Advancements in Nanotechnology for Orthopedic Applications: A Comprehensive Overview of Nanomaterials in Bone Tissue Engineering and Implant Innovation †
Abstract
1. Introduction
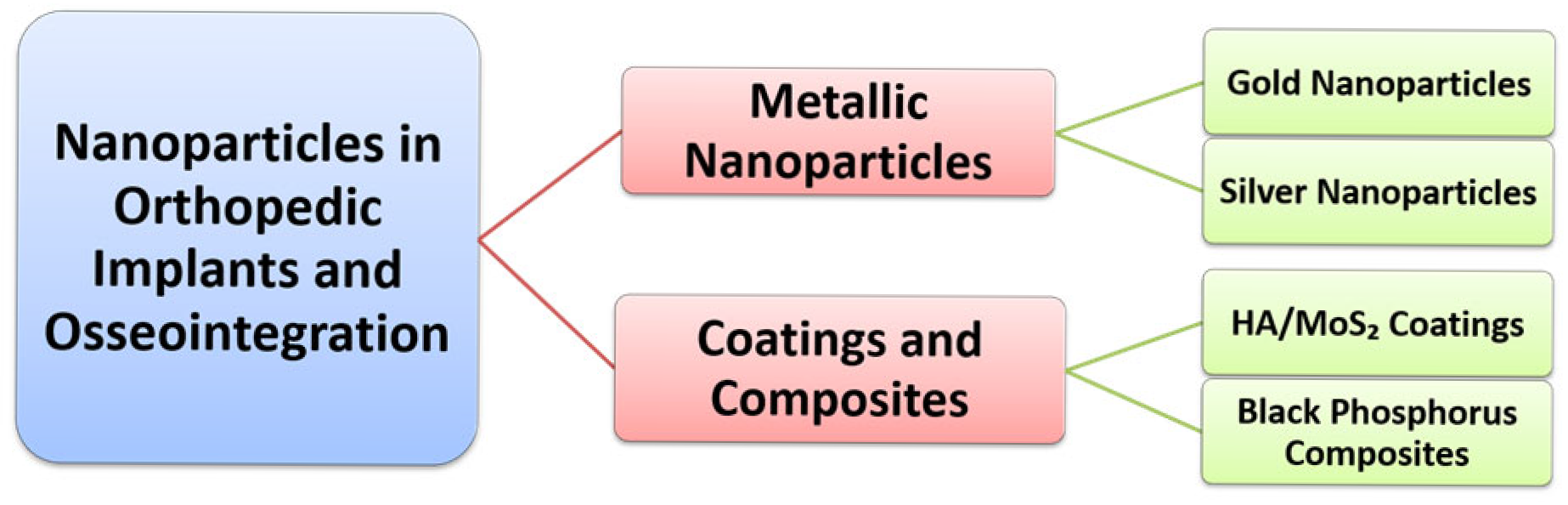
2. Nanoparticles in Orthopedic Implants and Osseointegration
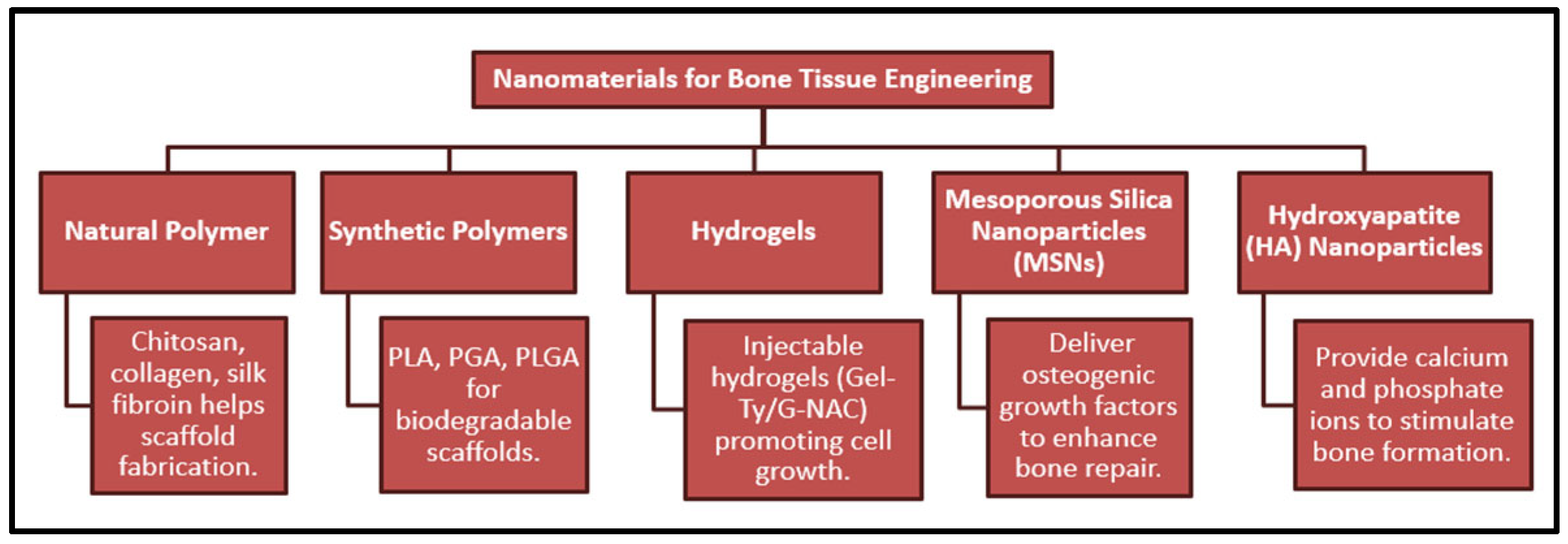
3. Nanomaterials for Bone Tissue Engineering
4. Nano–Bio Interactions: Biochemical and Biomechanical Perspectives

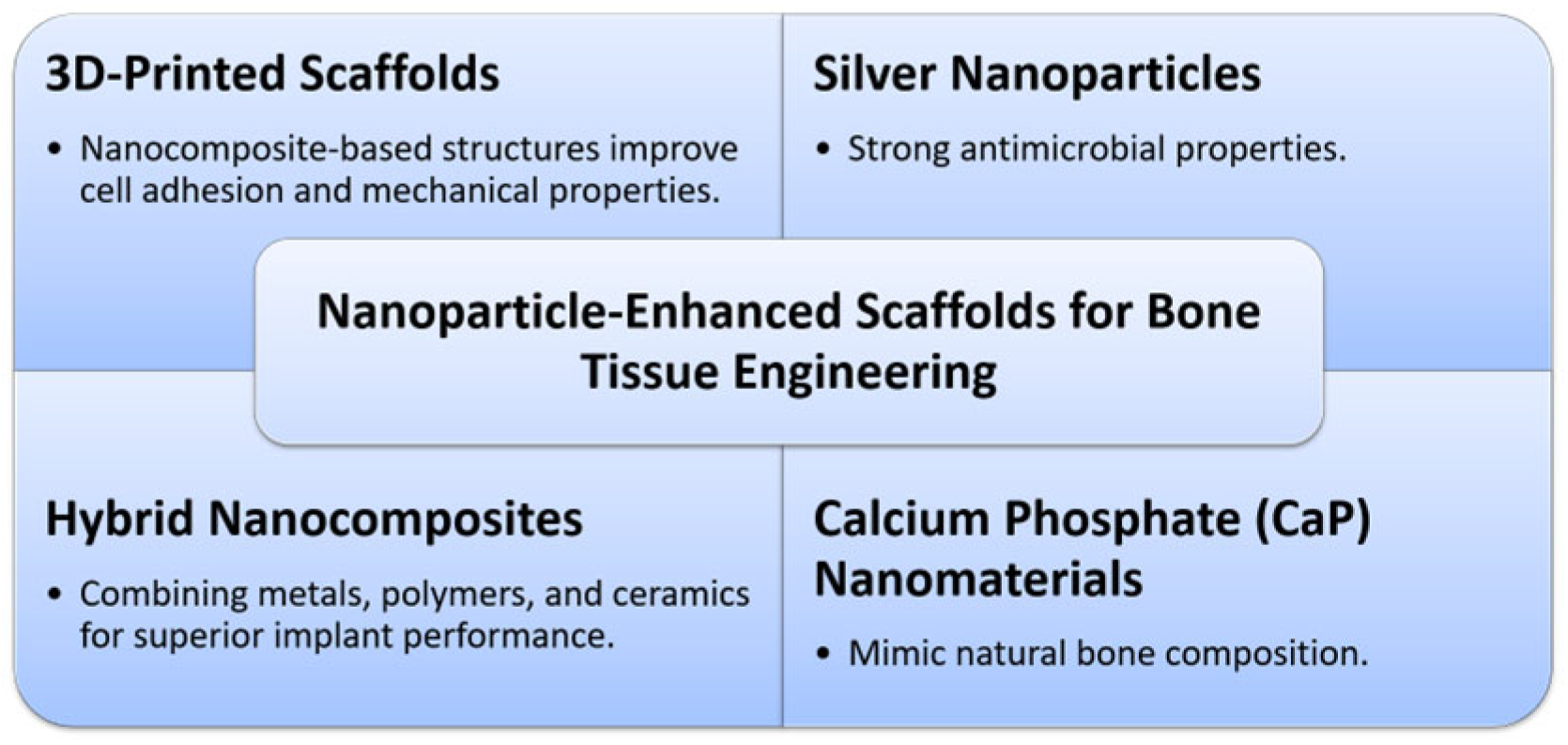
5. Nanoparticle-Enhanced Scaffolds for Bone Tissue Engineering
6. Nanoparticles in Bone Regeneration
7. Clinical Case Studies on Nanomaterials in Orthopedics
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, Y. Toward Fully Automated Personalized Orthopedic Treatments: Innovations and Interdisciplinary Gaps. Bioengineering 2024, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- Kyllönen, L.; D’Este, M.; Alini, M.; Eglin, D. Local Drug Delivery for Enhancing Fracture Healing in Osteoporotic Bone. Acta Biomater. 2015, 11, 412–434. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, W.; Liu, X.; Liang, C.; Zheng, Y.; Li, Z.; Liang, Y.; Zheng, D.; Zhu, S.; Cui, Z.; et al. Self-Activating Anti-Infection Implant. Nat. Commun. 2021, 12, 6907. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-K.; Heo, D.N.; Moon, H.-J.; Lee, S.J.; Bae, M.S.; Lee, J.B.; Sun, I.-C.; Jeon, H.B.; Park, H.K.; Kwon, I.K. The Effect of Gold Nanoparticle Size on Osteogenic Differentiation of Adipose-Derived Stem Cells. J. Colloid Interface Sci. 2015, 438, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Hindy, O.A.; Goker, M.; Yilgor Huri, P. Nanoscale Agents within 3D-Printed Constructs: Intersection of Nanotechnology and Personalized Bone Tissue Engineering. Emergent Mater. 2022, 5, 195–205. [Google Scholar] [CrossRef]
