1. Introduction
The human body is exposed to air pollutants not only by inhalation but also by the dermal route. This exposure route is gaining increasing interest with some works reporting it as a relevant carcinogenic route [
1].
Chlorpyrifos is a broad-spectrum pesticide revised by the European Food Safety Authority and by the Environmental Protection Agency as representing a risk to human health [
2]. This pesticide is a lipophilic compound and we recently showed that the (aqueous) skin permeability coefficient is higher than previously reported [
3]. In addition, there are also great differences in the experimental flux (
J) of chlorpyrifos through ex vivo human skin, depending on the receptor fluid employed in the diffusion cell [
3].
A few studies investigated the permeation of chlorpyrifos through the skin by either using ex vivo animal skin or human skin, but alternatives to animal and human skin urge for a more ethical mode of action in scientific research.
The Organization for Economic Cooperation and Development (OECD) provides guidelines defining the experimental conditions to be used when assessing the skin permeation of compounds [
4,
5]. The
J and lag time (Tlag) are important permeation parameters defined in the OECD guidelines [
4,
5].
The purpose of this study was to test the suitability of two synthetic membranes as non-animal alternatives to study the dermal permeation of chlorpyrifos in human health risk assessment.
2. Materials and Methods
All chemicals were from Sigma-Aldrich (St. Louis, MO, USA), Fisher Chemical (Geel, Belgium), or Chem-Lab/Honeywell (Seelze, Germany). STRAT-M® membrane was from Millipore and silicone membrane was a gift from Lintec.
The permeation of chlorpyrifos through synthetic membranes was performed in static diffusion Franz cells [
5]. The membranes—silicone and STRAT-M
®—were mounted between the donor and receptor compartments with a permeation area of 0.64 cm
2. After membranes’ conditioning, chlorpyrifos was applied in acetone at a dose of 400 μg/cm
2 (1 μmol/cm
2), representing a similar dose to the one tested for the permeation of this pesticide in human skin of volunteers [
