Development of Alginate Capsules with Bioactive Compounds from Lactobacillus plantarum GP108 and Evaluation of Their Effect Against Escherichia coli †
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining the Substrate with Bioactive Compounds (SB)
2.2. Sensitivity Tests Against E. coli
2.3. Preparation of Capsules with Bioactive Compounds (BCCs)
2.4. Sensitivity Testing of BCCs Against E. coli
2.5. Statistical Analysis
3. Results and Discussion
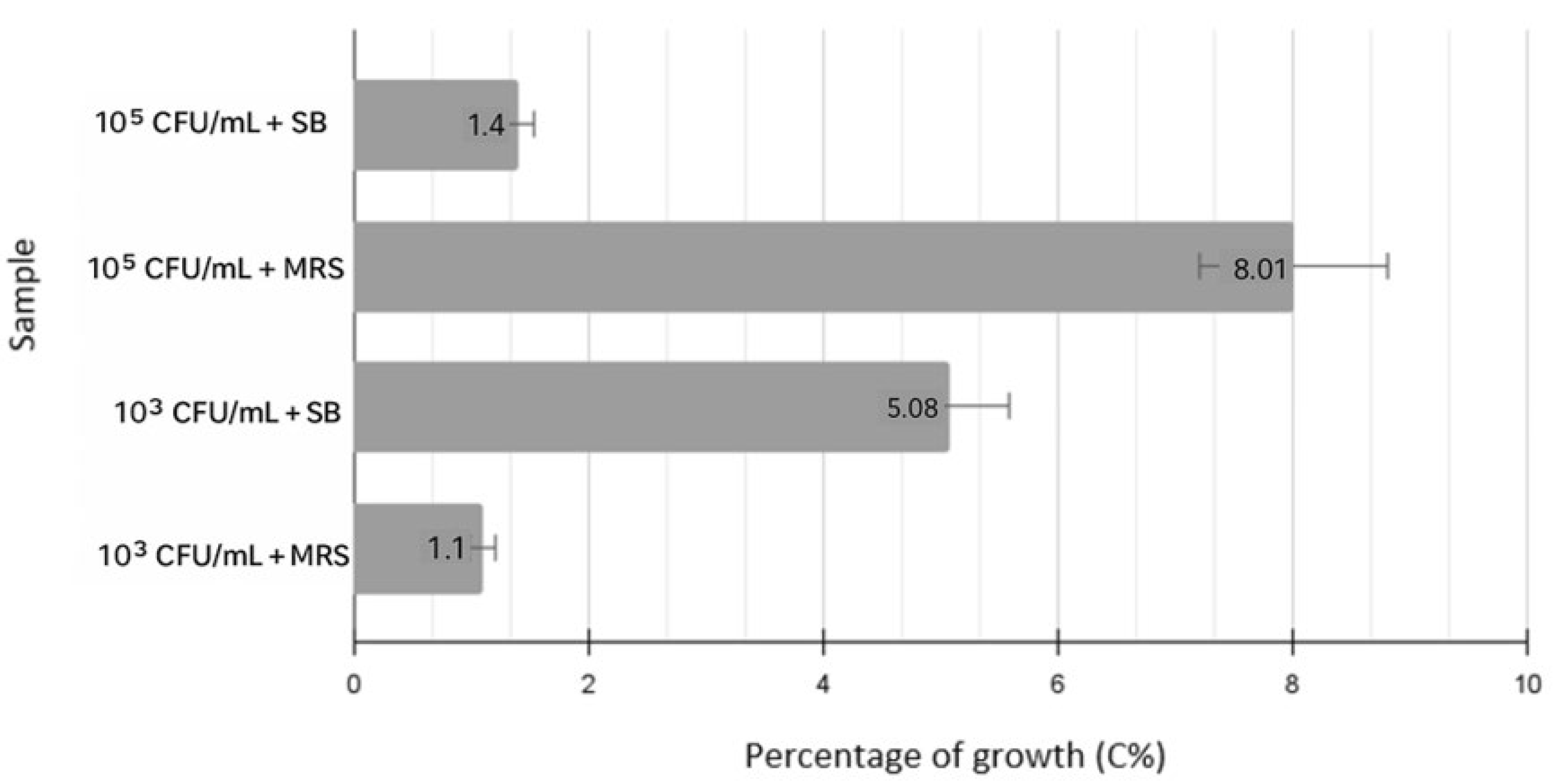
3.1. SB Sensitivity Testing Against E. coli
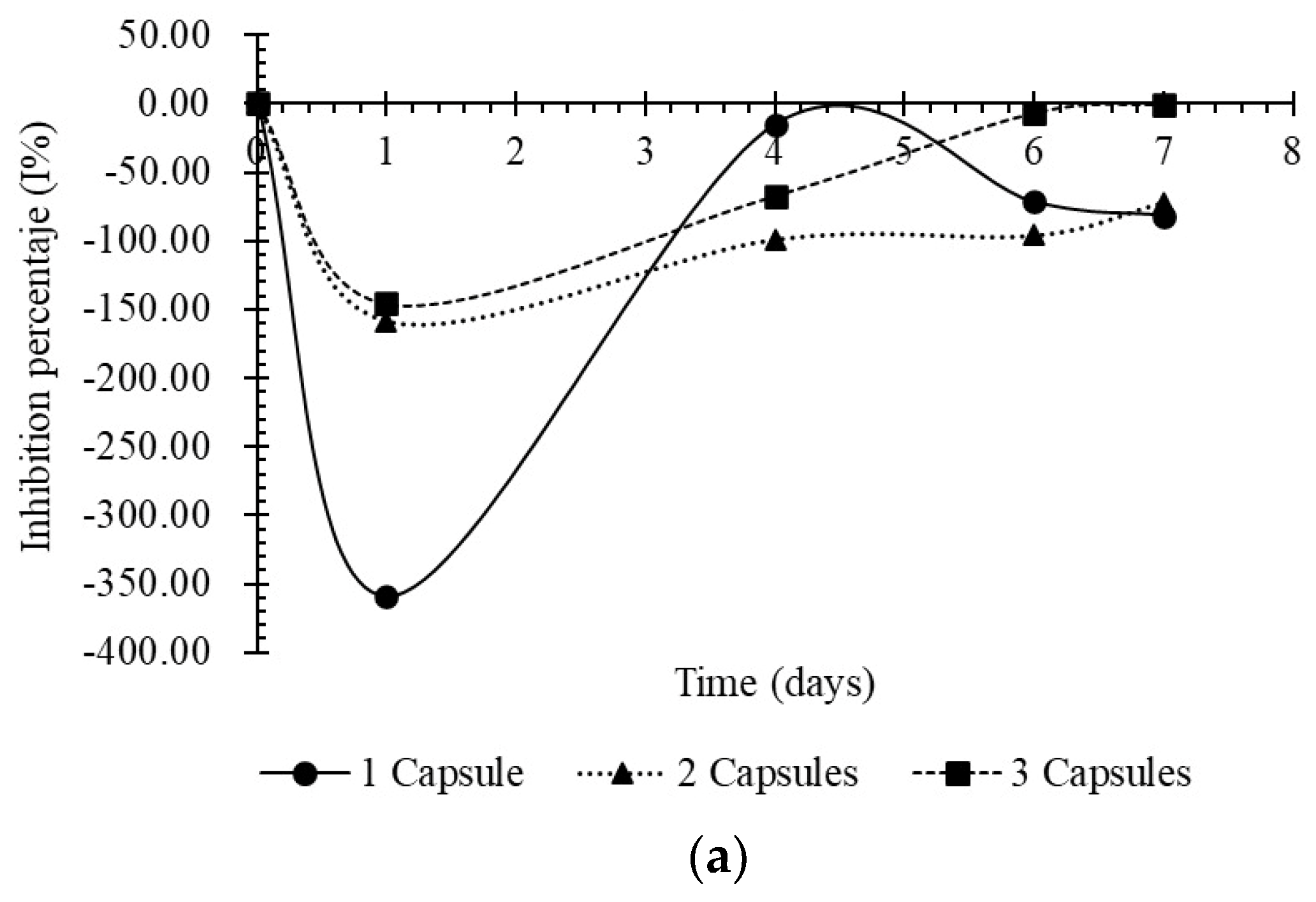
3.2. Sensitivity Testing of BCCs Against E. coli
3.3. Analysis of Variance and Tukey’s Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministerio de Salud Pública. 2021. Available online: https://www.salud.gob.ec/wp-content/uploads/2021/01/Etas-SE-03.pdf (accessed on 5 September 2025).
- Majowicz, S.E.; Scallan, E.; Jones-Bitton, A.; Sargeant, J.M.; Stapleton, J.; Angulo, F.J.; Yeung, D.H.; Kirk, M.D. Global incidence of human Shiga toxin–producing Escherichia coli infections and deaths: A systematic review and knowledge synthesis. Foodborne Pathog. Dis. 2014, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Gomaa, A.; Subirade, M.; Kheadr, E.; St-Gelais, D.; Fliss, I. Novel design for alginate/resistant starch microcapsules controlling nisin release. International J. Biol. Macromol. 