- Wang, N.; Fuh, J.Y.H.; Dheen, S.T.; Senthil Kumar, A. Functions and Applications of Metallic and Metallic Oxide Nanoparticles in Orthopedic Implants and Scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 160–179. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging Carriers for Drug Delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial Nanoparticles: Current Landscape and Future Challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Ko, W.; Kim, S.; Heo, D.; Han, I.; Kim, S.; Kwon, I.K.; Sohn, S. Double Layers of Gold Nanoparticles Immobilized Titanium Implants Improve the Osseointegration in Rabbit Models. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102129. [Google Scholar] [CrossRef]
- Iqbal, N.; Pant, T.; Rohra, N.; Goyal, A.; Lawrence, M.; Dey, A.; Ganguly, P. Nanobiotechnology in Bone Tissue Engineering Applications: Recent Advances and Future Perspectives. Appl. Biosci. 2023, 2, 617–638. [Google Scholar] [CrossRef]
- Lee, D.; Heo, D.N.; Nah, H.R.; Lee, S.J.; Ko, W.-K.; Lee, J.S.; Moon, H.-J.; Bang, J.B.; Hwang, Y.-S.; Reis, R.L.; et al. Injectable Hydrogel Composite Containing Modified Gold Nanoparticles: Implication in Bone Tissue Regeneration. Int. J. Nanomed. 2018, 13, 7019–7031. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Polymeric Scaffolds for Bone Tissue Engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef]
- Kumah, E.A.; Fopa, R.D.; Harati, S.; Boadu, P.; Zohoori, F.V.; Pak, T. Human and Environmental Impacts of Nanoparticles: A Scoping Review of the Current Literature. BMC Public Health 2023, 23, 1059. [Google Scholar] [CrossRef]
- Bauso, L.V.; La Fauci, V.; Longo, C.; Calabrese, G. Bone Tissue Engineering and Nanotechnology: A Promising Combination for Bone Regeneration. Biology 2024, 13, 237. [Google Scholar] [CrossRef]
