First Interim Analysis of the CABONEXT Study: A Retrospective Evaluation of Treatment Patterns Following Cabozantinib Treatment for Advanced Renal Cancer †
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Outcomes from Group A
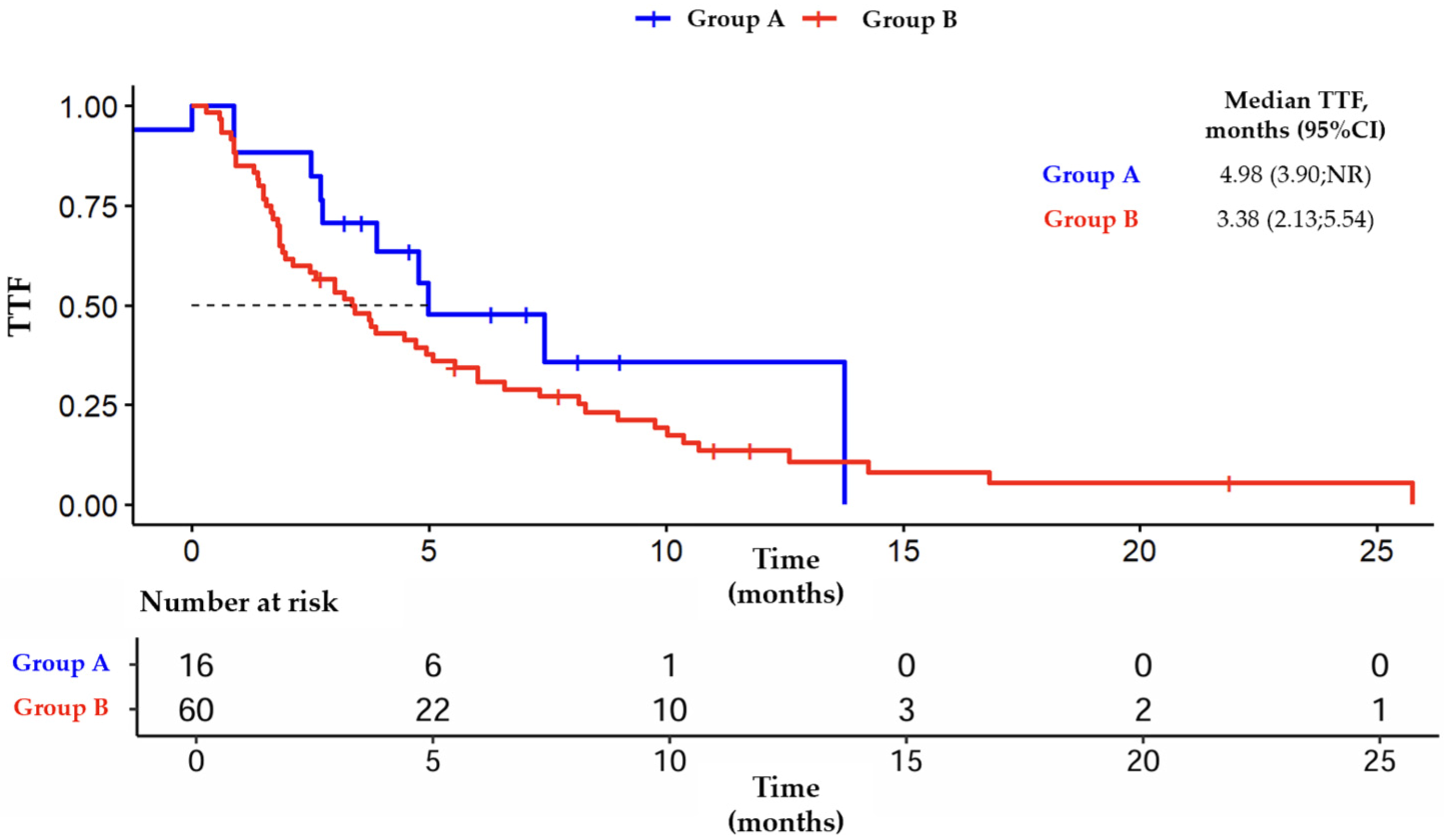
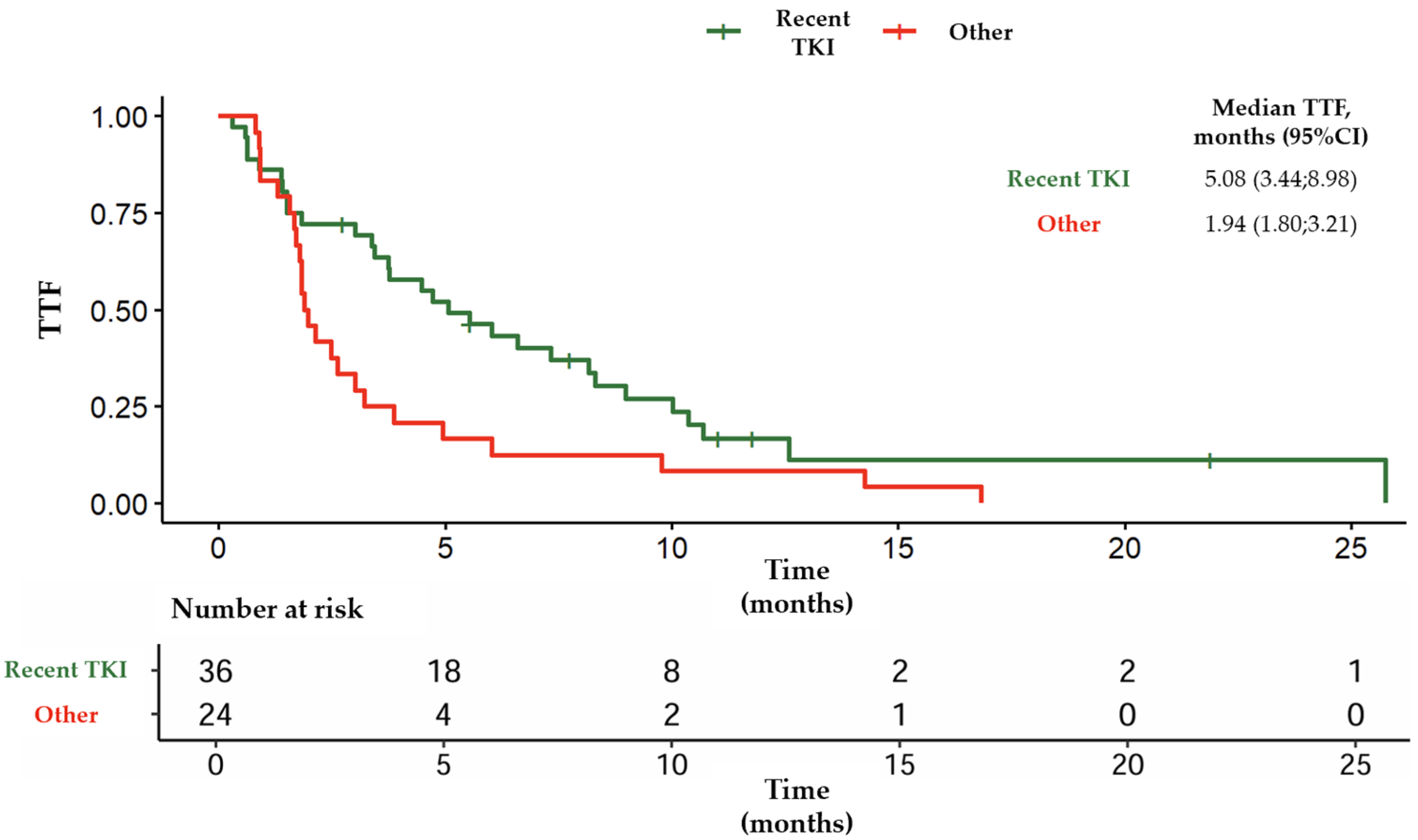
3.3. Outcomes from Group B
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DCR | Disease control rate |
| IMDC | International metastatic renal cell carcinoma database consortium |
| ORR | Overall response rate |
| OS | Overall survival |
| PFS | Progression free survival |
| RCC | Renal cell carcinoma |
| TKI | Tyrosine kinase inhibitor |
| TTF | Time to treatment failure |
| VEGF | Vascular endothelial growth factor |
References
- Yang, Y.; Psutka, S.P.; Parikh, A.B.; Li, M.; Collier, K.; Miah, A.; Mori, S.V.; Hinkley, M.; Tykodi, S.S.; Hall, E.; et al. Combining immune checkpoint inhibition plus tyrosine kinase inhibition as first and subsequent treatments for metastatic renal cell carcinoma. Cancer Med. 2022, 11, 3106–3114. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grünwald, V.; Gillessen, S.; Horwich, A.; ESMO Guidelines Committee. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Aren Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab Versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Mainwaring, P.N.; Rini, B.I.; Donskov, F.; Hammers, H.; Hutson, T.E.; Lee, J.-L.; Peltola, K.; et al. Cabozantinib Versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; Tomczak, P.; Hutson, T.E.; Michaelson, M.D.; Negrier, S.; Oudard, S.; Gore, M.E.; Tarazi, J.; Hariharan, S.; et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013, 14, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Navani, V.; Wells, J.C.; Boyne, D.J.; Cheung, W.Y.; Brenner, D.M.; McGregor, B.A.; Labaki, C.; Schmidt, A.L.; McKay, R.R.; Meza, L.; et al. CABOSEQ: The Effectiveness of Cabozantinib in Patients With Treatment Refractory Advanced Renal Cell Carcinoma: Results From the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC). Clin. Genitourin. Cancer 2023, 21, 106.e1–106.e8. