Abstract
The continuous increase in daily exposure to ultraviolet radiation, which influences the redox state of skin cells, may contribute to the damage to the structure and function of cellular macromolecules, which favors the search for protective compounds. One promising compound is cannabidiol (CBD), a phytocannabinoid which has antioxidant and anti-inflammatory properties. Therefore, the aim of this study was to compare the effect of CBD applied after (treatment) as well as before and after (pre-treatment + treatment) keratinocyte irradiation with UVB on the proteomic profile of membrane proteins. The obtained data show that both UVB radiation and CBD treatment significantly modified the proteomic profile of keratinocyte membranes. UVB was shown to dramatically increase the expression of proteins involved in the regulation of cell translation and proliferation (S3a/L13A/L7a ribosomal proteins), calcium ion homeostasis and inflammatory response (S100/S100-A6 proteins) and cellular redox state (peroxireoxin-1). The long action of CBD (pre-treatment + treatment) was more effective in preventing changes caused by UVB, compared to the action of CBD used only after UVB irradiation. The strong activity of CBD applied before and after UVB irradiation suggests that this phytocannabinoid is effective in protecting skin cells against UVB-induced changes, in the keratinocyte proteome.
Keywords:
cannabidiol; UVB; skin cells; keratinocytes; oxidative stress; membrane; proteomic analysis 1. Introduction
Ultraviolet radiation (UVR), as a harmful physical factor, can damage the skin by disturbing the intracellular redox balance and intensifying inflammation mainly in epidermal cells—keratinocytes [1]. Moreover, by inducing precancerous mutations, it may initiate carcinogenesis [2]. Along with these issues, our exposure to UV radiation increases day by day due to the activity of the natural sun source and additional artificial sources, as well as the average decrease in the ozone content [3]. In order to prevent skin diseases caused by constant exposure to UV radiation, compounds with a protective and/or therapeutic effect are sought.
A promising antioxidant and anti-inflammatory compound is cannabidiol (CBD), which is a pharmacologically active but non-psychoactive phytocannabinoid [4]. Interactions between CBD and its molecular targets, including cannabinoid receptors and other components of the endocannabinoid system, bring the therapeutic properties of CBD, which have been evaluated in cardiovascular, neurodegenerative and metabolic diseases and cancer, which are usually accompanied by oxidative stress and inflammation [4]. It has been shown that CBD can reduce the UV-induced level of superoxide anions, increase the antioxidant capacity and, consequently, inhibit the lipid peroxidation process by inducing transcription by the nuclear factor associated with erythroid 2 (Nrf2) and by inhibiting the transcription of the nuclear factor kappa B (NF-κB) [5,6]. In addition, it has already been observed that CBD is able to penetrate keratinocytes, accumulating in the cell membrane [5]. In this way, it protects keratinocytes, preventing changes in the composition of the cell membrane associated with damage caused by UVB, which include a reduction in the level of polyunsaturated fatty acids, and an increase in the level of sialic acid and lipid peroxidation products (malondialdehyde and 8-isoprostanes). Consequently, CBD protects the integrity of cell membranes and prevents the release of lactate dehydrogenase into the environment [5].
Therefore, the main aim of this study was to analyze the effect of CBD, applied only after as well as before and after UV irradiation of keratinocytes, on the proteomic profile of the membrane of these cells.
2. Experiment
SDS-Page/nanoHPLC/QExactiveOrbiTrap analysis was performed on prepared membrane fractions obtained from experimental keratinocyte cell culture groups which are control cells (CTR), cells treated with 4 μM CBD for 48 h (h) (CBD; 48 h), cells treated with CBD for 24 h (CBD; 24 h), cells exposed to UVB (312 nm) at 60 mJ/cm2 (UVB), cells cultured for 24 h before and after UVB irradiation in medium containing 4 μM CBD (CBD + UVB + CBD) and cells cultured for 24 h after UVB irradiation in medium containing 4 μM CBD (UVB + CBD).
Samples containing 30 µg of proteins were separated using 10% Tris-Glycine SDS-PAGE gels. Following gel digestion (using trypsin), the obtained final peptide mixtures were separated using nanoHPLC (analytical C18 column with 2 µm particle size using an Ultimate 3000 (Dionex, Idstein, Germany)). The gradient started at 3 min and was raised to 60% eluent B (90% acetonitrile + 0.03% formic acid) for 40 min (at this time, 40% eluent A contained 5% acetonitrile + 0.1% formic acid). Additionally, the eluted peptides from the column were analyzed using a Q Exactive HF mass spectrometer with an electrospray ionization source (ESI) (Thermo Fisher Scientific, Bremen, Germany). The data were acquired with the Xcalibur software (Thermo Fisher Scientific, Bremen, Germany). The whole comprehensive procedure of analysis using liquid chromatography-tandem mass spectrometry (LC-MS/MS) for peptide identification has been described previously in Gęgotek’s study [7]. Generated raw data from LC-MS/MS were analyzed using Proteome Discoverer 2.0 (Thermo Fisher Scientific, Bremen, Germany).