- Nanotechnologies: 6. What Are Potential Harmful Effects of Nanoparticles? Available online: https://ec.europa.eu/health/scientific_committees/opinions_layman/en/nanotechnologies/l-2/6-health-effects-nanoparticles.htm (accessed on 4 June 2025).
- Engin, A.B.; Nikitovic, D.; Neagu, M.; Henrich-Noack, P.; Docea, A.O.; Shtilman, M.I.; Golokhvast, K.; Tsatsakis, A.M. Mechanistic Understanding of Nanoparticles’ Interactions with Extracellular Matrix: The Cell and Immune System. Part. Fibre Toxicol. 2017, 14, 22. [Google Scholar] [CrossRef]
- Sohrabi Kashani, A.; Packirisamy, M. Cancer-Nano-Interaction: From Cellular Uptake to Mechanobiological Responses. Int. J. Mol. Sci. 2021, 22, 9587. [Google Scholar] [CrossRef]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in Bone Tissue Engineering. Nanomedicine 2015, 11, 1253–1263. [Google Scholar] [CrossRef]
- Ganguly, P.; Jones, E.; Panagiotopoulou, V.; Jha, A.; Blanchy, M.; Antimisiaris, S.; Anton, M.; Dhuiège, B.; Marotta, M.; Marjanovic, N.; et al. Electrospun and 3D Printed Polymeric Materials for One-Stage Critical-Size Long Bone Defect Regeneration Inspired by the Masquelet Technique: Recent Advances. Injury 2022, 53, S2–S12. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Song, L.; Zhou, R.; Luan, S. An Enzyme-Responsive and Photoactivatable Carbon-Monoxide Releasing Molecule for Bacterial Infection Theranostics. J. Mater. Chem. B 2020, 8, 9325–9334. [Google Scholar] [CrossRef]
- Chen, T.-C.; Wong, C.-W.; Hsu, S.H. Three-Dimensional Printing of Chitosan Cryogel as Injectable and Shape Recoverable Scaffolds. Carbohydr. Polym. 2022, 285, 119228. [Google Scholar] [CrossRef] [PubMed]
