1. Introduction
Thickening and gelling agents provide structure when dissolved or dispersed. They are used in the food industry to create soups, sauces, and jam, and resulting viscosity depends on the agent used [
1]. These additives are usually added at concentrations below 1% to improve eating experience. Food gels are mostly made from proteins (gelatin, egg proteins) or from polysaccharides (agar, starch) [
2].
Viscosity is a critical parameter in process engineering because it affects mixing, pumping, and heat transfer [
3,
4]. High-viscosity materials require more energy for mixing, impacting equipment design and operational costs [
5,
6]. Furthermore, high viscosity can hinder heat transfer [
7]. Thus, achieving reproducible viscosity on a large scale requires precise control over formulation and processing conditions, so that energy input is minimized without compromising texture. Low-molecular-weight gelators (LMWG), like phenylalanine (PHE), are being investigated for their potential to be used in pharmaceuticals, bioremediation, and cosmetics, providing significant benefits. PHE structural properties allow for the formation of three-dimensional networks through hydrogen bonding and π-π stacking interactions [
8], yielding supramolecular, reversible gels. PHE solution–gel systems exhibit fine-tuned control over viscosity [
9], allowing for their mechanical behavior and texture to be customized by controlling external physical and chemical factors (like temperature, pH, and concentration). Their adaptable and reversible properties facilitate process engineering optimization.
Another benefit of substituting traditional gelling agents with PHE gels is that PHE is an essential amino acid which must be obtained from diets, usually through protein-rich foods, such as meat, fish, eggs, dairy, and some plant-based sources. It is crucial for protein synthesis and neurotransmitter production, and plays a key role in brain function and mood regulation, contributing to cognitive health. Its nutritional benefits extend to supporting skin, hair, and tissue growth [
10].
A further benefit of substituting gelling agents with PHE is the possibility of generating aroma compounds or colorants in the presence of reducing sugars through Maillard reaction [
11]. Maillard reaction (MR) can generate compounds that improve food sensory quality, but for some products it can be a cause of food deterioration. In both scenarios, understanding its kinetics is essential [
12].
Most present applications for pharmaceutical, water, or residue treatments include PHE derivatization [
13] and use of organic solvents [
14], which limit their application in food production. This study proposes new formulations of tunable PHE gels using GRAS substances. The aim of this work was solution or gel preparation and characterization, using different PHE concentrations in water and propylene glycol 50:50 as solvent.
2. Materials and Methods
Aqueous solutions of PHE (Biopack, Zárate, Province of Buenos Aires, Argentina) were prepared in bidistilled water; solutions were stirred and gently heated until completely dissolved. Then, an equal volume of propylene glycol (Dorwil, Martínez, Province of Buenos Aires, Argentina.) was added, and the resulting mixture was stirred, thus keeping solvent composition constant at water: propylene glycol 50:50. Concentration was expressed as percentage mass—volume concentration (solute mass in 100 mL solution, % m/V) in all cases. To obtain PHE gels, these systems were either stirred at high speeding rates or cooled at 4 °C for at least 72 h.
To study the influence of stirring speed on mechanically induced gelation, 1.0% m/V PHE solutions in solvent mixture were prepared, weighing 0.20 g PHE for a 20 mL final volume solution, and were each subjected to a different stirring rate. Higher PHE concentration solutions started to gel before stirring and lower PHE concentration solutions required much longer experimental times or higher stirring rates to gel by agitation. Viscosity was measured as a function of time with a rheometer (MCR 302 ANTON PAAR, Graz, Austria). Temperature and stirring time were held constant at 20 °C and 30 min, respectively. The speeding rate varied between 250 and 1000 rpm. Maximum viscosity was considered an indication of gelation, given that systems transformed from transparent solutions to white gels. Measurements were carried out in duplicates.
pH influence on mechanically induced gelation was studied in 1.0% m/V PHE solutions in solvent mixture, by weighing 0.50 g PHE for a 50 mL final volume solution. Solutions thus prepared presented pH = 5.95. Solution pH values were adjusted by HCl (c) or NaOH (c) addition (pH = 3.20; 5.95; 8.12; 8.50 or 10.48) and were each subjected to stirring. Viscosity was measured as a function of time with a rheometer (MCR 302 ANTON PAAR). Temperature, stirring time, and stirring speed were kept constant at 20 °C, 30 min, and 800 rpm, respectively. Maximum viscosity was considered an indication of gelation, given that systems transformed from transparent solutions to white gels. Measurements were carried out in duplicates.