The effects of CBD pre-treatment (CBD+UVB+CBD) and CBD treatment (UVB+CBD) on the membranes obtained from keratinocytes exposed to UVB were analyzed separately. The results from individual protein label-free quantification were normalized by Z-score and log-transformed Perseus (Perseus 1.6.5.0, https://maxquant.net/perseus/, accessed on 5 December 2020). Quality control and biostatistical analysis including one-way ANOVA, post hoc analysis, principle component analysis (PCA), volcano plots and heat maps were completed using Perseus and open-source software MetaboAnalyst 4.0 (http://www.metaboanalyst.ca, accessed on 5 December 2020). Additionally, protein annotations were conducted using open-source PANTHER (http://pantherdb.org/, accessed on 5 December 2020), comparing with the NCBI database (https://www.ncbi.nlm.nih.gov/, accessed on 5 December 2020) and open-source STRING (https://string-db.org/, accessed on 5 December 2020).
3. Results and Discussion
Keratinocytes are an effective physicochemical barrier against environmental stressors [8]. Their plasma membrane and the cell membranes of other cellular organelles, because of their key components including lipids, proteins and sugars, as in other cell types, play a vital role in cell structure, organization and signaling [9]. Due to its high-energy characteristics, UVB radiation is mainly absorbed by the epidermis and may induce an inflammatory response, DNA damage and even carcinogenesis [10]. Our results clearly show that the keratinocyte membrane proteome undergoes a critical change after UVB irradiation. On the other hand, CBD used after the irradiation of cells, and especially both before and after UVB irradiation, can modulate these changes.
In this study, a total of 513 proteins were identified in membrane fractions of keratinocytes. According to statistical analysis, 70 proteins were found with a significantly changed protein expression within experimental groups. Additionally, the PCA demonstrated that CBD pre-treatment brought along clustering of experimental groups more in themselves (CBD pre-treatment: Component 1—45.2%; Component 2—11.7%; CBD treatment: Component 1—37%; Component 2—15.9%, Figure 1). Volcano graphs comparing the control group and the group exposed to UVB radiation with the groups treated with CBD, before and after or only after irradiation, and also between groups treated with CBD, before and after or only after UVB irradiation, show that the proteomes of the keratinocyte membranes differentiated significantly between groups (Figure 2). In addition, the primary split in the upper hierarchical dendrogram in the heat map showed that samples that were treated with CBD for 48 h (CBD (48 h)) and that were treated with CBD before and after UVB irradiation (CBD + UVB + CBD) cluster closer independently of samples exposed to UVB radiation (Figure 3).
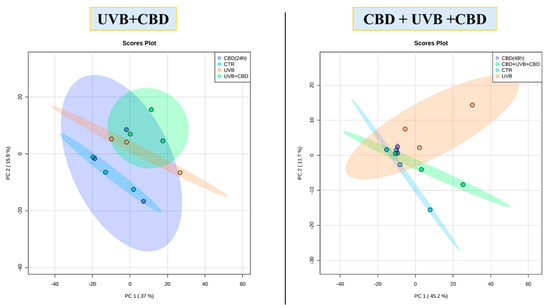
Figure 1.
Principal component analysis (PCA) of membrane fractions obtained from keratinocytes from control group (CTR), cell group treated with 4 μM CBD for 48 h (CBD 48 h), cell group treated with CBD for 24 h (CBD 24 h), cell group exposed to UVB (312 nm) at 60 mJ/cm2 (UVB), cell group cultured for 24 h before and after UVB irradiation in medium containing 4 μM CBD (CBD + UVB + CBD) and cell group cultured for 24 h after UVB irradiation in medium containing 4 μM CBD (UVB + CBD).
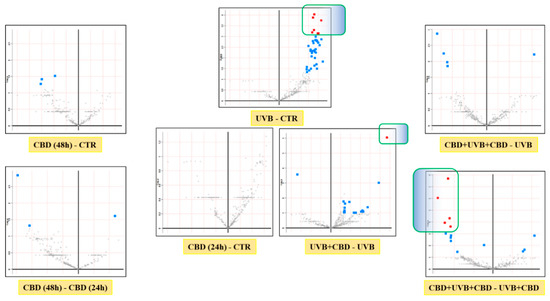
Figure 2.
