics and Outcome Research, Turin, Italy
- 4
Roche SpA, Monza, Italy
Background: Non-clear cell renal cell carcinoma (nccRCC) is an uncommon and heterogeneous entity characterised by distinctive histological, genetic, and molecular features. This results in nccRCCs exclusion from randomised clinical trials and the adoption of the same therapeutic strategies used for ccRCC in clinical practice with unfavourable clinical outcomes.
Methods: We analysed real-world data from the nationwide deidentified Flatiron Health-Foundation Medicine nccRCC clinico-genomic database, which integrates clinical and genomic data from approximately 280 US cancer clinics. The genomic profiles of 300 patients with metastatic nccRCC were evaluated, and the prognostic value (overall survival, OS) of the most frequent genomic alterations (GA) was assessed using the Kaplan–Meier method and Cox proportional hazards model.
Results: The most prevalent nccRCC subtypes were papillary RCC (pRCC, 74.3%) and chromophobe RCC (chRCC, 16.0%), followed by translocation RCC (tRCC, 4.0%), collecting duct RCC (cdRCC, 4.0%), and renal medullary RCC (RMC, 1.7%). In pRCC, the most common GAs were CDKN2A (30%), CDKN2B (25.1%), TERT (20.2%), and NF2 (15.7%). Among the chromatin-modifying genes, SETD2, BAP1, and PBRM1 mutations were observed in 14.3%, 9.0%, and 5.8%, respectively. In chRCC, the most prevalent GAs were TP53 (68.8%), PTEN (33.3%), and RB1 (18.8%), and other GAs, including CDKN2C, MYC, TERT, VHL, ATRX, CHEK2, and TSC1, were identified in less than 10% of chRCC patients. Survival analyses revealed three genes with prognostic value in pRCC cases: CDKN2A/B GAs (HR 2.59 and 2.45, respectively; p < 0.001) and TERT GAs (HR 1.66, p = 0.022) were significantly associated with poor prognosis. MET and PBRM1 GAs showed a trend towards better OS, while TP53 appeared to portend a poor prognosis.
Conclusions: This real-world analysis provides insights into the genomic landscape of nccRCC. The distinctive mutational assets identified in this study warrant further investigation to support the tailoring of treatment strategies and improve outcomes for nccRCC patients.
4.12. Relevance of IMDC Risk Model in Real-World Setting: Single Institution Experience
Salil Vengalil 1, Kyaw Kyaw Tun 1, Kanchana Wickramasinghe 1, Hasanthi Nillegoda 1, Sumera Butt 1, Jananie Perera 1, Rakhul Raveendran 1, Herman Fernando 1 and Anurag Golash 1
- 1
University Hospital North Midlands NHS Trust
Background: Systemic therapy landscape for metastatic renal cell carcinoma (mRCC) has evolved rapidly over the recent years, and the use of combination treatment regimens has shown improved outcomes compared to VEGF TKIs. IMDC has been validated and widely used as a prognostic tool for patients with mRCC treated with VEGF TKI and immunotherapy (IO). The aim of this study was to assess outcomes of patients treated with IO-based combination treatments and VEGF TKI in relation to IMDC prognostic groups and the impact of previous nephrectomy (Cyto-reductive or radical) in a real-world setting.
Methods: This single centre retrospective study included 112 patients with mRCC receiving first-line systemic therapy as of January 2018 to March 2022. PFS and OS were analysed using descriptive statistics and Kaplan–Meier curves.
Results: The median age was 66 years (35–85). More than half of the patients were in the IMDC intermediate-risk group (54.5%). Combination treatments were used in about 30%, and 42% received only one line of therapy.
There was a statistically significant difference in the median PFS and OS based on IMDC risk groups [median PFS 31.5, 21, and 8.7 months and median OS 87, 36, and 9 months, respectively, in favourable, intermediate, and poor groups; (p < 0.0001)].
Previous nephrectomy was performed in 48.2% of patients. Median PFS and OS were significantly longer in the nephrectomy group. [PFS was 29Vs 10.9 months and OS was 55 vs. 21 months; (p < 0.0001)]
Discussion: Our study included more patients treated with VEGF TKI monotherapy, as it included patients treated in the pre-combination therapy era. Similarly to other reports, our study also indicates a higher proportion of dropouts between lines of therapy.
The results of our retrospective study highlight that IMDC risk groups can be readily used in clinics and remain relevant to predict the prognosis in a real-world setting. Our study also suggests that patients who had a previous nephrectomy had a possible overall survival and progression-free survival advantage.
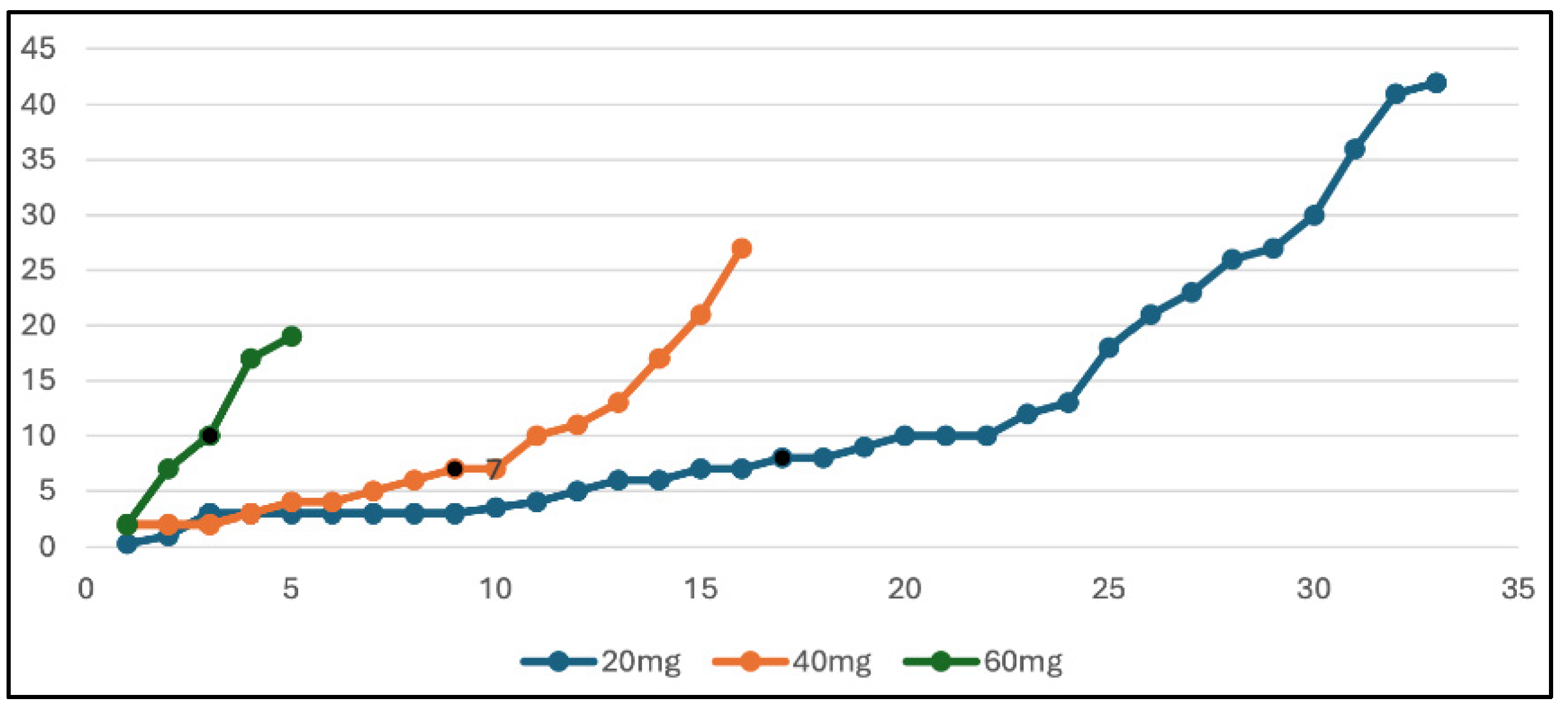
4.13. Real-World Usage of Cabozantinib at University Hospitals Plymouth NHS Trust Between 2020 and 2024: Does Dose Reduction Affect Progression-Free Survival (PFS)?