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, P.L.; Suri, Y.; Basu, A.; Koshkin, V.S.; Desai, A. Mechanisms of tyrosine kinase inhibitor resistance in renal cell carcinoma. Cancer Drug Resist. 2023, 6, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, R.; Ciccarese, C.; Facchini, G.; Milella, M.; Urbano, F.; Basso, U.; De Giorgi, U.; Sabbatini, R.; Santini, D.; Berardi, R.; et al. Cabozantinib After a Previous Immune Checkpoint Inhibitor in Metastatic Renal Cell Carcinoma: A Retrospective Multi-Institutional Analysis. Target. Oncol. 2020, 15, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Albiges, L.; Schmidinger, M.; Taguieva-Pioger, N.; Perol, D.; Grünwald, V.; Guemas, E. CaboPoint: A phase II study of cabozantinib as second-line treatment in patients with metastatic renal cell carcinoma. Future Oncol. 2022, 18, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Vano, Y.; Phan, L.; Gravis, G.; Korakis, I.; Schlürmann, F.; Maillet, D.; Bennamoun, M.; Houede, N.; Topart, D.; Borchiellini, D.; et al. Cabozantinib-nivolumab sequence in metastatic renal cell carcinoma: The CABIR study. Int. J. Cancer 2022, 151, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Gavira, J.; Auclin, E.; Rey-Cardenas, M.; Roy, P.; Tapia, J.C.; Nay, P.; Vinceneux, A.; Lefort, F.; Nannini, S.; Randis, A.M.d.C.; et al. Activity of Lenvatinib-Based Therapy in Previously Treated Patients with Metastatic Renal Cell Carcinoma: A European Multicenter Study (LENVA-LAT). Eur. J. Cancer 2025, 220, 115389. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Chen, Y.W.; Panian, J.; Yuen, K.; McKay, R.R. Emerging innovative treatment strategies for advanced clear cell renal cell carcinoma. Oncologist 2025, 30, oyae276. [Google Scholar] [CrossRef] [PubMed]
| All Patients | Group A | Group B | ||
|---|---|---|---|---|
| Number of patients, N | 77 | 17 | 60 | |
