New Compound Combining an Integrase-Targeting Aptamer and a Small Interfering RNA Targeting the Trans-Activation Response/Poly A Region of HIV-1 Potently Suppresses HIV-1 Replication †
Abstract
1. Introduction
2. Materials and Methods
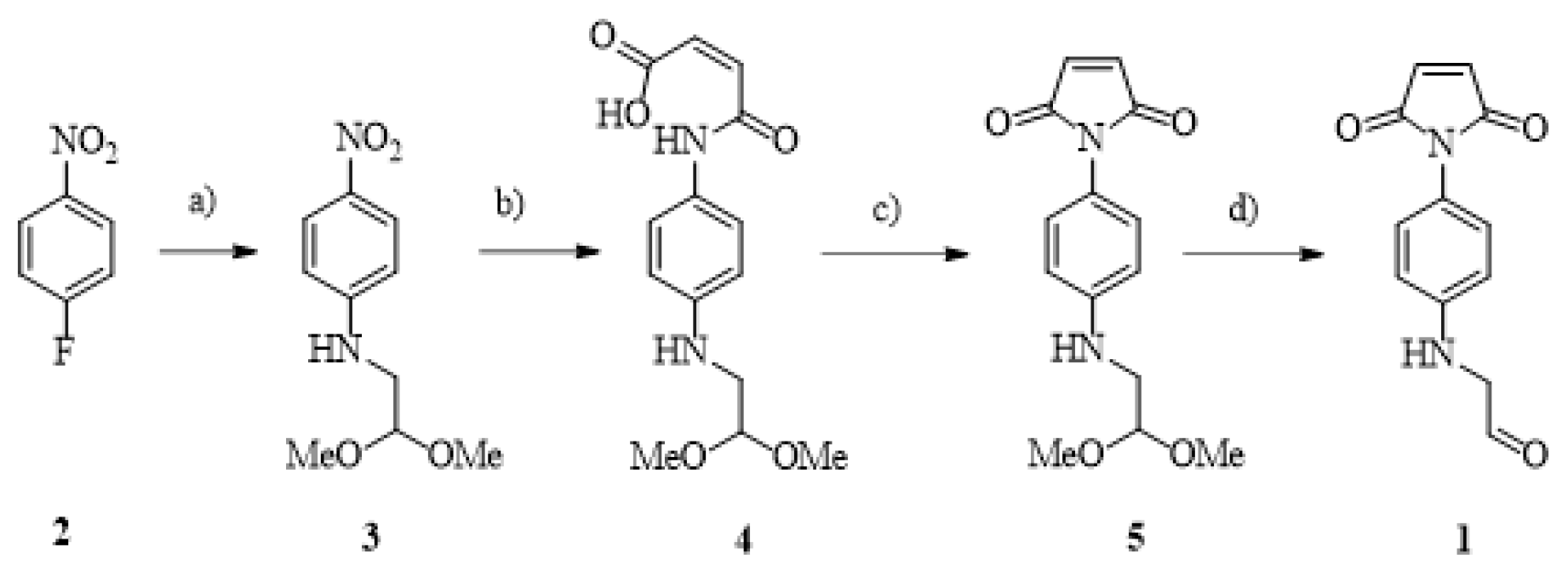
2.1. Apsi510 Synthesis
2.2. Production of Virus and Antiviral Assays
3. Results
3.1. Production of Apsi510
3.2. Activity of siRNA510, Ap(T30695) and Apsi510 against HIV-1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moranguinho, I.; Valente, S.T. Block-And-Lock: New Horizons for a Cure for HIV-1. Viruses 2020, 12, 1443. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Miesen, P.; van Rij, R.P. Antiviral RNAi in Insects and Mammals: Parallels and Differences. Viruses 2019, 11, 448. [Google Scholar] [CrossRef]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Chen, M.J.; Gatignol, A.; Scarborough, R.J. The discovery and development of RNA-based therapies for treatment of HIV-1 infection. Expert Opin. Drug Discov. 2023, 18, 163–179. [Google Scholar] [CrossRef]
- Naito, Y.; Nohtomi, K.; Onogi, T.; Uenishi, R.; Ui-Tei, K.; Saigo, K.; Takebe, Y. Optimal design and validation of antiviral siRNA for targeting HIV-1. Retrovirology 2007, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, B.; Yu, B.; Zhong, W.; Lu, Y.; Zhang, J.; Liao, J.; Liu, J.; Pu, Y.; Qiu, L.; et al. Advances in the development of aptamer drug conjugates for targeted drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotech. 2017, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Seo, J.M.; Shin, K.J.; Yang, S.G. Design and clinical developments of aptamer-drug conjugates for targeted cancer therapy. Biomater. Res. 2021, 25, 42. [Google Scholar] [CrossRef] [PubMed]
- Rozina, A.; Anisenko, A.; Kikhai, T.; Silkina, M.; Gottikh, M. Complex Relationships between HIV-1 Integrase and Its Cellular Partners. Int. J. Mol. Sci. 2022, 23, 12341. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Burnett, J.C.; Rossi, J.J. Aptamer-siRNA chimeras for HIV. Adv. Exp. Med. Biol. 2015, 848, 211–234. [Google Scholar] [PubMed]
- Zhou, J.; Neff, C.P.; Liu, X.; Zhang, J.; Li, H.; Smith, D.D.; Swiderski, P.; Aboellail, T.; Huang, Y.; Du, Q.; et al. Systemic Administration of Combinatorial dsiRNAs via Nanoparticles Efficiently Suppresses HIV-1 Infection in Humanized Mice. Mol. Ther. 2011, 19, 2228–2238. [Google Scholar] [CrossRef] [PubMed]
