Abstract
The objective of this work was to optimize subcritical water extraction (SWE) conditions of phenolic compounds and antioxidant activity from vineyard pruning residues. For that, a central composite design (CCD) was conducted to investigate the influence of temperature (123–307 °C) and time (14–56 min). The optimal extraction conditions were 33 min and 280 °C, revealing a high TPC (229 ± 23 mgGAE/g dw) and antioxidant activity by FRAP and ABTS assays (228 ± 20 and 236 ± 11 mgAAE/g dw). The phenolic composition revealed high amounts of catechin, gallic acid and quercetin. SWE demonstrated to be a powerful extraction technique for polyphenols recovery from vine-canes.
1. Introduction
Vineyard pruning residues, produced in high amounts in all viticulture areas, represent an important waste that should be re-used with innovative applications; in fact, vine-canes are typically incorporated in the soil or incinerated [1]. Recently, it was demonstrated that Portuguese vine-canes represent a good source of polyphenolic compounds, which have been associated with several health benefits [2,3].
The analysis and determination of the bioactive compounds can be divided into different steps, namely sample pretreatment, extraction, isolation and purification. However, it has been evident that the choice of the proper extraction technique represents one of the most crucial step [4]. Subcritical water extraction (SWE) is an environmentally friendly extraction technique that employs high temperatures and pressures changing the polarity and dielectric constant of solvents. This enhances the penetration of the solvent into the matrix, improving the extraction efficiency while reducing the extraction time and maintaining the biological activities from the obtained extracts [5].
The present work aimed to optimize the SWE process of vineyard pruning residues using a central composite design (CCD). The influence of the process parameters, namely temperature and extraction time, on total phenol content (TPC) and antioxidant activity was investigated. Following, the phenolic composition from the optimal extract was assessed.
2. Materials and Methods
2.1. Vine-Cane Samples
Vine-canes from Touriga Nacional variety were randomly collected at Quinta dos Carvalhais (Dão region) in 2015, dried at 50 °C for 24 h, milled to 1 mm and stored at room temperature.
2.2. Subcritical Water Extraction
SWE was performed in a Parr Series 4560 Reactor connected to a Parr 4848 Reactor Controller. The extractions were performed using 20 g of sample and 200 mL of deionized water at temperatures ranging from 150 to 280 °C and at times from 20 to 50 min, as defined by the RSM design (Table 1). After SWE, the system was cooled down, and the extracts were filtered, centrifuged (5000 rpm for 15 min at 4 °C) and lyophilized for 48 h. Afterwards, the extracts were stored at 4 °C until further use.

Table 1.
Experimental and predicted values of TPC (Y1, mg GAE/g dw), ABTS (Y2, mg AAE/g dw) and FRAP (Y2, mg AAE/g dw) of vine-canes’ SWE extracts obtained by central composite design (CCD).
2.3. Total Phenolic Content and Antioxidant Activity
The TPC and antioxidant activity evaluated by the ferric reduction antioxidant power (FRAP) and 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt (ABTS) assays were performed as previously described [1,6]. Results were expressed as milligrams of gallic acid equivalents (GAE) and ascorbic acid equivalents (AAE) per gram of dry weight (dw) depending on the assay.
2.4. Qualitative and Quantitative Polyphenol Characterization by HPLC-PDA
The phenolic profile of the optimal extract was characterized by HPLC with a photodiode array detector and a C18 column as described in detail by Moreira et al. [1]. The extract was analyzed three times, and the results were expressed as mg of compound/100 g of dw.
2.5. Statistical Analysis
All experimental results were expressed as means ± standard deviation (SD) of three parallel measurements, and all calculations were carried out using Design Expert (Version 7.0). The validated extraction at the predicted optimal conditions was repeated three times; results were statistically analyzed by analysis of variance (ANOVA) and Tukey’s multiple range test using the SPSS statistic software, version 24.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was accepted at a level of p < 0.05.
3. Results and Discussion
3.1. Total Phenolic Content and Antioxidant Activity
Table 1 shows the obtained content of phenolics and antioxidant activity for the proposed experiments by the CCD depending on two factors: temperature and time. Regarding the evaluated activities of the extracts, TPC ranged from 32.7 (extraction 1, 215 °C, 35 min) to 243 mg GAE/g dw (extraction 6, 280 °C, 20 min); ABTS varied between 40.8 (extraction 5, 307 °C, 55 min) and 257 mg AAE/g dw (extraction 6, 280 °C, 20 min) and FRAP ranged from 33.6 (extraction 1, 215 °C, 35 min) to 264 mg AAE/g dw (extraction 6, 280 °C, 20 min). These results are in line with previous studies [2,7], which also reported that the use of higher temperatures resulted in higher amounts of bioactive compounds as well as higher antioxidant properties.