Volcano plots comparing the effects of cannabidiol treatments (4 μM CBD for 48/24 h; 4 μM CBD for 24 h before and 24 h after UVB irradiation; 4 μM CBD for 24 h after UVB irradiation) on keratinocyte membranes from control and UVB (312 nm, at 60 mJ/cm2)-irradiated groups. (Significant features (in blue) had p < 0.05; significant features (in red) had FDR-adjusted p-value < 0.05.)
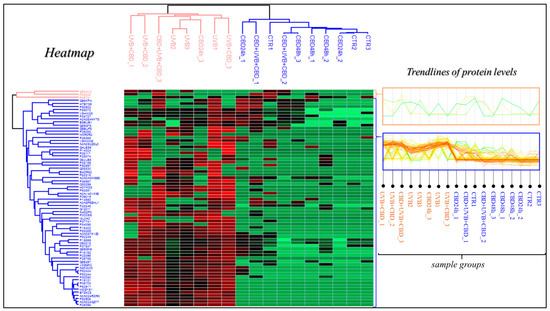
Figure 3.
Heat map and clustering for significant proteins from control group (CTR), cell group treated with 4 μM CBD for 48 h (CBD 48 h), cell group treated with CBD for 24 h (CBD 24 h), cell group exposed to UVB (312 nm) at 60 mJ/cm2 (UVB), cell group cultured for 24 h before and after UVB irradiation in medium containing 4 μM CBD (CBD + UVB + CBD) and cell group cultured for 24 h after UVB irradiation in medium containing 4 μM CBD (UVB + CBD).
Proteins whose expression changed significantly after UVB irradiation, as well as after the use of CBD, both as treatment as well as pre-treatment and treatment, are mainly ribosomal proteins that participate in translation and proteosomal activity, proteins with catalytic activity, proteins involved in cell signaling, DNA/RNA binding proteins and protein structural proteins which are obtained from the membrane fractions including the plasma membrane and the membranes of cellular organelles. In addition, some of these proteins were found to be involved in the cellular response to UVB radiation, including regulation of the cell redox status, inflammation and apoptosis, with a significant change after CBD treatment before and after UVB exposure (Table 1).

Table 1.
Fold changes between cell groups for level of proteins whose expressions were significantly changed in membrane fractions obtained from keratinocytes (control group (CTR), cell group treated with 4 μM CBD for 48 h (CBD 48 h), cell group treated with CBD for 24 h (CBD 24), cell group exposed to UVB (312 nm) at 60 mJ/cm2 (UVB), cell group cultured for 24 h before and after UVB irradiation in medium containing 4 μM CBD (CBD + UVB + CBD) and cell group cultured for 24 h after UVB irradiation in medium containing 4 μM CBD (UVB + CBD)).
In this study, it was found that UVB radiation induces levels of ribosomal proteins such as 40S ribosomal protein S3a, and 60S ribosomal proteins L13a and L7a, which are likely derived from the nuclear membrane or extracellular exosomes and which are involved in ribosomal complex formation, translation and NF-κB signaling. It has been shown that overexpression of the S3a ribosomal protein prevents drug-induced apoptosis and promotes neoplastic transformation [11]. On the other hand, increased expression of the L13a protein as well as L7a can induce apoptosis due to their participation in the cell cycle and translation through the accumulation of p53 [12]. Along with the induction, by CBD application, of the S3a protein level in UVB-exposed membranes, CBD also induces levels of other pro-apoptotic ribosomal proteins. The tendency of a cell to apoptosis or to differentiate is highly dependent on the metabolic environment and the specificity of the cells. Even if it is not possible to interpret one of these directions on the basis of these data alone, this situation may indicate that CBD, through its pro-apoptotic effects, protects cells from neoplastic transformation.
In addition, we have shown that the use of CBD after irradiation of keratinocytes with UVB radiation slightly increased the level of peroxiredoxin-1, a member of the antioxidant proteins, while the use of CBD both before and after irradiation with UVB cells reduced UVB-induced overexpression. In addition to its antioxidant effect, peroxiredoxin-1 also has anti-inflammatory properties through interaction with the DNA repair protein APE1, which may affect the DNA binding activity of NF-κB [13]. However, it is known that inhibition of APE1 activity also increases the level of Nrf2 target genes. Although this antioxidant and anti-inflammatory response from keratinocytes to UVB radiation may be induced by the relatively short duration of CBD’s action in the cellular response to UVB-induced stress, on the other hand, longer exposure to CBD can counteract these changes. A similar trend to the reduction in antioxidant protein expression caused by the action of CBD has recently been demonstrated in the case of superoxide dismutase in the keratinocytes of rats exposed to UVA/B radiation [14].