Dominique Parslow 1
- 1
University Hospitals Plymouth NHS Trust
A total of 54 patients (15 women, 39 men), ranging in age from 42 to 83 (median age = 64), were treated with single-agent Cabozantinib between 2020 and 2024 at University Hospitals Plymouth NHS Trust. The majority of these were treated in the second-line setting. Approximately 11 (20%) were favourable risk, 18 (33%) were intermediate risk, and 25 (46%) were poor risk (which was a much more unfavourable patient group than in the 2015 METEOR trial, where 43% were favourable risk, 43% intermediate risk, and 14% poor risk). Only 5 out of 54 (9%) patients completed their Cabozantinib course at 60 mg. The majority were eventually dose reduced to 20 mg (33/54 patients) due to side effects, and 16/54 ended up taking 40 mg daily. Dose reductions usually occurred early, most frequently for cycle 2 or 3. In the majority of cases (32/55), Cabozantinib was being utilised in the second-line setting, most commonly after progression of the first-line immunotherapy. The median PFS of our patients was 7 months. The median PFS is reflective of the 7.4-month median PFS seen in the METEOR trial. The METEOR trial showed an ORR of 21%, which is lower than the ORR seen in our real-world patients, which is 43%. From our data, the dose of Cabozantinib made no difference to the median overall survival, as shown in the graph below (
Figure 4); in fact, most of our long-term responders are taking only 20 mg. This data shows that we are achieving similar PFS results and improved ORR compared to the trial despite having a much older patient population with poorer risk disease. These impressive results are almost certainly achieved with lower levels of treatment toxicity given timely and aggressive dose reductions.
4.14. Trends in Kidney Cancer: Exploring the Impact of Sex and Age on Stage of Disease and Prognosis During the Past Three Decades in Denmark—A DaRenCa Study
Johanne Ahrenfeldt 1, Jesper Jespersen 1, Jens Ejrnæs Tønder 2, Laura Iisager 1, Anna Krarup Keller 3, Niels Fristrup 4, Tinne Laurberg 2 and Iben Lyskjær 5
- 1
Department of Molecular Medicine, Aarhus University Hospital, Aarhus, Denmark
- 2
Steno Diabetes Centre Aarhus, Aarhus University Hospital, Aarhus, Denmark
- 3
Department of Clinical Medicine, Aarhus University, Aarhus, Denmark and Department of Urology, Aarhus University Hospital, Aarhus, Denmark
- 4
Department of Clinical Medicine, Aarhus University, Aarhus, Denmark and Department of Oncology, Aarhus University Hospital, Aarhus, Denmark
- 5
Department of Molecular Medicine, Aarhus University Hospital, Aarhus, Denmark and Department of Clinical Medicine, Aarhus University, Aarhus, Denmark
Background and Objective: Renal cell carcinoma (RCC) management has advanced due to increased imaging-based diagnoses and improved therapies for metastatic disease. This nationwide registry-based cohort study investigates how the number of RCC cases, stage at diagnosis, and prognosis have changed during the past 30 years in Denmark and how these are associated with sex and age.
Methods: All Danish patients aged 18 and older diagnosed with RCC from 1992 to 2021 and without prior cancer history, except for non-melanoma skin cancer, were included and followed from diagnosis until death or the end of follow-up (31 December 2023).
Results: 17,423 RCC patients were identified. RCC cases increased from 2244 in 1992–1996 to 3947 in 2017–2021. The proportion of male patients increased from 59% in 1992–1996 to 72% in 2017–2021 (p < 0.001), and male patients were younger at diagnosis than female patients (median age 65 vs. 69 years, p < 0.001). Localized cancer cases increased from 51% (N = 983) in 1992–1996 to 79% (N = 2766) in 2017–2021, while metastatic cases declined from 34% (N = 640) to 19% (N = 652). The median survival for metastatic RCC improved from 4.1 months in 1992–1996 to 13.3 months in 2017–2021.
Conclusions: During the past 30 years, the number of RCC cases in Denmark has increased, particularly in male patients, primarily driven by localized tumors, providing additional pressure for urological departments. The number of metastatic cases has remained stable while survival has increased, highlighting the impact of early detection and treatment advancements over time.