According to the obtained results in Table 1 and information from 3D surface plots (data not shown), the optimal SWE conditions which simultaneously maximized the TPC and antioxidant activity were 280 °C and 33 min (R2 = 0.9198). The experimental values for the TPC, ABTS and FRAP assays determined at optimal conditions were 229 mg GAE/g dw, 236 mg AAE/g dw and 228 mg AAE/g dw. The obtained values were similar to the ones predicted by the model (p < 0.05), suggesting that the models were valid for the optimization of antioxidant compounds and polyphenols extraction from vine-canes using SWE.
The comparison of the results with the published data showed that dry extract of “Greco” grape canes obtained by conventional extraction with 20 mM of KCl/NaOH pH 13 at 50 °C for 20 min under continuous stirring [8] contained lower amounts of total phenols (104 mg GAE/g dw) than extracts from the present study. In another study [7], a TPC of 181 mg GAE/g DW was reported for vine-canes extracted at 250 °C for 50 min, which were similar to the values obtained in the present study.
3.2. HPLC-DAD Analysis
An HPLC-DAD analysis to the extract obtained at the optimal SWE conditions was performed to know which individual phenolic compounds were the main contributors to the exhibited antioxidant properties. Figure 1 presents the HPLC chromatogram obtained at 280 nm for the polyphenol’s standard mixture. In Table 2, the obtained content for the individual phenolic compounds identified in the optimal vine-cane extract are reported.
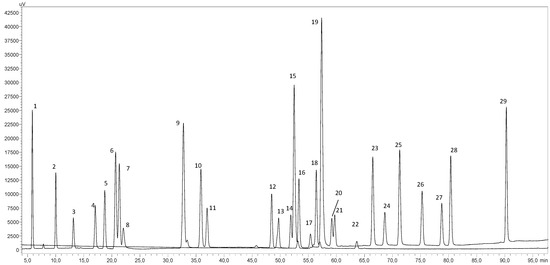
Figure 1.
HPLC-DAD chromatogram monitored at 280 nm for a polyphenol standard mixture of 5 mg/L; peak identification: (1) gallic acid, (2) protocatechuic acid, (3) (+)-catechin, (4) chlorogenic acid, (5) vanillic acid, (6) caffeic acid, (7) syringic acid, (8) (−)-epicatechin, (9) p-coumaric acid, (10) trans-ferulic acid, (11) sinapic acid, (12) naringin, (13) 3,5-di-caffeoylquinic acid, (14) quercetin-3-O-galactoside, (15) rutin, (16) phloridzin, (17) ellagic acid, (18) 3,4-di-O-caffeoylquinic acid; (19) myricetin, (20) cinnamic acid, (21) kaempferol-3-O-glucoside, (22) kaempferol-3-O-rutinoside, (23) naringenin, (24) quercetin, (25) phloretin, (26) tiliroside, (27) kaempferol, (28) apigenin and (29) chrysin.

Table 2.
Content of the individual polyphenols in vine-cane extract obtained at the optimal SWE conditions (250 °C, 33 min). Results are expressed as mean ± standard deviation (milligrams of compound/100g dw, n = 3).
The phenolic composition determined by HPLC-DAD revealed the presence of compounds belonging to different families, with gallic acid (300 ± 15 mg/100 g dw), catechin (468 ± 23 mg/100 g dw), kaempferol-3-O-glucoside (195 ± 10 mg/100 g dw) and quercetin (153 ± 8 mg/100 g dw) being the major contributors to the demonstrated antioxidant properties of the produced vine-cane extracts. On the contrary, 3,4-di-O-caffeoylquinic acid, phloretin and protocatechuic acid were present in the lowest amount, with values below 15.6 mg/100 g dw. These phenolic compounds have been previously identified in vine-canes [3,7]; however, different amounts have been quantified depending on the variety, as well as from the extraction conditions employed. For instance, in a recent study [3] approximately a three-fold higher amount of gallic acid was extracted (1041 versus 300 mg/100 g dw), while, on the contrary, a 10-fold lower amount of quercetin was recovered from vine-canes (16.1 versus 153 mg/100 g dw).