- Celikkin, N.; Mastrogiacomo, S.; Walboomers, X.F.; Swieszkowski, W. Enhancing X-Ray Attenuation of 3D Printed Gelatin Methacrylate (GelMA) Hydrogels Utilizing Gold Nanoparticles for Bone Tissue Engineering Applications. Polymers 2019, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.M.; Sultana, N.; Baba, S.; Hamdan, S.; Ismail, A.F. Porous PCL/Chitosan and NHA/PCL/Chitosan Scaffolds for Tissue Engineering Applications: Fabrication and Evaluation. J. Nanomater. 2015, 2015, 357372. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun Poly(Lactic Acid-Co-Glycolic Acid) Scaffolds for Skin Tissue Engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef]
- Mok, H.; Zhang, M. Superparamagnetic Iron Oxide Nanoparticle-Based Delivery Systems for Biotherapeutics. Expert Opin. Drug Deliv. 2013, 10, 73–87. [Google Scholar] [CrossRef]
- Van Hengel, I.A.J.; Tierolf, M.W.A.M.; Valerio, V.P.M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Self-Defending Additively Manufactured Bone Implants Bearing Silver and Copper Nanoparticles. J. Mater. Chem. B 2020, 8, 1589–1602. [Google Scholar] [CrossRef]
- Fan, J.H.; Li, W.T.; Hung, W.I.; Chen, C.P.; Yeh, J.M. Cytotoxicity and Differentiation Effects of Gold Nanoparticles to Human Bone Marrow Mesenchymal Stem Cells. Biomed. Eng. Appl. Basis Commun. 2012, 23, 141–152. [Google Scholar] [CrossRef]
- Iqbal, N.; Kadir, M.R.A.; Mahmood, N.H.; Salim, N.; Froemming, G.R.A.; Balaji, H.R.; Kamarul, T. Characterization, Antibacterial and in Vitro Compatibility of Zinc–Silver Doped Hydroxyapatite Nanoparticles Prepared through Microwave Synthesis. Ceram. Int. 2014, 40, 4507–4513. [Google Scholar] [CrossRef]
- Aghajani Derazkola, H.; Simchi, A. Effects of Alumina Nanoparticles on the Microstructure, Strength and Wear Resistance of Poly(Methyl Methacrylate)-Based Nanocomposites Prepared by Friction Stir Processing. J. Mech. Behav. Biomed. Mater. 2018, 79, 246–253. [Google Scholar] [CrossRef]