4.15. Comparison of IO-TKI vs. IO-IO Combinations in IMDC Poor-Risk Metastatic Renal Cell Carcinoma (mRCC) Patients (Meet-URO 33 Analysis)
Sara Elena Rebuzzi 1, Sara Elena Rebuzzi 1, Alessio Signori 2, Sebastiano Buti 3, Alberto Dalla Volta 4, Martina Fanelli 5, Valeria Sardaro 6, Marilena Di Napoli 7, Cristina Masini 8, Annalisa Guida 9, Roberto Filippi 10, Silvia Chiellino 11, Carlo Messina 12, Emanuela Fantinel 13, Lucia Bonomi 14, Vincenza Conteduca 15, Filippo Maria Deppieri 16, Mariella Sorarù 17, Silvia Puglisi 18 and Davide Bimbatti 19
- 1
Medical Oncology Unit, Ospedale San Paolo, Savona, Italy
- 2
Department of Health Sciences (DISSAL), Section of Biostatistics, University of Genova, Genova, Italy
- 3
Medical Oncology Unit, University Hospital of Parma, Parma, Italy; Department of Medicine and Surgery, University of Parma, Parma, Italy
- 4
Unit of Medical Oncology, Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, ASST Spedali Civili di Brescia, University of Brescia, Brescia, Italy
- 5
Department of Oncology, Azienda Sanitaria Universitaria Friuli Centrale Santa Maria Della Misericordia, Udine, Italy
- 6
Medical Oncology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
- 7
Department of Urology and Gynecology, Istituto Nazionale Tumori IRCCS Fondazione G. Pascale, Naples, Italy
- 8
Medical Oncology Unit, AUSL-IRCCS of Reggio Emilia, Reggio Emilia, Italy
- 9
Oncologia Medica e Traslazionale, Azienda Ospedaliera Santa Maria di Terni, Terni, Italy
- 10
Department of Oncology, University of Turin, Torino, Italy
- 11
Medical Oncology Unit, IRCCS Policlinico San Matteo, Pavia, Italy
- 12
Ospedale Arnas Civico, Clinical Oncology, Palermo, Italy Sarah Scagliarini, Department of Medical Oncology, AORN “A. Cardarelli”, Naples, Italy
- 13
Section of Oncology, Department of Medicine, University of Verona, Azienda Ospedaliera Universitaria Integrata, Verona, Italy
- 14
Unit of Oncology, ASST Papa Giovanni XXIII Hospital, Bergamo, Italy
- 15
Medical Oncology and Biomolecular Therapy Unit, Department of Medical and Surgical Sciences, University of Foggia, Foggia, Italy
- 16
Medical Oncology Unit, AULSS 3 Serenissima, Mestre-Venice, Italy
- 17
UOC Oncologia, AULSS 6 Euganea, Ospedale di Camposampiero, Padua, Italy
- 18
Medical Oncology Unit 1, IRCCS Ospedale Policlinico San Martino, Genova, Italy
- 19
Oncology Unit 1, Istituto Oncologico Veneto, IOV—IRCCS, Padua, Italy
Background: Immune combinations are the first-line treatment cornerstone of mRCC patients, but head-to-head comparisons are lacking and few real-world data are available. In this context, little evidence is available for IMDC poor-risk patients, who have the worst prognosis and lowest response to standard treatments.
Methods: The Meet-URO 33 study is an Italian ambispective registry in the first-line mRCC setting (CESC-IOV-2023-78, PMID: 38914928) to answer as many clinical questions as possible. This analysis focused on the different baseline characteristics, responses, and survival performances of patients receiving IO-TKI and IO-IO combinations.
Results: 892 patients were enrolled at 40 centres; 772 (87%) were evaluable for survival analyses and 160 (21%) had IMDC poor risk: 42 (26%) received IO-IO, 105 (66%) IO-TKI, and 13 (8%) TKI. Poor-risk patients receiving IO-IO were older (mean age: 68 vs. 64, p = 0.03), had more cardiovascular comorbidities (74% vs. 56%, p = 0.048), and lower frequency of bone metastases (29% vs. 57%, p = 0.002) compared to IO-TKI-treated patients. After a mFU of 6.9 months, the overall mOS was 11.3 months, which was higher with IO-IO than with IO-TKI [20.6 vs. 11 months, HR 1.65 (0.97–2.82); p = 0.067]. The overall mPFS was 6.4 months, which was higher with IO-IO than IO-TKI [11 vs. 5.8 months, HR 1.70 (1.05–2.75); p = 0.031]. After multivariable analysis, the difference between IO-IO and IO-TKI was not statistically significant for both OS [HR 1.37 (0.78–2.40); p = 0.27] and PFS [HR 1.58 (0.95–2.63); p = 0.078]. The general ORR was 46%, which was higher with IO-TKI than with IO-IO [71% vs. 29%, OR 0.70 (0.21–1.56); p = 0.38].
Conclusions: These first analyses show no clear survival advantage in choosing an IO-TKI combination instead of an IO-IO combination in IMDC poor-risk patients. These results are in line with the well-known small benefit of TKI in poor-risk patients (more immunogenic/less angiogenic; COSMIC-313 trial results). Future analyses with longer follow-up are planned.
10. Therapeutics
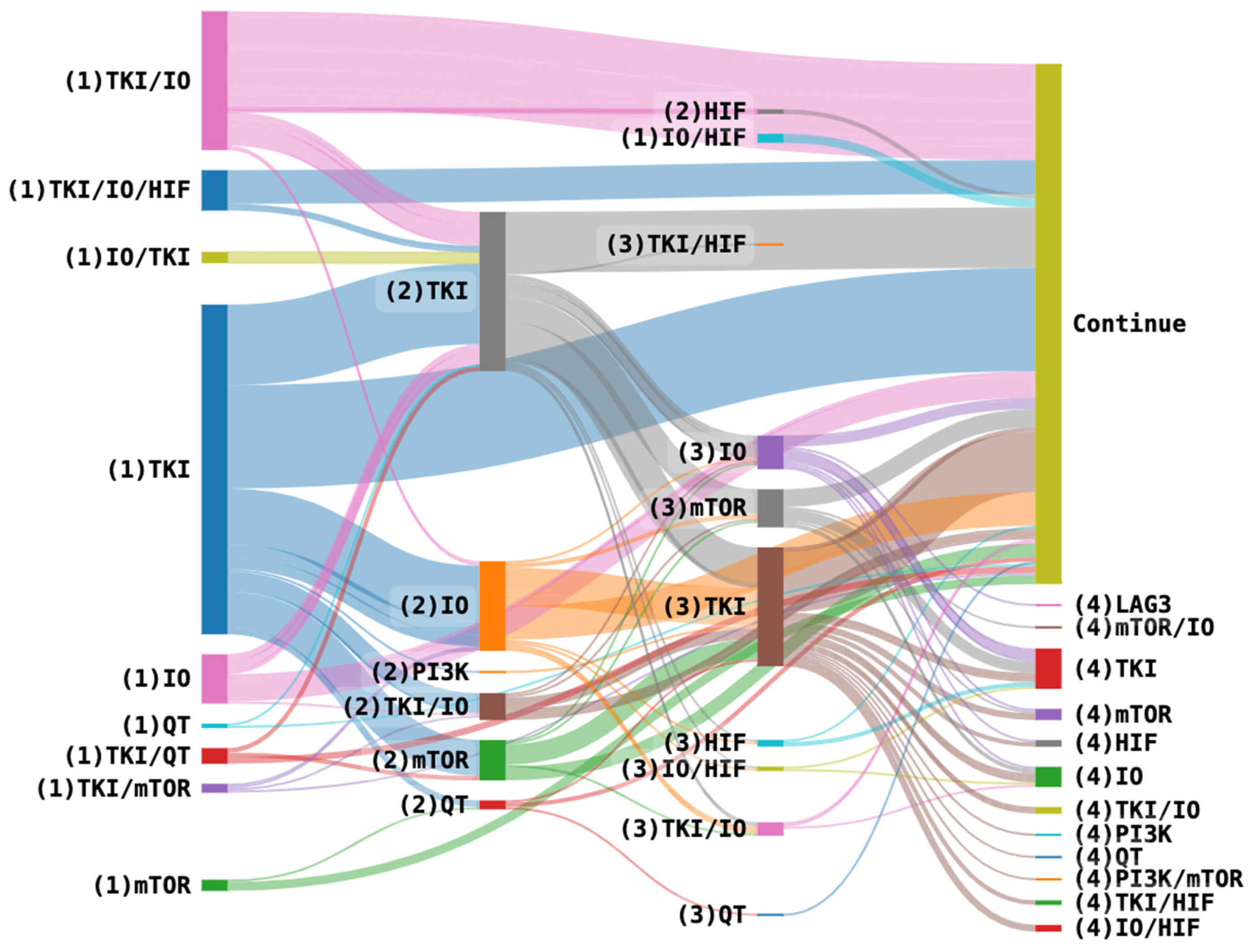
10.1. Management Sequences and Clinical Outcomes in Advanced Clear Cell Renal Cell Carcinoma (ccRCC): A Real-World Analysis
Josette Staufert Gutiérrez 1, Laia Catalán 1, David Marmolejo Castañeda 1, Nely Díaz Mejia 1, Macarena González Rodríguez 1, Rafael Morales Barrera 1, Joan Carles 1 and Cristina Suárez 1
- 1
Vall d’Hebron University Hospital, Barcelona, Spain
Background: Over the years, we have witnessed the advancement of treatments for patients with ccRCC. However, uncertainties remain regarding the optimal treatment sequence to follow in this context
Methods: A retrospective analysis of ccRCC patients treated at Vall d’Hebron Hospital from 2004 to 2024 assessed their clinical characteristics and outcomes. Descriptive and univariate analyses were performed, and PFS2 was estimated using the Kaplan–Meier method and compared with the log-rank test.