2020, 153, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Bach, L.G.; Nguyen, D.C.; Le, H.X.; Pham, K.H.; Nguyen, D.H.; Thi, T.H. Evaluation of Factors Affecting Antimicrobial Activity of Bacteriocin from Lactobacillus plantarum Microencapsulated in Alginate-Gelatin Capsules and Its Application on Pork Meat as a Bio-Preservative. Int. J. Environ. Res. Public Health 2019, 16, 1017. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Pulla, J.S. Aislamiento y Purificación de Bacteriocinas a Partir de Lactobacillus plantarum para su uso como Conservantes en carne de res. Master’s Thesis, Universidad del Azuay, Cuenca, Ecuador, 2020. [Google Scholar]
- Dey, S.K.; De, P.K.; De, A.; Ojha, S.; De, R.; Mukhopadhyay, A.K.; Samanta, A. Floating mucoadhesive alginate beads of amoxicillin trihydrate: A facile approach for H. pylori eradication. Int. J. Biol. Macromol. 2016, 89, 622–631. [Google Scholar] [CrossRef]
- Earle, R.D.; McKee, D.H. Process for Treating Fresh Meats. U.S. Patent US3991218A, 9 September 1976. Available online: https://patents.google.com/patent/US3991218A/en (accessed on 5 September 2025).
