Abstract
A drug is defined as a substance capable of influencing biological functions through chemical reactions, either by agonism or antagonism, to achieve the desired therapeutic effect. During this process, several pharmacokinetic and pharmacodynamic events occur, which can be potential sites for drug interactions to occur. For patients undergoing hospital treatment, drugs of different classes are usually prescribed, and it is necessary to understand the risk of interactions between these drugs and their possible change in therapeutic efficacy or safety. Therefore, the study sought to identify the main classes of drugs, and their combinations, used in the treatment of pneumonia in pediatric patients at a teaching hospital in Brazil. This is a cross-sectional, retrospective and descriptive study, from September 2017 to December 2020, based on data obtained from medical records provided by the Júlio Bandeira University Hospital. Regarding the most used antibiotics in the analyzed period, we reported the following drugs: ampicillin (61.76%) and azithromycin (23.53%) in 2017; ampicillin (41.04%) and ceftriaxone (23.51%) in 2018; ampicillin (45.70%) and ceftriaxone (25.50%) in 2019; ceftriaxone (39.20%) and azithromycin (31.49%) in 2020. The main combinations identified in the study were as follows: dipyrone and fenoterol (60.70%), dipyrone and hydrocortisone (47.92%), dipyrone and ondansetron (34.66%) and dipyrone, hydrocortisone and fenoterol (37.38%). It is important to highlight that 58 different drugs were found in the prescriptions of this period, alerting us to the possibility of drug interactions of various types. In view of this, it is possible to highlight the combination of azithromycin and ondansetron as a potentially moderate risk of drug interaction, since both increase the QT interval, (The measurement from the beginning of the QRS complex to the end of the T wave), requiring patient monitoring by means of ECG (electrocardiogram). Therefore, an in-depth analysis of these data may be useful to prepare technical material and assist in therapeutic decision-making, improving the quality of prescriptions and the patient’s clinical response, adopt even more effective conducts and as close to what is expected from the pharmacological characteristics of drugs.
1. Introduction
The role that antibiotics play in health since their discovery is undeniable, and this class of drug is currently among the most prescribed in the hospital environment, whether for the treatment of bacterial infections installed or for prophylactic use, preventing the installation of the microorganism [1]. However, its use requires caution, because, when used indiscriminately, it becomes the basis of a problem with major consequences for health and the economy, such as the development of superbugs, that is, bacteria resistant to more than one class of antibiotics [2].
A complication of great importance for global public health is the emergence of resistant bacteria, which are directly linked to the inappropriate use of antibiotics, with drug interactions being one of the factors that corroborate the development of resistant strains, either by interfering with the effectiveness of the drug or by causing adverse reactions that lead to discontinuation of treatment [2].
The science that studies substances, their chemical interactions with living organisms, either by activating or inhibiting the known effect is called pharmacology. Through chemical reactions, this modification can occur as activation (agonism) or as inhibition (antagonism) [3].
However, from administration to therapeutic effect, the drug goes through several steps and procedures, which make it susceptible to changes in its bioavailability and its mechanism of action [4]. These interferences are called drug interactions (MI), which can be beneficial, when they increase efficacy or decrease side effects, or negative, causing harm to the patient [3]. Self-medication and polypharmacy are risk factors for the occurrence of drug interactions, both common in Brazil, demanding attention from health professionals during the prescription and administration of drugs, due to the negative consequences arising from this irrational use of drugs [5].
In view of the diversity of pathologies of bacterial etiology that require adequate therapy, as well as the high rates of hospitalizations due to bacterial infections, the scientific search is constant to develop drugs that fight the pathogen or reduce the symptoms of these infections. In this sense, it is essential to understand the effect of the use of these drugs, whether at a socioeconomic or scientific level.
In this context, it is extremely important to develop research that seeks to understand the main types of interactions to which patients treated with antibiotics in a hospital environment are subjected, in order to improve prescriptions and reduce the negative impacts that drug interactions in polytherapy can cause.
Therefore, this article wants to discuss the main combinations between drugs and warn about the risk of drug interactions that involve the use of antibiotics in the therapeutic management of bacterial pneumonia, whether community-acquired pneumonia (CAP) or hospital-acquired pneumonia, in patients hospitalized in the pediatric sector from the Júlio Bandeira University Hospital (HUJB) in Cajazeiras-PB, Brazil.