6]. During the assay, the Franz cells were kept at 32 °C with an agitation of 600 rpm. Samples were collected for the pesticide analysis. Chlorpyrifos was quantified by reverse-phase HPLC (Agilent 1100) with a C18 column and detection at 225 nm. The mobile phase was acetonitrile and water (85:15) and the flux was 1 mL/min.
3. Results and Discussion
One of the experimental conditions recommended by the OECD guidelines for lipophilic compounds, such as chlorpyrifos, is the use of 50% (
v/
v) ethanol in the receptor fluid [
4]. However, there is no clear evidence that this percentage of ethanol is appropriate to reproduce human skin absorption of chlorpyrifos, so we decided to test different ratios of ethanol:saline in the receptor fluid of the Franz cells. The results are presented below.
3.1. The Composition of the Receptor Fluid Affects the Permeation of Chlorpyrifos
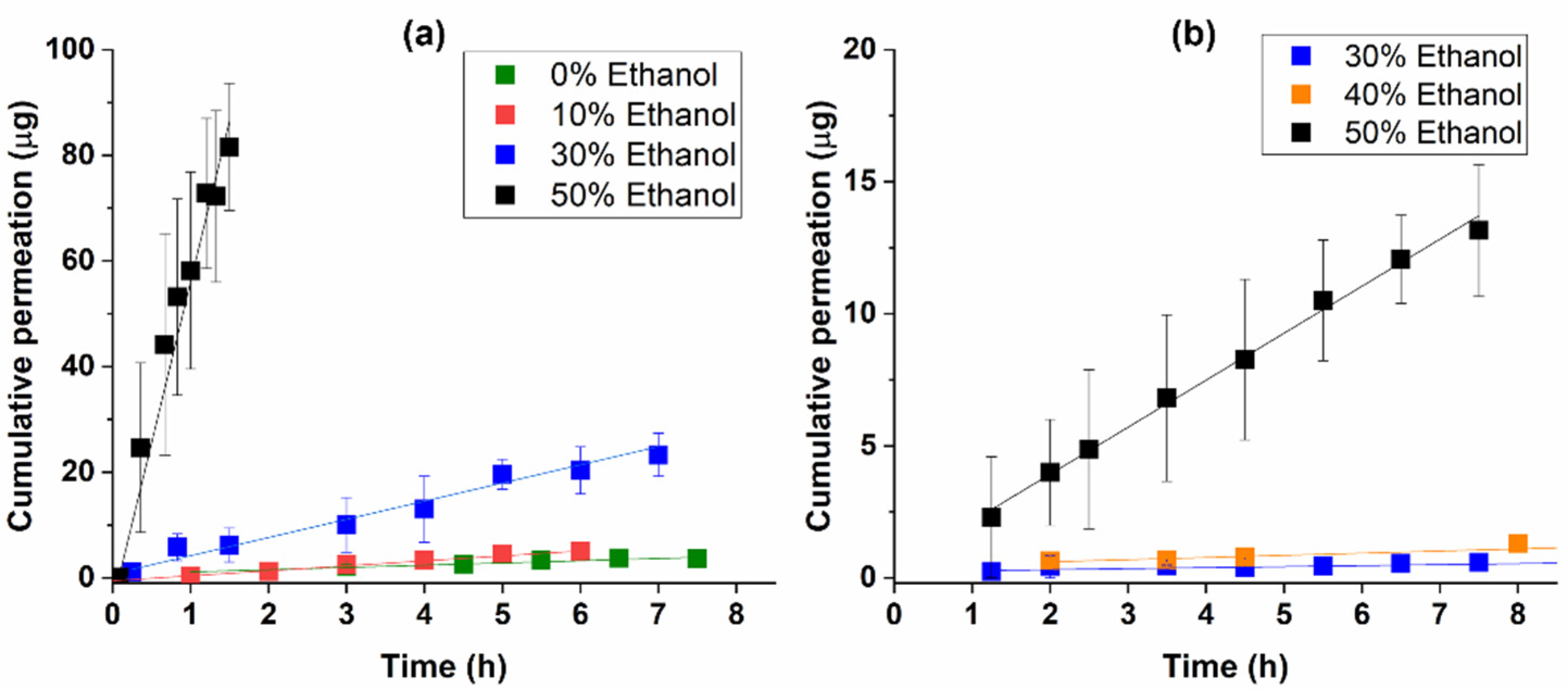
The permeation of chlorpyrifos through both synthetic membranes was studied with different percentages of ethanol in the receptor fluid (10, 30, 40, and 50%). As shown in
Figure 1, the receptor composition influenced the permeation kinetics. Higher ethanol percentages in the receptor contributed to a faster permeation of the pesticide either through the silicone membrane (
Figure 1a) or through the STRAT-M
® membrane (
Figure 1b). Consequently, at 8 h—a time point simulating a work-shift—the quantity of chlorpyrifos that crossed the membranes was also higher for receptors richer in ethanol (
Table 1).
3.2. Flux and Tlag Obtained for the Chlorpyrifos’ Permeation through the Membranes
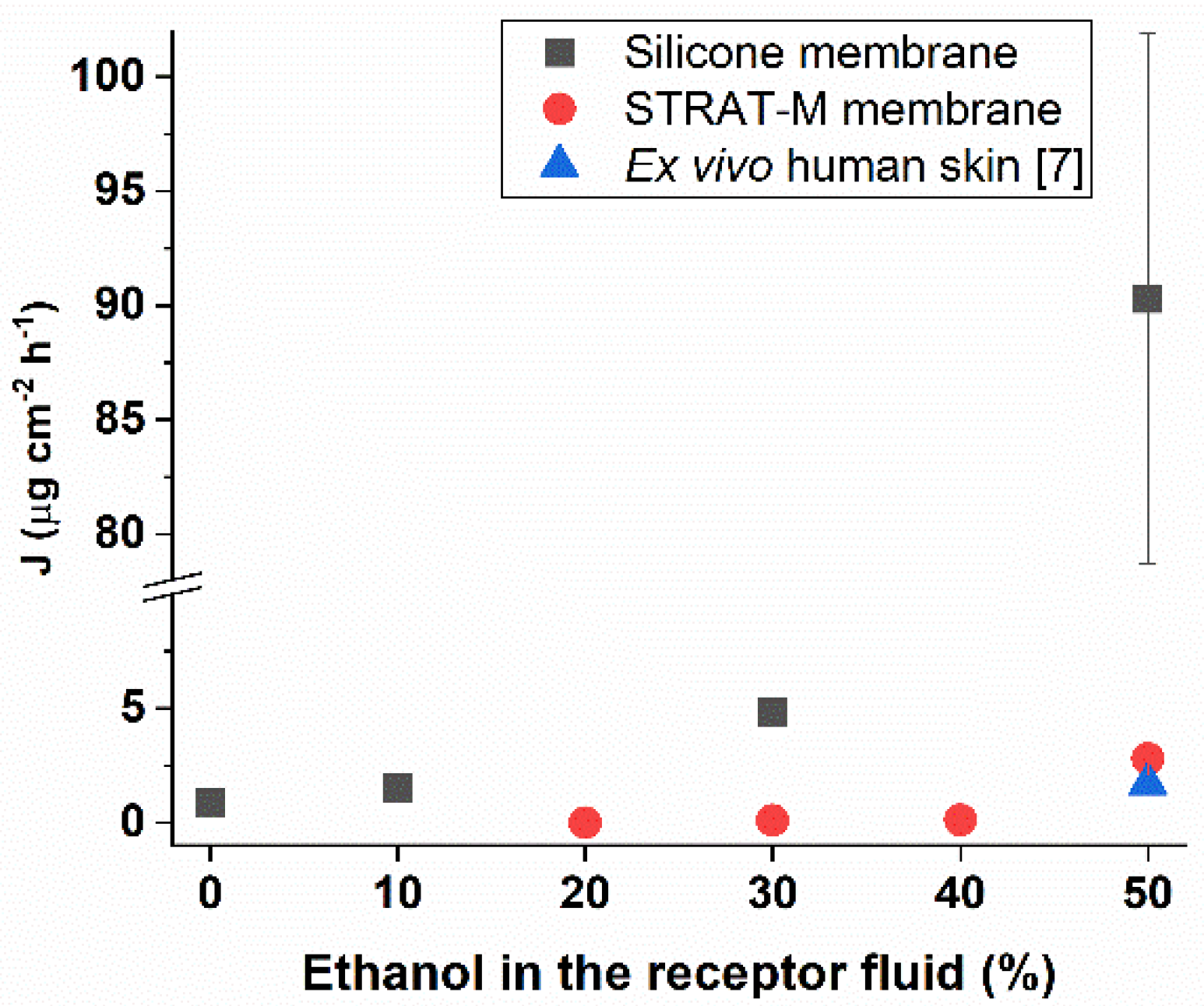
The kinetics in
Figure 1 were used to calculate the parameters J and Tlag of the pesticide permeation. Flux values are represented in
Figure 2 for the different receptors tested, showing their variation with the percentage of ethanol present in the receptor fluid. This effect is more pronounced for the silicone membrane. Regarding Tlag (
Table 2), all the values obtained were inferior to 1 h, including when the silicone membrane was tested with saline fluid (no ethanol) in the receptor. These results indicate that Tlag with the synthetic membranes is not influenced by the percentage of ethanol present in the receptor fluid.