Results: 281 patients with advanced ccRCC were included (
Figure 7). The mean age was 54 years (23–81). A total of 63% were metastatic at diagnosis or had less than one year between diagnosis and the initiation of systemic therapy. First-line treatment primarily consisted of tyrosine kinase inhibitor (TKI) in 53%, TKI/PD1i in 6.1%, PD1 inhibitor (PD1i)/CTL4 inhibitor (CTL4i) in 5.4%, TKI/PD1/HIF inhibitor (HIFi) in 6.5%, TKI/PD1i/LAG3 inhibitor in 5.7%, and TKI/PD1i/CTL4i in 4.3%. Second-line treatment was administered to 141 patients (50%), third-line to 100 patients (35%), and fourth-line to 52 patients (18%). The most common treatment sequences were TKI followed by another TKI (26%), TKI followed by immunotherapy (IO) (24%), and TKI/IO followed by TKI (12%).
The median PFS2 for initiating treatment with an IO and switching to TKI upon progression was 23.5 months [15-NA, 95% Confidence Interval (CI)]. When compared to other approaches, the only sequence that showed a significant difference was TKI/IO followed by TKI (PFS2 40.5 months [26–61, 95% CI], HR = 0.45 [0.21–0.99, p = 0.046]). Among other combinations, no significant differences were observed; however, a trend toward improved PFS2 was noted in certain sequences, including TKI/IO followed by HIFi (66 months; p = 0.09), TKI followed by an mTOR inhibitor (29 months; p = 0.34), TKI followed by TKI/IO (28 months; p = 0.19), and TKI followed by IO (26 months; p = 0.09).
Conclusions: Despite TKIs being the most commonly used drugs since their approval in 2006, new combinations with checkpoint inhibitors have led to improved survival outcomes. Furthermore, we observed a trend toward improved PFS2 in patients who initially received combinations. Further analysis is required.
10.2. A Phase II Study of Nivolumab Combined with Metformin in Pre-Treated Metastatic Renal Cell Carcinoma (mRCC) Patients:NivoMet TWINS-GU002 Study
Luigi Roca 1, Fortuna Migliaccio 2, Daniela Arduini 3, Denis Occhipinti 2, Romina Rose Pedone 2, Gloria Messina 2, Valeria Sardaro 2, Chiara Ligato 2, Rachele Belletto 2, Davide Di Leo 2, Alessio Neri 2, Alessandro Strusi 2, Ileana Sparagna 2, Iolanda Bisogno 2, Ylenia Antonicelli 2, Susanna Yedro 4, Giampaolo Tortora 2, Roberto Iacovelli 2 and Chiara Ciccarese 2
- 1
Department of Medical Oncology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy. Francesca Primi, Department of Medical Oncology, Belcolle Hospital of Viterbo, Viterbo, Italy
- 2
Department of Medical Oncology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
- 3
Department of Medical Oncology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
- 4
Department of Medical Oncology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy. Giovanni Schinzari, Department of Medical Oncology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
Background: Recent evidence suggests a potential synergistic antitumor effect of the combination of immune checkpoint inhibitors targeting PD-1/PD-L1 with the oral hypoglycemic agent metformin. We designed a prospective study to evaluate the activity of nivolumab plus metformin in pre-treated mRCC patients.
Materials and Methods: The NivoMet TWINS-GU002 Study was a prospective, multicentre, single-arm, phase II trial involving pre-treated mRCC patients eligible for nivolumab without a diagnosis of diabetes mellitus. Patients received nivolumab (flat dose of 240 mg every 2 weeks) plus metformin (500 mg twice daily) until disease progression. The primary endpoint was a 9-month progression-free survival (PFS) rate. The study aimed to identify a 25% increase (from 30% to 55%), requiring 21 patients to show this difference. Secondary endpoints included median PFS, overall survival (OS), overall response rate (ORR), and safety.
Results: A total of 12 patients from two sites in Italy were enrolled between November 2020 and April 2023. The trial was terminated early due to slow accrual. The median PFS was 2.7 months (95% confidence interval [CI], 2.6–3.2 months). The 9-month PFS rate was 8%. The median OS was 14.9 months (95% CI, 2.5–27.4 months). The ORR in the overall population was 8%, and the disease control rate was 25%. Adverse events (AEs) of any grade occurred in nine patients (75%), with grade 3 or 4 occurring in only one patient (grade 3 fatigue), which was determined to be unrelated to treatment. No treatment-related deaths were reported.
Conclusions: The concomitant use of nivolumab and metformin showed marginal activity. Nevertheless, recent genomic and transcriptomic evidence confirms the key role of metabolic pathways in RCC pathogenesis, suggesting the need for further investigations to identify new therapeutic targets.