- Giz, A.S.; Berberoglu, M.; Bener, S.; Aydelik-Ayazoglu, S.; Bayraktar, H.; Alaca, B.E.; Catalgil-Giz, H. A detailed investigation of the effect of calcium crosslinking and glycerol plasticizing on the physical properties of alginate films. Int. J. Biol. Macromol. 2020, 148, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, Y.; Zhang, H.; Jin, J.; Zhang, H. Quantitative proteomic analysis reveals the influence of plantaricin BM-1 on metabolic pathways and peptidoglycan synthesis in Escherichia coli K12. PLoS ONE 2020, 15, e0231975. [Google Scholar] [CrossRef] [PubMed]
- Yates, G.T.; Smotzer, T. On the lag phase and initial decline of microbial growth curves. J. Theor. Biol. 2007, 244, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Kanipes, M.I.; Lin, S.; Cotter, R.J.; Raetz, C.R. Ca2+-induced Phosphoethanolamine Transfer to the Outer 3-Deoxy-d-manno-octulosonic Acid Moiety of Escherichia coli Lipopolysaccharide. J. Biol. Chem. 2001, 276, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Hauben, K.J.; Bernaerts, K.; Michiels, C.W. Protective effect of calcium on inactivation of Escherichia coli by high hydrostatic pressure. J. Appl. Microbiol. 1998, 85, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Niaz, T.; Shabbir, S.; Noor, T.; Abbasi, R.; Raza, Z.A.; Imran, M. Polyelectrolyte Multicomponent Colloidosomes Loaded with Nisin Z for Enhanced Antimicrobial Activity against Foodborne Resistant Pathogens. Front. Microbiol. 2018, 8, 2700. [Google Scholar] [CrossRef] [PubMed]
- Narsaiah, K.; Jha, S.N.; Wilson, R.A.; Mandge, H.M.; Manikantan, M.R. Optimizing microencapsulation of nisin with sodium alginate and guar gum. J. Food Sci. Technol. 2014, 51, 4054–4059. [Google Scholar] [CrossRef] [PubMed]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Alginate–calcium films: Water vapor permeability and mechanical properties as affected by plasticizer and relative humidity. LWT-Food Sci. Technol. 2008, 41, 359–366. [Google Scholar] [CrossRef]

| Concentration (% w/w) | |||
|---|---|---|---|
| Minimum | Average | Maximum | |
| Alginate | 1.2 | 1.8 | 2.4 |
| Maltodextrin | 10.8 | 10.2 | 9.6 |
| Drinking water | 78 | 73 | 68 |
| SB/MRS | 10 | 15 | 20 |
| Glycerol | 20 * | 20 * | 20 * |
| Concentration (% w/w) | |||
|---|---|---|---|
| Minimum | Average | Maximum | |
| CMC | 0.76 | 0.75 | 0.74 |
| Drinking water | 93.7 | 92.6 | 91.6 |
| CaCl2 | 5.55 | 6.61 | 7.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campoverde, A.X.; Rosales, M.F.; Avilés, J.; Tacuri, J.; Montero, D.H.; Tejedor, R. Development of Alginate Capsules with Bioactive Compounds from Lactobacillus plantarum GP108 and Evaluation of Their Effect Against Escherichia coli. Biol. Life Sci. Forum 2025, 50, 7. https://doi.org/10.3390/blsf2025050007
Campoverde AX, Rosales MF, Avilés J, Tacuri J, Montero DH, Tejedor R. Development of Alginate Capsules with Bioactive Compounds from Lactobacillus plantarum GP108 and Evaluation of Their Effect Against Escherichia coli. Biology and Life Sciences Forum. 2025; 50(1):7. https://doi.org/10.3390/blsf2025050007
Chicago/Turabian StyleCampoverde, Antonio Xavier, Maria Fernanda Rosales, Jonnatan Avilés, Johanna Tacuri, Diego Hernán Montero, and René Tejedor. 2025. "Development of Alginate Capsules with Bioactive Compounds from Lactobacillus plantarum GP108 and Evaluation of Their Effect Against Escherichia coli" Biology and Life Sciences Forum 50, no. 1: 7. https://doi.org/10.3390/blsf2025050007
APA StyleCampoverde, A. X., Rosales, M. F., Avilés, J., Tacuri, J., Montero, D. H., & Tejedor, R. (2025). Development of Alginate Capsules with Bioactive Compounds from Lactobacillus plantarum GP108 and Evaluation of Their Effect Against Escherichia coli. Biology and Life Sciences Forum, 50(1), 7. https://doi.org/10.3390/blsf2025050007