3.3. Comparison of Study Results with Chlorpyrifos Permeation through Human Skin
To understand how synthetic membranes can be useful as alternative skin models, we compared the values of permeation parameters obtained in this work with those reported in [
7] for the permeation of the pesticide through ex vivo human skin (
Table 3).
Since the experimental conditions (ethanol in the receptor fluid) influence the kinetics of chlorpyrifos permeation through the membranes, we selected the flux and corresponding Tlag that best approximates the ex vivo human skin data [
7]. In the case of the silicone membrane, this was achieved by using 10% of ethanol in the receptor fluid, while for STRAT-M
® closer values were obtained for 50% of ethanol in the receptor (
Table 3).
Although not identical, the results obtained in the selected conditions with each membrane afforded permeation parameters close to the values measured with ex vivo human skin (
Table 3).
4. Conclusions
In this work, we have explored different experimental conditions using synthetic membranes as possible alternatives to animal and human skin when investigating the permeation of an organophosphorus pesticide. Both membranes in selected conditions could provide results close to ex vivo human skin. However, having in mind the goal of this study, the results achieved by the silicone membrane are more attractive in terms of the quantity of permeated pesticide and flux obtained when compared to ex vivo human skin.
Author Contributions
Conceptualization, D.M.-d.-S. and R.L.; investigation, D.M.-d.-S.; writing—original draft preparation, writing—review and editing, D.M.-d.-S. and R.L.; visualization, D.M.-d.-S.; supervision, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FUNDAÇÃO PARA A CIÊNCIA e TECNOLOGIA (FCT—Portugal), grant number PTDC/BIA-MIB/31864/2017 and by LA/P/0045/2020 (ALiCE), UIDB/50020/2020 and UIDP/50020/2020 (LSRE-LCM), funded by national funds through FCT/MCTES (PIDDAC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, L.Y.; Shu, X. Aggregate human health risk assessment from dust of daily life in the urban environment of Beijing. Risk Anal. 2014, 34, 670–682. [Google Scholar] [CrossRef] [PubMed]
- EPA. Chlorpyrifos: Third Revised Human Health Risk Assessment for Registration Review. 2020. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2008-0850-0944 (accessed on 6 January 2022).
- Silva, J.; Marques-da-Silva, D.; Lagoa, R. Reassessment of the experimental skin permeability coefficients of polycyclic aromatic hydrocarbons and organophosphorus pesticides. Environ. Toxicol. Pharmacol. 2021, 86, 103671. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guidance Notes on Dermal Absorption, Series on Testing and Assessment, 2nd ed.; Organisation for Economic Co-Operation and Development: Paris, France, 2019; p. 156. Available online: https://www.oecd.org/chemicalsafety/testing/Guidance%20Notes%20Dermal%20Absorption%20156_Oct2019_clean.pdf (accessed on 14 February 2021).
- OECD. Guidance Document for the Conduct of Skin Absorption Studies. Series on Testing and Assessment, No. 28; Organisation for Economic Co-Operation and Development: Paris, France, 2004. [Google Scholar] [CrossRef]
- Griffin, P.; Mason, H.; Heywood, K.; Cocker, J. Oral and dermal absorption of chlorpyrifos: A human volunteer study. Occup Environ Med. 1999, 56, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Griffin, P.; Payne, M.; Mason, H.; Freedlander, E.; Curran, A.D.; Cocker, J. The in vitro percutaneous penetration of chlorpyrifos. Hum. Exp. Toxicol. 2000, 19, 104–107. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).