10.3. PErioperative PEmbrolizumab in Patients with Resectable Metastases from Kidney Cancer: The PE-PE Study
Fortuna Migliaccio 1, Roberto Iacovelli 2, Emanuela Fantinel 3, Davide Bimbatti 4, Sebastiano Buti 5, Lucia Bonomi 6, Mimma Rizzo 7, Caterina Accettura 8, Consuelo Buttigliero 9, Matteo Santoni 10, Paolo Andrea Zucali 11, Elisa Zanardi 12, Giuseppe Procopio 13, Sergio Bracarda 14, Lorenzo Antonuzzo 15 and Chiara Ciccarese 2
- 1
Department of Clinical Medicine and Surgery, University of Naples “Federico II”, 80138 Naples, Italy
- 2
Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
- 3
Oncology Unit, Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy
- 4
Oncology Unit 1, Istituto Oncologico Veneto, IOV—IRCCS, Padua, Italy
- 5
University Hospital of Parma, Parma, Italy
- 6
ASST Papa Giovanni XXIII, Bergamo, Italy
- 7
Division of Medical Oncology, A.O.U Consorziale Policlinico di Bari, Bari, Italy
- 8
Vito Fazzi Hospital Lecce, Lecce, Italy
- 9
Medical Oncology, San Luigi Gonzaga Hospital, Orbassano, Italy Francesco Massari, Department of Experimental, Diagnostic and Specialty Medicine, S. Orsola-Malpighi University Hospital, University of Bologna, Bologna, Italy
- 10
Medical Oncology Unit, Macerata General Hospital, Macerata, Italy
- 11
Department of Biomedical Science, Humanitas University, Pieve Emanuele, Milan, Italy
- 12
UO Clinica di Oncologia Medica, IRCCS Ospedale Policlinico San Martino, Genova, Italy Alessandra Mosca, Oncology, Candiolo Cancer Institute FPO-IRCCS, Candiolo, Torino, Italy Francesca Primi, Ospedale Belcolle-Viterbo, Viterbo, Italy Francesco Spina, Department of Hematology & Oncology, Niguarda Cancer Center, Ospedale Niguarda Ca’ Granda, Milan, Italy
- 13
Genitourinary Medical Oncology, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy, Milano, Italy
- 14
Medical and Translational Oncology Unit, Department of Oncology, Azienda Ospedaliera Santa Maria, Terni, Italy Fabio Calabrò, IFO Istituto Regina Elena, Rome, Italy
- 15
Oncology Unit, Azienda Ospedaliera Careggi, Florence, Italy
Radical metastasectomy and other local treatment strategies (including definitive radiotherapy, RT) can be employed as alternatives to systemic therapies for selected metastatic renal cell carcinoma (mRCC) patients (pts) with the aim of achieving patient healing, even in the advanced stage. Recently, adjuvant pembrolizumab has shown significant efficacy in the M1 NED pts. Furthermore, the combination of RT with a short course of pembrolizumab has demonstrated activity in oligometastatic RCC pts. However, no randomised trial has investigated the concomitant use of pembrolizumab with radical metastasis directed therapy (MDT; metastasectomy or RT) in mRCC compared to local therapy alone.
The PE-PE study (NCT05578664) is a randomised, open-label, multicentre, phase 2 study evaluating the efficacy of pembrolizumab in delaying tumour progression in oligometastatic RCC patients who have undergone previous nephrectomy and have a maximum of three metastases in the same or in different sites and are considered eligible for MDT. The study will include patients who have had recurrence of disease within 5 years from prior nephrectomy or metastasectomy. Participants will be randomly assigned to receive pembrolizumab at a flat dose of 400 mg every six weeks for nine cycles, followed by MDT from day 21 to day 42 of cycle 1 (Arm A) or MDT alone within 42 days of randomisation (Arm B).
The primary endpoint is relapse-free survival (RFS), defined as the time from randomisation to the appearance of radiological progression of kidney cancer in patients who received pembrolizumab or not. Secondary endpoints include the following: distant relapse-free survival (dRFS), defined as the time from randomisation to the appearance of distant metastases outside of those already treated; overall survival (OS), defined as the time from randomisation to the patient’s death or last contact; and the overall incidence of adverse events and incidence of local progression in both arms.
The study is actively enrolling until today (February 2025) with 49 pts (60.5%).
10.4. The AxIn Study: Phase II Study of Axitinib Intensification Compared to Nivolumab Alone After Induction with Ipilimumab Plus Nivolumab in Patients with mRCC Without Previous Complete Response
Gloria Messina 1, Chiara Ciccarese 1, Elena Verzoni 2, Davide Bimbatti 3, Sebastiano Buti 4, Luca Galli 5, Giuseppe Fornarini 6, Cinzia Baldessari 7, Gaetano Facchimi 8, Cristina Masini 9, Maria Olga Giganti 10, Franco Nolè 11, Alfredo Berruti 12, Michele Milella 13, Lorenzo Antonuzzo 14 and Roberto Iacovelli 1
- 1
Oncologia Medica, Fondazione Policlinico Universitario Agostino Gemelli Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Roma, Italy
- 2
Genitourinary Medical Oncology, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
- 3
Oncology Unit 1, Istituto Oncologico Veneto, IOV-IRCCS, Padua, Italy
- 4
University Hospital of Parma, Parma, Italy Fabio Calabrò, IFO Istituto Regina Elena, Rome, Italy
- 5
UO Oncologia Medica 2 Universitaria, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy Sarah Scagliarini, Departement of Oncology, Cardarelli Hospital, Napoli, Italy
- 6
IRCCS Ospedale Policlinico San Matteo, Genoa, Italy
- 7
Oncology Unit, Azienda Ospedaliero Universitaria Policlinico di Modena, Modean, Italy Domenico Azienda Ospedaliera S. Carlo, Potenza, Italy, Bilancia
- 8
Medical Oncology unit, SM delle Grazie Hospital, Pozzuoli, Italy
- 9
AUSL-IRCCS di Reggio Emilia, Reggio Emilia, Italy
- 10
Ospedale di Cremona, Cremona, Italy Giuseppa Scandurra, Humanitas Centro Catanese di Oncologia, Catania, Italy
- 11
IRCCS Istituto Europeo di Oncologia, Milano, Italy
- 12
Medical Oncology Unit, Department of Medical and Surgical Specialities, Radiological Sciences and Public Health University of Brescia, ASST-Spedali Civili, Brescia, Italy
- 13
Oncology Unit, Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy
- 14
Oncology Unit, Azienda Ospedaliera Careggi, Florence, Italy
Background: The CheckMate214 study demonstrated that nivolumab plus ipilimumab led to a significant increase in overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) compared to sunitinib (SUN) in patients with metastatic renal cell carcinoma (mRCC) with intermediate or poor prognosis. Recently, a higher rate of partial responses was obtained with the combination Pembrolizumab-Axitinib (45.9%) compared with that reported with N + I (30.6%). Moreover, analysis of outcomes based on the depth of response strongly revealed a connection between response and survival. The aim of this study is to investigate the efficacy of an intensified strategy with axitinib added to nivolumab in patients with mRCC without a complete response at the end of N + I induction.