2. Materials and Methods
2.1. Type of Study
It consists of retrospective, descriptive research that involves human beings, being a cross-sectional study that is an integral part of a larger project entitled profile of antimicrobial use in a university hospital in the high sertão of Paraíba. The present study seeks to evaluate the main combinations between drugs and the main antimicrobials administered during the period of hospitalization in patients in the pediatric sector of the Hospital Universitário Júlio Bandeira (HUJB) from 1 September 2017 to 31 December 2020.
2.2. Place of Research
Data were collected at the Hospital Universitário Júlio Bandeira (HUJB), Cajazeiras-PB, a public hospital, a reference for the municipalities of the 9th health region of Paraíba, whose demand for urgent and emergency services available in this environment comes from the surrounding municipalities, including the state of Ceará, due to the geographic location of the city in which it is located.
2.3. Population
The population studied was children and adolescents aged from 0 to 17 years and 11 months of age hospitalized in the pediatric sector who received drug therapy, including antimicrobials, from September 2017 to December 2020; the reason that justifies the selected period is the fact that the hospital was restructured in terms of its physical and professional constitution, in order to facilitate data collection and search for adequate information for the study in question.
2.4. Procedures
Data collection was carried out at the HUJB through the availability of medical prescriptions and electronic medical records through the Management Application for University Hospitals (AGHU), which were carefully analyzed and entered into a spreadsheet containing the following information:
- -
- Epidemiological and clinical data of the patient;
- -
- Antimicrobials prescribed during the period of hospitalization;
- -
- Prescription drugs in general.
2.5. Statistical Analysis
Initially, it was carried out using the Microsoft Excel 2019 program (Microsoft Corporation, Washington, DC, USA), for data tabulation, creating tables and graphs to improve the visualization of the analyzed results in a statistical, descriptive and qualitative way.
2.6. Ethical Aspects
The research was approved by the Research Ethics Committee, Opinion: 3,686,831, to ensure that the information collected has a guarantee of confidentiality and to ensure the privacy and anonymity of the subjects regarding the confidential data involved in the research, thus complying with resolution number 510/2016, from the National Health Council.
3. Results
With regard to the gender, based on what is shown in Figure 1, it was observed that in the years 2017 and 2020, there was a prevalence of males of 59% and 57%, respectively; in 2018 and 2019, females predominated, with values of 52% and 59%, respectively. With regard to the sociodemographic profile of patients who were hospitalized due to pneumonia during this period, the main parameters were the age group, based on the division proposed by the pneumonia protocol of the Hospital Universitário Júlio Bandeira (HUJB), as can be observed through the Figure 2.
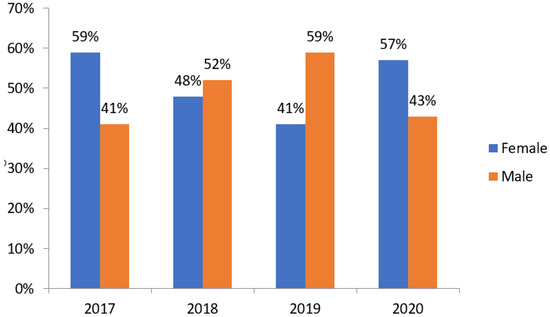
Figure 1.
Evolution of the percentage of pneumonia cases from 2017 to 2020 according to sex. Source: own authorship.
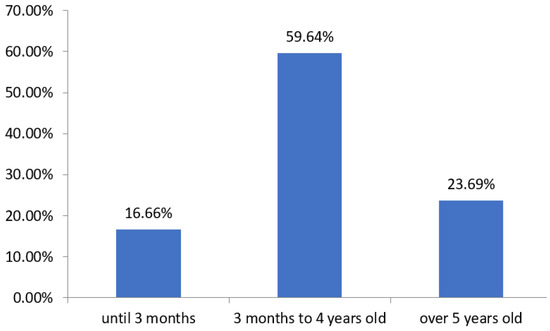
Figure 2.
Relation of the number of cases in percentage and the corresponding age group of patients hospitalized for pneumonia at the HUJB from 2017 to 2020. Source: own authorship.