- Zafar, B.; Mottaghitalab, F.; Shahosseini, Z.; Negahdari, B.; Farokhi, M. Silk Fibroin/Alumina Nanoparticle Scaffold Using for Osteogenic Differentiation of Rabbit Adipose-Derived Stem Cells. Materialia 2020, 9, 100518. [Google Scholar] [CrossRef]
- Lih, E.; Kum, C.H.; Park, W.; Chun, S.Y.; Cho, Y.; Joung, Y.K.; Park, K.S.; Hong, Y.J.; Ahn, D.J.; Kim, B.S.; et al. Modified Magnesium Hydroxide Nanoparticles Inhibit the Inflammatory Response to Biodegradable Poly(Lactide-Co-Glycolide) Implants. ACS Nano 2018, 12, 6917–6925. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neogi, N.; Choudhury, K.P.; Hossain, S.; Hossain, I. Advancements in Nanotechnology for Orthopedic Applications: A Comprehensive Overview of Nanomaterials in Bone Tissue Engineering and Implant Innovation. Med. Sci. Forum 2025, 32, 4. https://doi.org/10.3390/msf2025032004
Neogi N, Choudhury KP, Hossain S, Hossain I. Advancements in Nanotechnology for Orthopedic Applications: A Comprehensive Overview of Nanomaterials in Bone Tissue Engineering and Implant Innovation. Medical Sciences Forum. 2025; 32(1):4. https://doi.org/10.3390/msf2025032004
Chicago/Turabian StyleNeogi, Newton, Kristi Priya Choudhury, Sabbir Hossain, and Ibrahim Hossain. 2025. "Advancements in Nanotechnology for Orthopedic Applications: A Comprehensive Overview of Nanomaterials in Bone Tissue Engineering and Implant Innovation" Medical Sciences Forum 32, no. 1: 4. https://doi.org/10.3390/msf2025032004
APA StyleNeogi, N., Choudhury, K. P., Hossain, S., & Hossain, I. (2025). Advancements in Nanotechnology for Orthopedic Applications: A Comprehensive Overview of Nanomaterials in Bone Tissue Engineering and Implant Innovation. Medical Sciences Forum, 32(1), 4. https://doi.org/10.3390/msf2025032004