To characterize PHE gels obtained by storage at low temperatures, dynamic oscillatory measurements were carried out with an Anton Paar rheometer (MCR 302) at 20 °C, for PHE 1% m/V or 1.7% m/V gels in water: propylene glycol 50:50. PHE solutions in solvent mixture were prepared, weighing 0.20 g or 0.34 g PHE for a 20 mL final volume solution. These solutions were each divided into three 6–7 mL aliquots and placed in 15 mL cylindrical containers. Samples thus prepared were stored at 4 °C for 72 h. The region of viscoelastic linearity (LVR) was determined by performing a stress amplitude sweep; the sample was subjected to a range of stress values (0.001% to 1%), and dynamic moduli were measured [
15]. Then, mechanical spectra were obtained, maintaining shear strain constant at 0.005%, recording storage or loss moduli G′ or G″ variation, with frequency ranging from 0.01 Hz to 100 Hz. Samples were placed between plates, keeping the gap between the plates so that the normal force between them was 0 N. Measurements were carried out in triplicates.
Gel transition temperature was determined by measuring G′ and G′ moduli variation with temperature. These tests were performed with an Anton Paar rheometer (MCR 302). Temperature scanning experiments were performed by applying heating scans with a Peltier system. In these conditions, storage (G′) and loss (G″) moduli were recorded between 10 and 70 °C. Gel transition temperature was taken as the temperature at which G′ equals G″, the crossover of dynamic moduli [
16]. To analyze the influence of PHE concentration on gelling temperature, PHE 0.7; 1.0; 1.3 or 1.7% m/V gels were prepared by weighing 0.14; 0.20; 0.26 or 0.34 g PHE for a 20 mL final volume solution. These solutions were each divided into three 6–7 mL aliquots and placed in 15 mL cylindrical containers. Samples thus prepared were stored at 4 °C for 72 h. XYL presence influence