- Das, A.T.; Harwig, A.; Berkhout, B. The HIV-1 Tat protein has a versatile role in activating viral transcription. J. Virol. 2011, 85, 9506–9516. [Google Scholar] [CrossRef] [PubMed]
- Jing, N.; Hogan, M.E. Structure-Activity of Tetrad-forming Oligonucleotides as a Potent Anti-HIV Therapeutic Drug. J. Biol. Chem. 1998, 273, 34992–34999. [Google Scholar] [CrossRef]
- Zhou, J.; Li, H.; Li, S.; Zaia, J.; Rossi, J.J. Novel Dual Inhibitory Function Aptamer–siRNA Delivery System for HIV-1 Therapy. Mol. Ther. 2008, 16, 1481–1489. [Google Scholar] [CrossRef]
- Duclair, S.; Gautam, A.; Ellington, A.; Prasad, V.R. High-affinity RNA Aptamers Against the HIV-1 Protease Inhibit Both In Vitro Protease Activity and Late Events of Viral Replication. Mol. Ther.-Nucleic Acids 2015, 4, e228. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moranguinho, I.; Borrego, P.; Lavrado, J.; Moreira, R.; Taveira, N. New Compound Combining an Integrase-Targeting Aptamer and a Small Interfering RNA Targeting the Trans-Activation Response/Poly A Region of HIV-1 Potently Suppresses HIV-1 Replication. Med. Sci. Forum 2023, 22, 23. https://doi.org/10.3390/msf2023022023
Moranguinho I, Borrego P, Lavrado J, Moreira R, Taveira N. New Compound Combining an Integrase-Targeting Aptamer and a Small Interfering RNA Targeting the Trans-Activation Response/Poly A Region of HIV-1 Potently Suppresses HIV-1 Replication. Medical Sciences Forum. 2023; 22(1):23. https://doi.org/10.3390/msf2023022023
Chicago/Turabian StyleMoranguinho, Inês, Pedro Borrego, João Lavrado, Rui Moreira, and Nuno Taveira. 2023. "New Compound Combining an Integrase-Targeting Aptamer and a Small Interfering RNA Targeting the Trans-Activation Response/Poly A Region of HIV-1 Potently Suppresses HIV-1 Replication" Medical Sciences Forum 22, no. 1: 23. https://doi.org/10.3390/msf2023022023
APA StyleMoranguinho, I., Borrego, P., Lavrado, J., Moreira, R., & Taveira, N. (2023). New Compound Combining an Integrase-Targeting Aptamer and a Small Interfering RNA Targeting the Trans-Activation Response/Poly A Region of HIV-1 Potently Suppresses HIV-1 Replication. Medical Sciences Forum, 22(1), 23. https://doi.org/10.3390/msf2023022023