The results obtained in the present work proved that SWE can be a useful extraction technique for obtaining phenolic compounds from vineyard pruning residues, which can be further safely applied to food or cosmetic industries, creating an added value to this residue.
Author Contributions
Conceptualization, M.M.M. and C.D.-M.; funding acquisition, C.D.-M. and C.F.; investigation, M.M.M., O.D., S.S., A.M.S., C.G., V.C.F., F.R., E.F.V. and A.F.P.; methodology, M.M.M., S.S., O.D., A.M.S., C.G., V.C.F., F.R. and A.F.P.; software, E.F.V.; project administration, M.M.M., A.F.P., C.F. and C.D.-M.; resources, C.D.-M. and C.F.; supervision, M.M.M. and C.D.-M.; writing—original draft preparation, M.M.M. and E.F.V.; writing—review and editing, M.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FCT/MCTES through national funds (UIDB/50006/2020 and UIDP/50006/2020.). This work was also financed by the FEDER Funds through the Operational Competitiveness Factors Program—COMPETE and by National Funds through FCT within the scope of the project “PTDC/BII-BIO/30884/2017—POCI-01-0145-FEDER-030884” and project “PTDC/ASP-AGR/29277/2017- POCI-01-0145-FEDER-029277”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
O.D. is thankful for the research grant from project PTDC/BII-BIO/30884/2017—POCI-01-0145-FEDER-030884. A.M.S. is thankful for the PhD grant (SFRH/BD/144994/2019) financed by POPH-QREN and subsidised by the European Science Foundation and Ministério da Ciência, Tecnologia e Ensino Superior. M.M.M. (CEECIND/02702/2017), E.F.V. (CEECIND/03988/2018), V.C.F. (SFRH/BPD/109153/2015), F.R. (CEECIND/01886/2020), C.G. (CEECIND/03436/2020) and A.F.P. (CEECIND/01614/2020) are grateful for the financial support financed by national funds through FCT—Fundação para a Ciência e a Tecnologia, I.P. and to REQUIMTE/LAQV. The supply of the vineyard pruning is acknowledged to Sogrape, S.A.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Dorosh, O.; Moreira, M.M.; Rodrigues, F.; Peixoto, A.F.; Freire, C.; Morais, S.; Delerue-Matos, C. Vine-Canes Valorisation: Ultrasound-Assisted Extraction from Lab to Pilot Scale. Molecules 2020, 25, 1739. [Google Scholar] [CrossRef]
- Moreira, M.M.; Rodrigues, F.; Dorosh, O.; Pinto, D.; Costa, P.C.; Švarc-Gajić, J.; Delerue-Matos, C. Vine-Canes as a Source of Value-Added Compounds for Cosmetic Formulations. Molecules 2020, 25, 2969. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Morais, S.; Delerue-Matos, C. Chapter 2—Environment-Friendly Techniques for Extraction of Bioactive Compounds from Fruits A2—Grumezescu, Alexandru Mihai. In Soft Chemistry and Food Fermentation; Holban, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 21–47. [Google Scholar]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Mendes, M.; Carvalho, A.P.; Magalhães, J.M.C.S.; Moreira, M.; Guido, L.; Gomes, A.M.; Delerue-Matos, C. Response surface evaluation of microwave-assisted extraction conditions for Lycium barbarum bioactive compounds. Innov. Food Sci. Emerg. Technol. 2016, 33, 319–326. [Google Scholar] [CrossRef]
- Dorosh, O.; Moreira, M.M.; Pinto, D.; Peixoto, A.F.; Freire, C.; Costa, P.; Rodrigues, F.; Delerue-Matos, C. Evaluation of the Extraction Temperature Influence on Polyphenolic Profiles of Vine-Canes (Vitis vinifera) Subcritical Water Extracts. Foods 2020, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Squillaci, G.; Zannella, C.; Carbone, V.; Minasi, P.; Folliero, V.; Stelitano, D.; Cara, F.; Franci, G.; Morana, A. Grape Canes from Typical Cultivars of Campania (Southern Italy) as a Source of High-Value Bioactive Compounds: Phenolic Profile, Antioxidant and Antimicrobial Activities. Molecules 2021, 26, 2746. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).