Methods: The AxIn study (NCT05817903) is a randomized, open-label, multicenter, phase 2 trial evaluating whether the intensification of therapy by adding axitinib to the standard nivolumab can increase the response rate and improve survival compared with nivolumab alone in 118 mRCC pts ≥ 18 years old, with an ECOG between 0 and 1, and who completed induction with N + I without a complete response or progressive disease and without any toxicity ≥ G2. Eligible patients will be randomized 1:1 to the intensification of therapy with axitinib in addition to nivolumab (Arm A) or nivolumab alone (Arm B). The primary endpoint is to assess the ORR of patients treated with the intensification of axitinib plus nivolumab compared to the standard of care of nivolumab monotherapy. Secondary endpoints include PFS, OS, depth of response, duration of response, quality of life, and safety. Exploratory biomarkers analysis will be performed. The trial is actively enrolling to date (31 patients as of February 2025).
10.5. Optimizing Sunitinib Treatment in Metastatic Renal Cell Carcinoma: Integrating Therapeutic Drug Monitoring and Pharmacogenetics into Clinical Practice
Giorgia Bortolus 1, Erika Cecchin 2, Sara Gagno 2, Bianca Posocco 2, Arianna Dri 1, Sandra Santarossa 3, Giuseppe Toffoli 2, Fabio Puglisi 1, Michele Spina 3 and Lucia Fratino 3
- 1
Department of Medicine (DMED), University of Udine, 33100 Udine, Italy
- 2
Experimental and Clinical Pharmacology Unit, Centro di Riferimento Oncologico di Aviano (CRO), IRCCS, 33081 Aviano, Italy
- 3
Unit of Medical Oncology and immune-related tumors, Department of Medical Oncology, Centro di Riferimento Oncologico di Aviano (CRO), IRCCS, 33081 Aviano, Italy
Background: Sunitinib (SUN) is a key treatment for metastatic renal cell carcinoma (mRCC), yet its fixed dosing results in substantial interpatient variability in drug exposure and toxicity. Integrating Therapeutic Drug Monitoring (TDM), pharmacogenetic (PGx) profiling, and drug–drug interaction (DDI) assessments may enhance treatment personalization. This study evaluated the feasibility and clinical impact of pharmacological counseling in optimizing SUN therapy.
Methods: Patients enrolled in the CRO-2022-14 trial provided steady-state plasma samples for the LC-MS/MS quantification of SUN levels (Cmin). Therapeutic target ranges were defined as 37.5–60/75 ng/mL (continuous dosing) and 50–80/87.5 ng/mL (intermittent dosing). Genetic polymorphisms affecting SUN metabolism and transport were analyzed, while potential DDIs were screened via UpToDate Lexi-Drug. Integrated pharmacological reports were provided to oncologists for treatment adjustments.
Results: Among the 11 patients (median treatment duration: 34 months), 10 were on reduced doses due to toxicity. Seven patients on a dose of 50 or 37.5 mg/day achieved therapeutic Cmin (53–70 ng/mL). Two female patients exhibited Cmin > 80 ng/mL despite dose reductions (25 mg/day), experiencing recurrent Grade 2/3 toxicities, suggesting further dose refinement. Conversely, two male patients on 25 mg/day had Cmin < 40 ng/mL with none/mild toxicity, indicating a need for dose escalation. Females (n = 2) exhibited higher SUN exposure (82 ng/mL) than males (n = 8, 55 ng/mL). PGx analysis was limited by sample size, and no significant DDIs were detected.
Conclusions: Toxicity-driven dose adjustments generally led to optimal SUN exposure. Females showed higher plasma levels, though the sample size limits conclusions. Early TDM integration could refine dose individualization, enhancing tolerability, adherence, and therapeutic efficacy. Pharmacological counseling was feasible and clinically valuable, supporting its implementation in routine mRCC management.
10.6. Newly Updated Data from the Tide-A Trial on Avelumab (Ave) Plus Intermittent Axitinib (Axi) in Previously Untreated Patients with Metastatic Renal Cell Carcinoma (mRCC)
Daniela Arduini 1, Chiara Ciccarese 1, Alessandro Acunzo 2, Matteo Perrino 3, Marco Maruzzo 4, Elena Verzoni 5, Caterina Accettura 6, Lucia Bonomi 7, Consuelo Buttigliero 8, Malvina Cremante 9, Stefania Pipitone 10, Cristina Masini 11, Veronica Mollica 12, Alessandro Strusi 1, Sebastiano Buti 13, Paolo Andrea Zucali 3, Emanuela Fantinel 14 and Roberto Iacovelli 1
- 1
Fondazione Policlinico Universitario A. Gemelli IRCCS, Dept. of Medical Oncology, Rome, Italy
- 2
University Hospital of Parma, Dept of. Medical Oncology, Italy
- 3
Humanitas Research Hospital, Dept. of Medical Oncology, Rozzano, Italy
- 4
Istituto Oncologico Veneto, Dept. of Medical Oncology, Padua, Italy
- 5
Fondazione IRCCS Istituto Nazionale dei Tumori, Dept. of Medical Oncology, Milano, Italy
- 6
Vito Fazzi Hospital, Dept. of Medical Oncology, Lecce, Italy
- 7
ASST Papa Giovanni XXIII Hospital, Dept. of Medical Oncology, Bergamo, Italy
- 8
San Luigi Gonzaga Hospital, Dept. of Medical Oncology, Orbassano, Italy
- 9
IRCCS Ospedale Policlinico San Martino, Dept. of Medical Oncology, Genova, Italy
- 10
University Hospital of Modena, Dept. of Oncology and Hemathology, Modena, Italy Mario Scartozzi, University Hospital of Cagliari, Dept. of Medical Oncology, Cagliari, Italy
- 11
Azienda USL-IRCCS di Reggio Emilia, Dept. of Medical Oncology, Reggio Emilia, Italy
- 12
IRCCS Azienda Ospedaliero-Universitaria di Bologna, Dept. of Medical Oncology, Bologna, Italy Francesca Primi, Central Hospital of Belcolle, Dept. of Medical Oncology, Viterbo, Italy
- 13
University Hospital of Parma, Dept of. Medical Oncology, Parma, Italy
- 14
University of Verona, Dept. of Medical Oncology, Verona, Italy
The combination of VEGFR-TKI (TKI) and anti-PD1/PDL1 immunotherapy (IO) is the standard first-line treatment for metastatic renal cell carcinoma (mRCC). The Tide-A trial demonstrated the feasibility of interrupting TKI while continuing IO in patients who responded to the combination, meeting its primary endpoint. This study presents updated results with extended follow-up.
Patients (pts) who underwent surgery for primary tumors and had no symptomatic/bulky disease or liver metastasis received an Ave 800 mg flat dose IV Q2W + Axi 5 mg PO BID for 36 weeks. At week 36, pts achieving partial response (PR) interrupted Axi and continued Ave until progression (PD) or unacceptable toxicity. If PD occurred on Ave, Axi was restarted and interrupted again in case of new PR. Pts without PR continued the combination until PD. The analysis focused on median progression-free survival (mPFS) and overall survival (mOS) in the overall population and those who interrupted Axi.