In Figure 3, we can observe, in total, 626 hospitalizations for pneumonia were analyzed in the pediatric sector of the HUJB in this period, being 29 in 2017, 224 in 2018, 258 in 2019 and 115 in 2020.
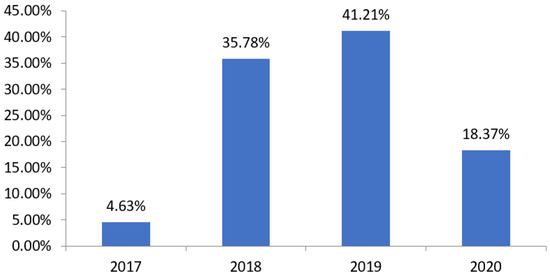
Figure 3.
Relation of the number of cases of hospitalization for pneumonia in the HUJB according to the year and the total number of cases in the analyzed period. Source: own authorship.
As for the evaluation based on the drugs used, there is a disposition between the main therapy, with antimicrobials, described in Figure 4, and the symptomatic therapy, described in Figure 5, performed with the other drugs. In Figure 6, we can observe that the main combinations identified in the study among the symptomatic drugs were as follows: dipyrone and fenoterol (60.70%), dipyrone and hydrocortisone (47.92%), dipyrone and ondansetron (34.66%) and dipyrone, hydrocortisone and fenoterol (37.38%). It is important to highlight that, in total, 71 different drugs were found in the prescriptions of this period, the most prescribed being dipyrone (82.91%), fenoterol (68.37%), hydrocortisone (53.99%), ampicillin (29.94%), ondansetron (34.82%), ceftriaxone (26.51%), simethicone (23.32%), prednisolone (21.56%) and azithromycin (20.93%), alerting us to the possibility of drug interactions of various types.
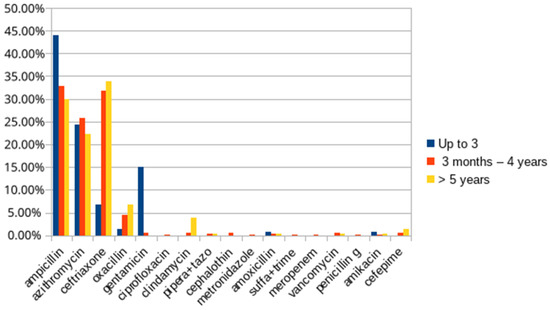
Figure 4.
List of the main antimicrobials used in the treatment of patients with pneumonia from 2017 to 2020. Source: own authorship.
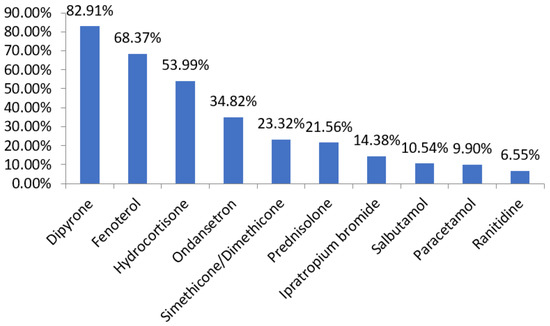
Figure 5.
List of the main drugs used in the treatment of patients with pneumonia from 2017 to 2020. Source: own authorship.
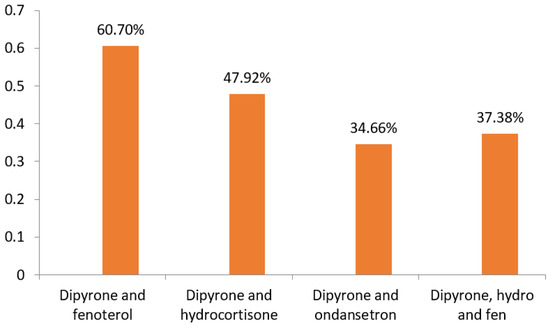
Figure 6.
List of the main combinations of drugs used in the treatment of patients with pneumonia from 2017 to 2020. Source: own authorship.