| Male N (%) | 62 (81) | 11 (65) | 51 (85) | |
| Median age [IQR] (years) | 67 [59; 76] | 67 [42; 83] | 68 [41; 86] | |
| Histology | Clear Cell, N (%) ° | 67 (87) | 14 (82) | 53 (88) |
| Papillary, N (%) | 5 (6) | 1 (6) | 4 (7) | |
| Chromophobe, N (%) | 4 (5) | 2 (12) | 2 (3) | |
| Sarcomatoïd feature °°, N (%) | 9 (13) | 2 (12) | 7 (12) | |
| IMDC °°°, N (%) | Favorable | 15 (31) | 1 (6) | 14 (23) |
| Intermediate | 26 (53) | 4 (24) | 22 (37) | |
| Poor | 8 (16) | 1 (6) | 7 (12) | |
| Median time between diagnosis and systemic treatment (months) | 5.5 | 4.6 | 6.4 | |
| De Novo metastatic disease, N (%) | 34 (44) | 8 (47) | 26 (43) | |
| Nephrectomy, N (%) | 50 (65) | 8 (47) | 42 (70) | |
| Metastatic site at Cabozantinib start, N (%) | Lung | 58 (75) | 11 (65) | 47 (78) |
| Bone | 34 (44) | 6 (35) | 28 (47) | |
| Lymph nodes | 45 (58) | 7 (41) | 38 (63) | |
| Brain | 10 (13) | 2 (12) | 8 (13) | |
| 1st systemic line, N (%) | Cabozantinib + Nivolumab | 17 (22) | 17 (100) | - |
| ICI + TKI * | 36 (47) | - | 36 (60) | |
| ICI + ICI | 24 (31) | - | 24 (40) |
| Group A (1 L Cabo-Nivo) | Group B (2 L Cabo) | |
|---|---|---|
| N | 17 | 60 |
| Median PFS (months) | 11.3 | 9.9 |
| ORR (%) | 35 | 35 |
| DCR (%) | 88 | 85 |
| Start of cabozantinib at full dose, N (%) * | 16 (94) | 45 (75) |
| Dose reduction (%) | 59 | 63 |
| Group A (1 L Cabo-Nivo) | Group B | Group B1 (ICI-TKI, 2 L Cabo) | Group B2 (dual ICI, 2 L Cabo) | |
|---|---|---|---|---|
| N | 17 | 60 | 36 | 24 |
| Axitinib | 12 * | 16 | 4 | 12 |
| Lenvatinib | 1 | 16 ° | 11 | 5 |
| Everolimus alone | 1 | 16 | 13 | 3 |
| ICI | 0 | 3 | 2 | 1 |
| Tivozanib | 0 | 3 | 2 | 1 |
| Other | 3 | 6 | 4 | 2 |
| Median TTF (months) | 5 | 3.4 | 3.1 | 3.9 |
| ORR (%) | 29 ** | 12 °° | 13 | 10 |
| DCR (%) | 86 ** | 48 °° | 41 | 60 |
| PD (%) | 14 ** | 52 °° | 59 | 40 |
| Variables | HR (95% CI) |
|---|---|
| Age | 1.04 (1.01; 1.08), p = 0.05 |
| IMDC score | 1.15 (0.72; 1.85), NS (p = 0.57) |