on gelling temperature was also studied for different XYL concentrations (0.1; 1.0, or 10.0% m/V), keeping PHE concentration constant (1.0% m/V). PHE-XYL solutions in solvent mixture were prepared, weighing 0.20 g PHE and 0.02, 0.20, or 2.00 g XYL for a 20 mL final volume solution. These solutions were each divided into three 6–7 mL aliquots and placed in 15 mL cylindrical containers. Samples thus prepared were stored at 4 °C for 72 h. Measurements were carried out in triplicates.
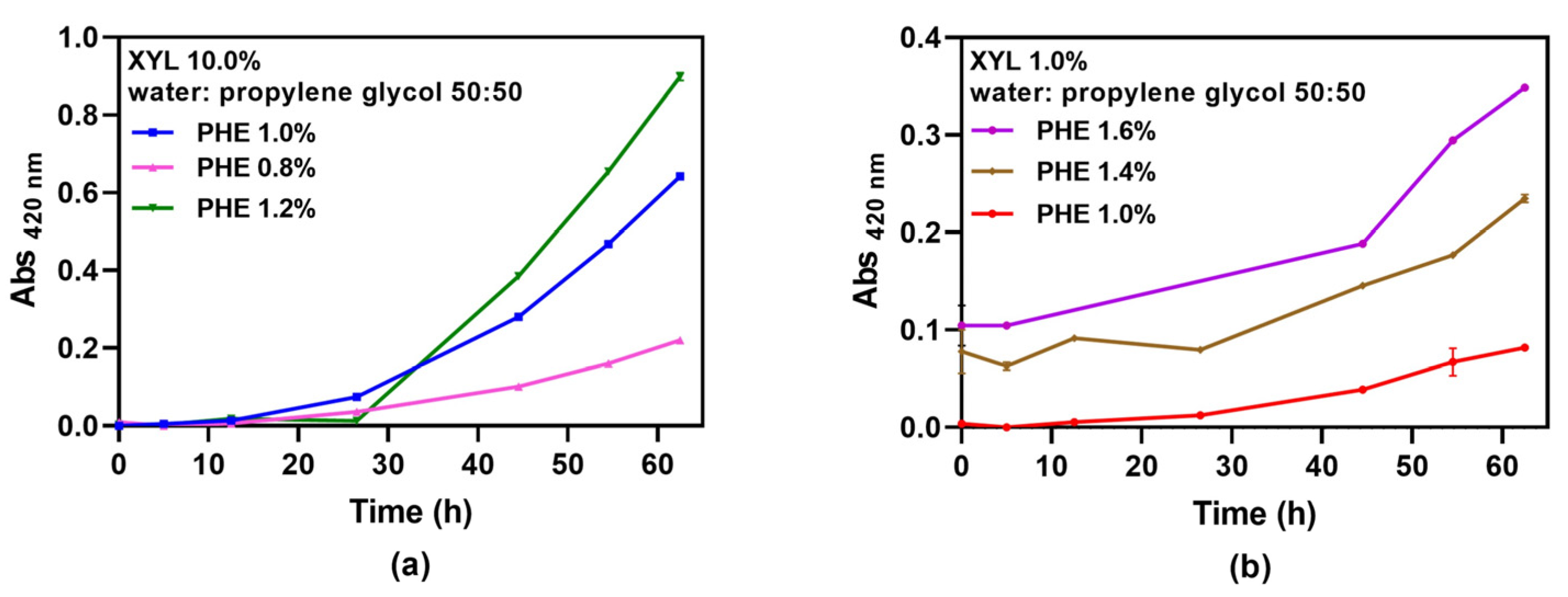
To characterize PHE non enzymatic browning aptitude in the presence of XYL, two sets of solutions were prepared. In the first solution set, XYL was added in approximately stoichiometric amount, 1.0% m/V (0.0667 M) for PHE concentrations 1.0, 1.4, or 1.6% m/V (0.0606 M; 0.0848 M; or 0.0970 M) by weighing 0.2 g XYL and 0.20, 0.28, or 0.32 g PHE for a 20 mL final volume solution. In the second solution set, XYL was added in excess amount, 10.0% m/V (0.667 M) for PHE concentrations 0.8% m/V (0.0485 M); 1.0% m/V (0.0606 M) or 1.2% m/V (0.0727 M), by weighing 2.0 XYL and 0.16, 0.20, or 0.24 g PHE for a 20 mL final volume solution. All systems were heated at 60 °C for 60 h, taking aliquots periodically and cooling them at room temperature. UV-VIS absorbance measurements were made at 420 nm (Shimadzu 1620, Kyoto, Japan) to analyze the kinetics of the final colored product [
17]. Measurements were carried out in duplicates.
3. Results
Mechanically induced gelation of PHE solutions depends on both stirring conditions, such as temperature, stirring time or rate, and physical and chemical characteristics, such as composition, pH, or additives [
18]. Gelation is mediated by mechanical energy and may be analyzed by following changes in viscosity during stirring time.
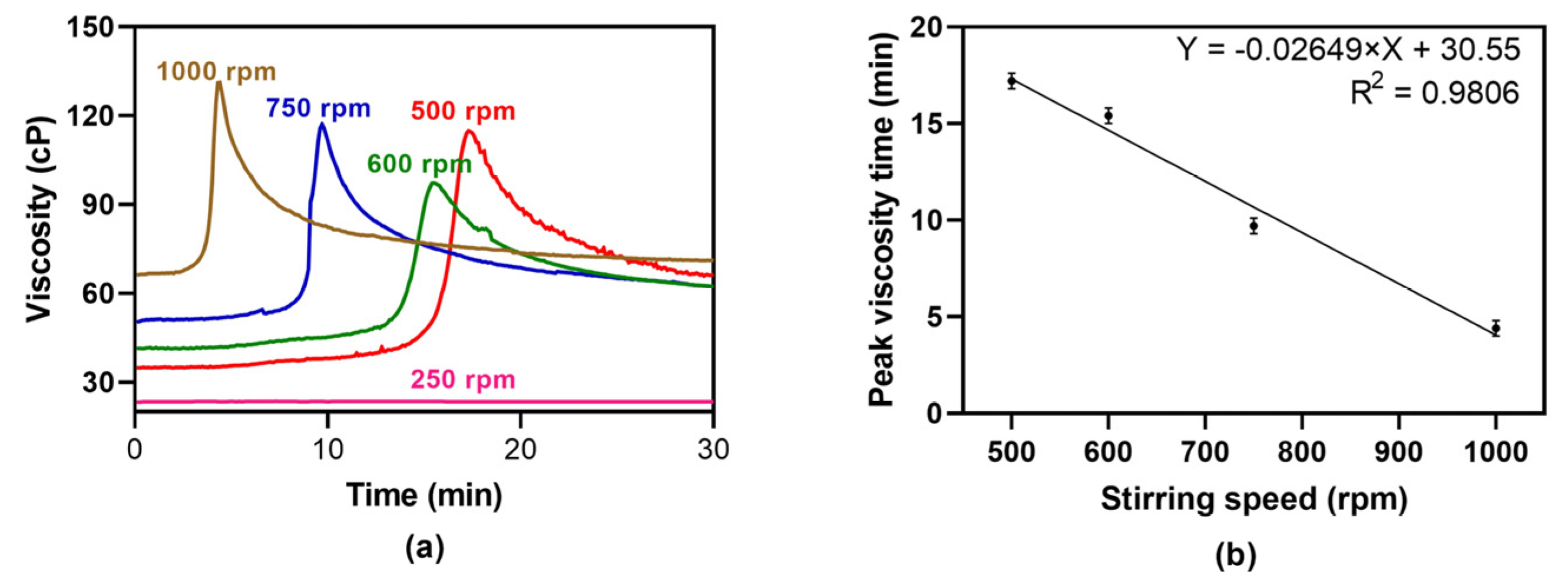
In this study, the influence of stirring rate (