A total of 75 pts (40% favorable risk, 60% intermediate/poor risk) were included in the efficacy analysis. After a median follow-up of 31.7 months, mPFS was 27.9 months (95% CI, 23.0–32.8). mOS was not reached, with a 24-month OS rate of 85%. The median duration of first avelumab maintenance was 16 weeks. Among the 29 pts who interrupted Axi at week 36, mPFS and mOS were not reached, and the 24-month PFS and OS rates were 68% and 82%, respectively. Five pts remained on avelumab without evidence of PD, and twenty-one restarted Axi. In these 21 patients, after restarting Axi, the ORR was 50% (10 PR, 8 SD, 2 PD, and 1 NA), and mPFS was 17.2 months (95% CI, 11.9–22.5).
Updated results confirmed that interrupting TKI therapy while maintaining IO is a feasible strategy for selected mRCC patients. Progression during IO maintenance does not affect the response to TKI reintroduction, supporting further investigation in randomized trials.
10.7. Two-Stage Estimation of Overall Survival (OS) in CheckMate 9ER (CM9ER), Adjusting for the Impact of Subsequent Therapy
Marc-Oliver Grimm 1, Emma Karim 2, Venediktos Kapetanakis 3, Mickael Lothgren 4, Alessia Ogareva 5, Evidera Pragya Shukla 6 and Camillo Porta 7
- 1
Department of Urology, Jena University Hospital, Friedrich-Schiller University Jena, Jena, Germany
- 2
Ipsen, London, UK
- 3
Evidera, London, UK
- 4
Ipsen, Zug, Switzerland
- 5
Ipsen, Boulogne-Billancourt, France
- 6
Evidera, Montreal, Canada James Truscott, Evidera, London, UK
- 7
Division of Medical Oncology, A.O.U. Consorziale Policlinico di Bari, Bari, Italy; Interdisciplinary Department of Medicine, University of Bari “Aldo Moro”, Bari, Italy
Background: In CM9ER (NCT03141177), first-line cabozantinib plus nivolumab (1L CaboNivo) demonstrated superiority over sunitinib in patients with advanced renal cell carcinoma (aRCC). Here, we report the outcomes of a secondary analysis using a two-stage estimation (TSE) approach to assess the efficacy of CaboNivo versus sunitinib after adjustment for the impact of subsequent treatment, which can confound cross-trial comparisons of OS. The 67.6-month median follow-up of CM9ER was used.
Methods: TSE was used to statistically estimate OS for patients who switched to any subsequent treatment after discontinuation of the randomized treatment, under the hypothetical scenario that they did not switch to a subsequent treatment. The TSE recognises that randomisation holds until the point of treatment discontinuation, which is treated as a “secondary baseline”, and beyond that, the trial becomes an observational study. The analysis included patients who discontinued treatment for any reason. Starting from the secondary baseline, a parametric acceleration failure time (AFT) model was fitted for each arm, controlling for prognostic covariates and a time-dependent covariate indicating treatment switching. The best fitting model was then used to estimate the expected survival deceleration factor, which indicates how many times post-discontinuation survival is extended due to initiating subsequent treatment and subsequently to estimate adjusted (i.e., reduced) post-discontinuation and overall survival times.
Results: Results are shown in
Table 2.
Conclusions: Adjusting for the effect of subsequent therapy had a limited impact on OS for CaboNivo but a larger impact for sunitinib. These results are consistent with clinical practice, further supporting 1L CaboNivo as a standard of care for aRCC.
11. Translational Research
11.1. Circulating Cytokine and Outcomes in Metastatic Clear Cell Renal Cell Carcinoma Treated with Front-Line Immunotherapy Combinations
Mariangela Calabrese 1, Macarena Rey-Cardenas 1, Jean Jouniaux 2, Javier Gavira Diaz 3, Nathalie Chaput-Gras 2, Lydie Cassard 2, Caroline Pradon 4, Mohamed-Amine Bani 4, Natacha Naoun 1, Bernard Escudier 1, Laurence Albiges 1 and Ronan Flippot 1
- 1
Department of Medical Oncology, Gustave Roussy, Paris Saclay University, Villejuif, France
- 2
Laboratory of Immunomonitoring in Oncology, Gustave Roussy, Paris Saclay University, Villejuif, France
- 3
Department of Medical Oncology, Institut Català d’Oncologia, Bellvitge Institute for Biomedical Research, Barcelona, Spain
- 4
Department of Medical Biology and Pathology, Gustave Roussy, Paris Saclay University, Villejuif, France
Introduction: Metastatic clear cell renal cell carcinoma (ccRCC) relies on immune checkpoint inhibitor (ICI)-based regimens including dual ICI and ICI plus tyrosine kinase inhibitor (TKI) combinations, but optimal patient selection remains challenging. Circulating cytokines may better reflect the broader tumor immune microenvironment than tissue biomarkers. We explored cytokine profiles at sequential timepoints in patients with metastatic ccRCC treated with ICI combinations.
Methods: Patients treated with first-line ICI combination for ccRCC were included (NCT04932525). Serum cytokine levels were measured at baseline (T0) and 12 weeks after treatment initiation (T1) using the Olink Target 96 Immuno-oncology platform. The main endpoints included overall response rate, overall survival (OS), and progression-free survival (PFS).
Results: Overall, 23 patients were included (10 dual ICI, 13 ICI-TKI). We identified an inflammatory cytokine cluster (CD5, IFN-γ, IL-13, NOS3, and IL-4) associated with early resistance to ICI-TKI combinations at baseline. In the dual ICI subgroup, the CCL23 cytokine related to myeloid inflammation was significantly elevated in non-responders (p = 0.02); notably, CCL23 showed significant associations with both PFS (p = 0.006) and OS (p = 0.028). Longitudinal analysis demonstrated that responders had significant increases in CCL19, CCL3, CD27, CXCL13, and PDCD1, irrespective of the treatment regimen.
Conclusions: These preliminary findings suggest that specific cytokine profiles may help predict benefits to first-line ICI combinations. Validation in larger cohorts is warranted.