We also observed that UVB radiation increased the level of the S100 protein, especially the S100-A6 protein, which participates in calcium-dependent binding of proteins and regulation of cell proliferation and apoptosis, as well as the cellular response to various stress factors due to the specificity of the cell type [15]. S100-A6 can induce or reduce apoptosis in several cell types [15]. On the other hand, it was shown that NF-κB and Nrf2 at the transcription level can activate the promoter of the S100A6 gene [15]. Thus, the increased level of S100-A6 caused by CBD treatment may be the result of a relationship between activation of the NF-κB promoter or Nrf2 and S100-A6. Moreover, along with its tendency to lower the peroxiredoxin-1 level, this situation may also support the long-term protective effect of CBD against cell proliferation in tumorigenesis through its pro-apoptotic effect, which is exacerbated by the level of pro-apoptotic factors such as the 60S ribosomal protein L7a [16]. However, since the use of CBD increased the level of the S100 protein as well as S100-A6 located in the nuclear membranes, the plasma membrane or the extracellular exosome, CBD’s long action increased this UVB-induced S100-A6 level, but it was two times lower than that after CBD treatment, and even CBD pre-treatment reduced the level of the S100 protein increased by UVB.
4. Conclusions
Our study shows that UVB radiation significantly modifies the proteome of keratinocyte membranes. The use of CBD can effectively modulate changes induced by UVB, mainly related to cell proliferation as well as oxidative and inflammatory states. Our data also show that the use of CBD both before and after irradiation of keratinocytes with UVB may be more effective in counteracting changes in the membrane proteome. The protective effect of CBD against stress induced by UVB may result from the induction of pro-apoptotic and ribosomal proteins. This effect may also be the result of CBD interference in the crosstalk between NF-κB and Nrf2.
Institutional Review Board Statement
Human cell keratinocytes line CDD 1102 KERTr were obtained from American Type Culture Collection.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This research was conducted within a project which has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 754432 (and the Polish Ministry of Science and Higher Education, from financial resources for science in 2018–2023, granted for the implementation of an international co-financed project). SA is supported by the above project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet radiation-induced skin aging: The role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Pedro, I.; Alonso-Lecue, P.; Sanz-Góme, N.; Freije, A.; Gandarillas, A. Sublethal UV irradiation induces squamous differentiation via a p53-independent, DNA damage-mitosis checkpoint. Cell Death Dis. 2018, 9, 1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol protects keratinocyte cell membranes following exposure to UVB and hydrogen peroxide. Redox Biol. 2020, 36, 101613. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol regulates the expression of keratinocyte proteins involved in the inflammation process through transcriptional regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gęgotek, A.; Domingues, P.; Wroński, A.; Wójcik, P.; Skrzydlewska, E. Proteomic plasma profile of psoriatic patients. J. Pharm. Biomed. Anal. 2018, 155, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Watson, H. Biological membranes. Essays Biochem. 2015, 59, 43–69. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Liao, W.J.; Liao, J.M.; Liao, P.; Lu, H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.W.; Ioannou, Y.A. Ribosomal proteins in cell proliferation and apoptosis. Int. Rev. Immunol. 1999, 18, 429–448. [Google Scholar] [CrossRef]
- Nassour, H.; Wang, Z.; Saad, A.; Papaluca, A.; Brosseau, N.; Affar, E.B.; Alaoui-Jamali, M.A.; Dindial, R. Peroxiredoxin 1 interacts with and blocks the redox factor APE1 from activating interleukin-8 expression. Sci. Rep. 2016, 6, 29389. [Google Scholar] [CrossRef] [Green Version]
- Atalay, S.; Gęgotek, A.; Wroński, A.; Domigues, P.; Skrzydlewska, E. Therapeutic application of cannabidiol on UVA and UVB irradiated rat skin. A proteomic study. J. Pharm. Biomed. Anal. 2021, 192, 113656. [Google Scholar] [CrossRef]
- Donato, R.; Sorci, G.; Giambanco, I. S100A6 protein: Functional roles. Cell Mol. Life Sci. 2017, 74, 2749–2760. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xiong, X.; Sun, Y. The role of ribosomal proteins in the regulation of cell proliferation, tumorigenesis, and genomic integrity. Sci. China Life Sci. 2016, 59, 656–672. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).