| Previous 1st line therapy (ICI-TKI vs. dual ICI) | 1.64 (0.78; 3.45), NS (p = 0.19) |
| Duration of cabozantinib | 0.95 (0.90; 1.00), NS (p = 0.08) |
| Dose reduction in cabozantinib | 2.33 (1.09; 4.98), p = 0.05 |
| Post cabozantinib treatment (2nd generation TKI vs. others) | 3.82 (1.64; 8.93), p = 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nannini, S.; Moinard-Butot, F.; Ladoire, S.; Bochaton, D.; Bigot, P.; Lefort, F.; Mahammedi, H.; Calcagno, F.; Richaud, J.-B.B.; Campedel, L.; et al. First Interim Analysis of the CABONEXT Study: A Retrospective Evaluation of Treatment Patterns Following Cabozantinib Treatment for Advanced Renal Cancer. Med. Sci. Forum 2025, 39, 2. https://doi.org/10.3390/msf2025039002
Nannini S, Moinard-Butot F, Ladoire S, Bochaton D, Bigot P, Lefort F, Mahammedi H, Calcagno F, Richaud J-BB, Campedel L, et al. First Interim Analysis of the CABONEXT Study: A Retrospective Evaluation of Treatment Patterns Following Cabozantinib Treatment for Advanced Renal Cancer. Medical Sciences Forum. 2025; 39(1):2. https://doi.org/10.3390/msf2025039002
Chicago/Turabian StyleNannini, Simon, Fabien Moinard-Butot, Sylvain Ladoire, Dorian Bochaton, Pierre Bigot, Félix Lefort, Hakim Mahammedi, Fabien Calcagno, Jean-Baptiste Barbe Richaud, Luca Campedel, and et al. 2025. "First Interim Analysis of the CABONEXT Study: A Retrospective Evaluation of Treatment Patterns Following Cabozantinib Treatment for Advanced Renal Cancer" Medical Sciences Forum 39, no. 1: 2. https://doi.org/10.3390/msf2025039002
APA StyleNannini, S., Moinard-Butot, F., Ladoire, S., Bochaton, D., Bigot, P., Lefort, F., Mahammedi, H., Calcagno, F., Richaud, J.-B. B., Campedel, L., Falkowski, S., Amrane, K., Charalambous, H., Cornillon, P., & Barthélémy, P. (2025). First Interim Analysis of the CABONEXT Study: A Retrospective Evaluation of Treatment Patterns Following Cabozantinib Treatment for Advanced Renal Cancer. Medical Sciences Forum, 39(1), 2. https://doi.org/10.3390/msf2025039002