4. Discussion
It was possible to observe that most patients took a high number of prescribed drugs, which justifies the diversity of drugs identified (71 different drugs) and the high frequency among the most prevalent drug combinations. This is consistent with what is described in the literature. For example, an Italian study on interactions identified an average of 8 drugs prescribed per patient, while research from Pará identified an average of 9 drugs per patient [6]. These data corroborate the hypothesis that hospitalized patients are at high risk of drug interactions due to polymedication.
In this context, it should be noted that drug interactions are classified as severe, moderate or mild; thus, among the most prescribed antimicrobials, we have azithromycin, a macrolide that is used to cover atypical bacteria in pneumonia and among the other drugs, we have ondansetron, which is used as an antiemetic in several pathological conditions; in the combination between azithromycin and ondansetron, there is a moderate drug interaction, since both increase the QT interval, requiring monitoring of the patient by means of ECG.
5. Conclusions
From the above, it is possible to determine that, among the prescriptions, there are chances of drug interactions due to the diversity of drugs administered during hospitalizations, which is useful, for example, in the analysis of a pharmaceutical professional in a multidisciplinary team, reducing the risk of interactions of serious or less effective drugs, as well as increasing patient safety.
This analysis may be useful to prepare technical material and assist in therapeutic decision-making, improving the quality of prescriptions and the patient’s clinical response, adopting even more effective conduct, with adequate care and as close as expected in relation to the characteristics of drugs.
Author Contributions
Conceptualization, F.L.E.d.A. and S.B.F.; methodology, S.M.d.A.O., S.M.G.B., M.F.B.d.S. and S.B.F.; software, S.M.d.A.O., S.M.G.B., R.R.L. and F.L.E.d.A.; validation, N.B.P. and S.B.F.; formal analysis, S.M.G.B.; investigation, S.M.G.B.; data curation, F.L.E.d.A., R.R.L., S.M.G.B. and S.M.d.A.O.; writing—original draft preparation, R.R.L., S.M.G.B. and S.M.d.A.O.; writing—review and editing, S.B.F.; visualization, S.M.G.B.; supervision, S.B.F.; project administration, S.B.F. and S.M.G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Ethics Committee of Pesquisa da Universidade Federal de Campina Grande protocol code n° 3.686.831 of 6 November 2019.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data collection was carried out at the HUJB (Hospital Universitário Júlio Bandeira) through the availability of medical prescriptions and electronic medical records through the Management Application for University Hospitals (AGHU).
Acknowledgments
CNPq—National Council for Scientific and Technological Development, through the Institutional Voluntary Institutional Program for Scientific Initiation (PIVIC); HUJB—Júlio Bandeira University Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miranda, M.M.; Simões, A.C.A.; Teixeira, C.D. Resistência a antimicrobianos em cepas de Enterococcus spp. Revista Univap. 2017, 22, 364. [Google Scholar] [CrossRef]
- Santos, B.S.; Silva, M.S.; Pereira, I.O.; Lemos, L.B.; Lemos, G.S. Potential drug interactions and profile of antimicrobials prescribed for outpatient use in the interior of Bahia. Res. Soc. Dev. 2021, 10, e44210414250. [Google Scholar] [CrossRef]
- Scrignoli, C.P.; Teixeira, V.C.M.; Leal, D.C.P. Drug interactions among the most prescribed drugs in adult intensive care unit. Rev. Bras. Farm. Hosp. Serv. Saúde 2019, 7, 1–19. [Google Scholar]
- De Leite, J.M.S.; Rocha, B.P.; de Moura, A.K.O.; Lins, I.V.F.; Cordeiro, G.B.C.; Paulo, P.T.C.; Teixeira, A. Potências de reações adversas e interações medicamentosas relacionadas ao uso de antibióticos em ambiente hospitalar. J. Biol. Pharm. Agric. Manag. 2020, 16, 177–195. [Google Scholar]
- Chimada, C.A.; Silva, E.M. The importance of pharmacotherapeutic follow-up in patients with heart failure. Res. Soc. Dev. 2020, 9, e1949108538. [Google Scholar] [CrossRef]
- Vilaça, S.O.; Santana, D.S.; Alves, E.S.; Penha, S.S.; Vale, V.V.; Ribeiro, C.H.M. Avaliação de potenciais interações medicamentosas em prescrições de antimicrobianos em um hospital no Estado do Pará. Res. Soc. Dev. 2021, 10, 1–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).