11.2. Elaboration and Clinical Validation of an AI Algorithm as Decision Support System in Kidney Cancer
Jesus Garcia-Donas 1, Jose Luis Ayala 2, Cristina Rodriguez-Antona 3, Natalia Vidal 4, Ignacio Duran 5, Olatz Etxaniz 6, Carlos Álvarez 7, Alfonso Gómez 8, Carmen Santander 9, Alavaro Pinto 10, Nuria Lainez 11, Veronica Calderero 12, Laura BASTERRETXEA 13, Ligia Montero 14 Miguel Rodrigo-Aliaga 15, Cristina Pernaut 16, Oscar Reig 17, Laura Villalobos 18, Amalia Gonzalez 19
- 1
Clara Campal Comprehensive Oncology Center
- 2
Universidad Complutense de Madrid
- 3
Institute for Biomedical Research Sols-Morreale
- 4
Hospital Clinico San Carlos
- 5
Hospital Marques de Valdecilla
- 6
Hospital Germa Trials i Pujol Badalona
- 7
Hospital Central de Asturias
- 8
Complejo Hospitalario Universitario Insular-Materno Infantil (CHUMI), Las Palmas de Gran Canaria Blanca Riesco, Hospital General Albacete
- 9
Hospital Universitario Miguel Servet Zaragoza
- 10
Hospital Universitario La Paz
- 11
Hospital Universitario Navarra
- 12
H.U. San Jorge
- 13
OSI Donostialdea-Onkologikoa
- 14
Clinica Universitaria de Navarra
- 15
Hospital General Universitario Castellon
- 16
Hospital Severo Ochoa
- 17
Hospital Clinic
- 18
Hospital Principe De Asturias
- 19
Hospital Virgen De La Salud
Background: Clear cell renal cell cancer (ccRCC) is a lethal disease with significant knowledge gaps to be addressed. Although it is genetically homogeneous (90% of cases involve VHL mutations), treatment response and tolerability are highly variable. Additionally, the development of new drugs has stalled because the most successful therapies interact with the tumor microenvironment (antiangiogenics and immunotherapy), which is difficult to replicate in vitro. In this project, we aim to integrate complex clinical data with multiple omics platforms (genomics and transcriptomics) using an artificial intelligence algorithm (named Clin-ART) to predict the response to treatments and select the best therapeutic option for every patient.
Methodology: Initially, the clinical and RNA sequencing data (43,893 transcripts) of 181 ccRCC patients treated with NIVOLUMAB, an immune checkpoint inhibitor, in the clinical trials Checkmate 009, 010, and 025 were recorded. After assessing different machine learning classifiers, logistic regression achieved the best results with an accuracy of 86.4%. Currently, we have added 804 patients from the Javelin 101 trial and 305 additional cases from the IMMOTION 150 trial to train the model. Finally, we have started a prospective study in 30 centers with members of the GUARD consortium, aiming to recruit 500 real-life cases (85 have already been included).
Conclusion: The Clin-ART algorithms seems to be accurate when predicting the response to treatments in ccRCC. Updated results will be presented at the symposium.
11.3. Baseline Histopathologic Biomarker for Predicting Front-Line Immunotherapy Combination Outcomes in Metastatic Clear Cell Renal Cell Carcinoma (mccRCC)
Yasser Ged 1, Ezra Baraban 1, Ardit Feinaj 1 and Nirmish Singla 2
- 1
Johns Hopkins University
- 2
Johns Hopkins University Julie Stein Deutsch, Johns Hopkins University
Background: The treatment landscape of mccRCC has evolved in recent years, with immune checkpoint inhibitor (ICI) combinations currently serving as the front-line backbone therapy. However, there remains a lack of robust biomarkers to predict treatment outcomes with ICI combinations. Previously, we demonstrated favorable outcomes with nivolumab monotherapy in previously treated mccRCC patients using the hematoxylin and eosin (H&E)-based scoring of tumor infiltrating immune cells (TILplus) and necrosis (Deutsch, Cell Rep Med, 2023). Herein, we investigated the utility of this biomarker in the context of first-line ICI combinations in mccRCC.
Methods: We conducted a retrospective analysis of mccRCC patients treated at our institution (2013–2024). Eligibility criteria included the following: confirmed ccRCC histology, metastatic disease, treatment with first-line ICI combinations (dual ICI or ICI plus tyrosine kinase inhibitor [TKI]), and availability of H&E-stained slides from metastatic lesions (excluding brain) prior to treatment. Using a previously published methodology, TILplus scores were generated by assessing the mononuclear immune infiltrate, including tumor infiltrating lymphocytes, macrophages, plasma cells, and other associated immune cells. TILplus was scored as “0” (no immune infiltrate identified interfacing with the tumor) or “1” (immune infiltrate involving the tumor was present). Necrosis was scored as “0” if necrosis involved ≤10% surface area and as “1” if >10%. Overall survival (OS) and progression-free survival (PFS) were calculated from ICI initiation using the Kaplan–Meier method. Associations between survival outcomes and the TILplus and necrosis scores were assessed.
Results: A total of 35 patients were included receiving first-line ICI combinations: ICI + ICI (n = 12) and ICI + TKI (n = 23). Across all patients, the OS and PFS were significantly improved in those with TILplus 1 compared to TILplus 0 (p = 0.04, log-rank test, and p = 0.01, Gehan–Breslow–Wilcoxon test, respectively). Necrosis scores did not associate with OS or PFS.
Conclusions: The baseline assessment of TILplus on H&E may serve as a novel biomarker for predicting outcomes to first-line ICI combinations in patients with mccRCC. Incorporation of this biomarker into a prospective clinical trial will be needed for validation.
11.4. Gut Microbiome Dynamics in Metastatic Renal Cell Carcinoma Patients Undergoing Dual Check-Point Inhibition
Cecilie Lindgaard 1, Victoria Norsell 2, Astrid K Hvidt 3, Johanne Ahrenfeldt 1, Jesper Jespersen 1, Niels Fristrup 2 and Iben Lyskjær 1
- 1
Department of Molecular Medicine, Aarhus University Hospital, Aarhus, Denmark. And Department of Clinical Medicine, Aarhus University, Denmark
- 2
Department of Oncology, Aarhus University Hospital, Aarhus, Denmark
- 3
Department of Molecular Medicine, Aarhus University Hospital, Aarhus, Denmark. And Department of Clinical Medicine, Aarhus University, Denmark Sara Schou, Department of Molecular Medicine, Aarhus University Hospital, Aarhus, Denmark. And Department of Clinical Medicine, Aarhus University, Denmark
Background: The gut microbiome is increasingly recognized as a key modulator of immune responses and may influence treatment outcomes in metastatic renal cell carcinoma (mRCC) patients receiving immune checkpoint inhibitors (ICIs). While previous studies suggest a link between microbial diversity and immunotherapy response, data from patients undergoing dual ICI treatment remain limited.
Methods: In this initial study, we performed shotgun metagenomic sequencing on fecal samples from 18 primary mRCC patients treated with Nivolumab and Ipilimumab in the NORDIC-SUN clinical trial (ClinicalGovTrials ID NCT03977571). Samples (n = 31) were collected before treatment initiation and after three months of therapy. Microbial composition, diversity, and functional profiles were analyzed to assess changes in response to treatment and potential associations with clinical outcomes.
Results: Preliminary findings indicate inter-individual variability in baseline microbiome composition, with shifts in microbial diversity and taxonomic profiles observed during treatment. No significant associations were found between the baseline microbiome composition and treatment response. However, patients who did not experience immune-related adverse events (n = 3) had a significantly higher alpha diversity at baseline compared to those who developed adverse events. No other microbiome characteristics showed a significant correlation with clinical outcomes.
Conclusion: While the study did not identify a strong link between microbiome composition and treatment response, the finding that patients without adverse events had higher microbial diversity warrants further investigation. Larger studies are needed to explore the potential role of gut microbiome diversity in predicting toxicity during dual ICI therapy.