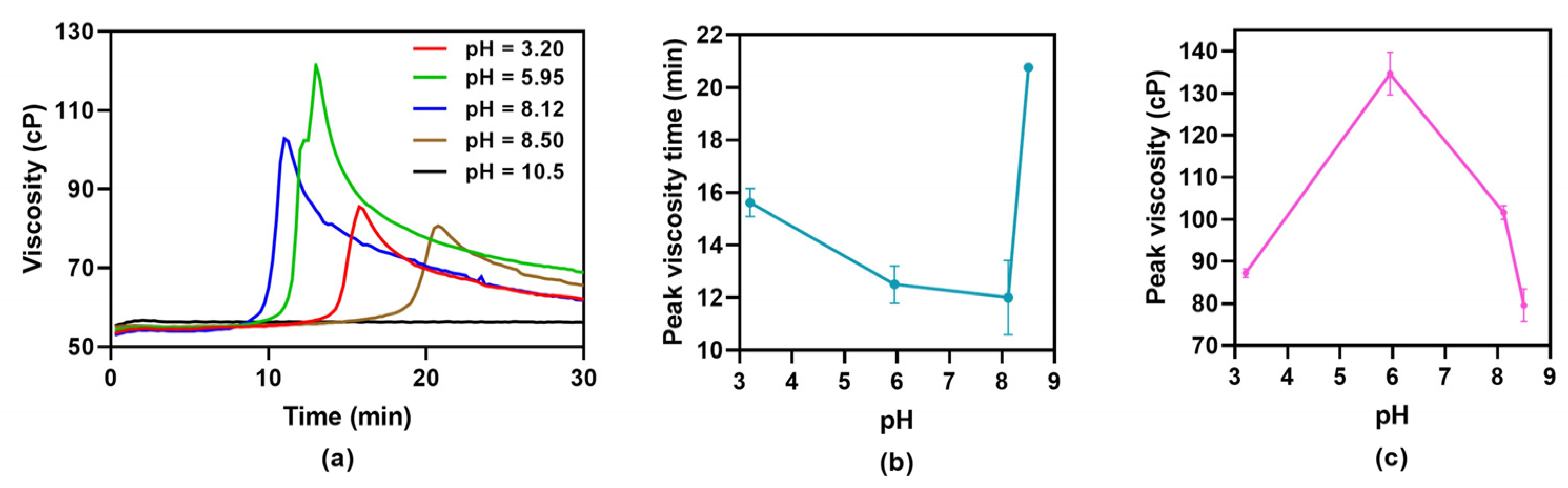
Figure 1) and pH (
Figure 2) on mechanical gelation was analyzed for the 1.0% PHE samples, keeping stirring time, temperature and solution composition constant.
Figure 1a and
Figure 2a show that, if gelation occurs, a maximum viscosity develops, indicating the time at which, under specific conditions, gel is formed. If stirring continues beyond gelation time, viscosity lowers, demonstrating that mechanically induced gelation is a reversible process.
Figure 1a shows that gelation time decreased when stirring rate was increased and gels thus formed presented different viscosities, implying that gel strength also depends on stirring rate. Given that, at 500 rpm, a maximum is reached, but not at 250 rpm, it can be concluded that there is a minimum stirring speed threshold below which gelation does not occur during the extent of the experiment.
Figure 1b exhibits the inversely proportional relationship between speeding rate and gelation time. These findings allow the prediction of the stirring speed required for gelation to occur, enabling the adjustment of the conditions to obtain either a solution or a gel.
PHE 1.0% m/V solutions in water: propylene glycol 50:50 exhibit pH = 5.95, which is close to phenylalanine isoelectric point, pI = 5.48 [
19]. To study the influence of pH on mechanically induced gelation, solution pH was adjusted in a wide range.
Figure 2b,c shows that, at pH = 5.95, maximum viscosity was reached faster (gelation process was fastest), and viscosity was highest, (gel strength was highest). At pH = 10.48, viscosity did not change over time (
Figure 2a), indicating that the gelation process did not occur over the 30 min duration of the test. Since at pH = 8.50 a maximum viscosity was observed but not at pH = 10.48, it follows that there is a maximum pH threshold beyond which gelation does not occur throughout the duration of the test.
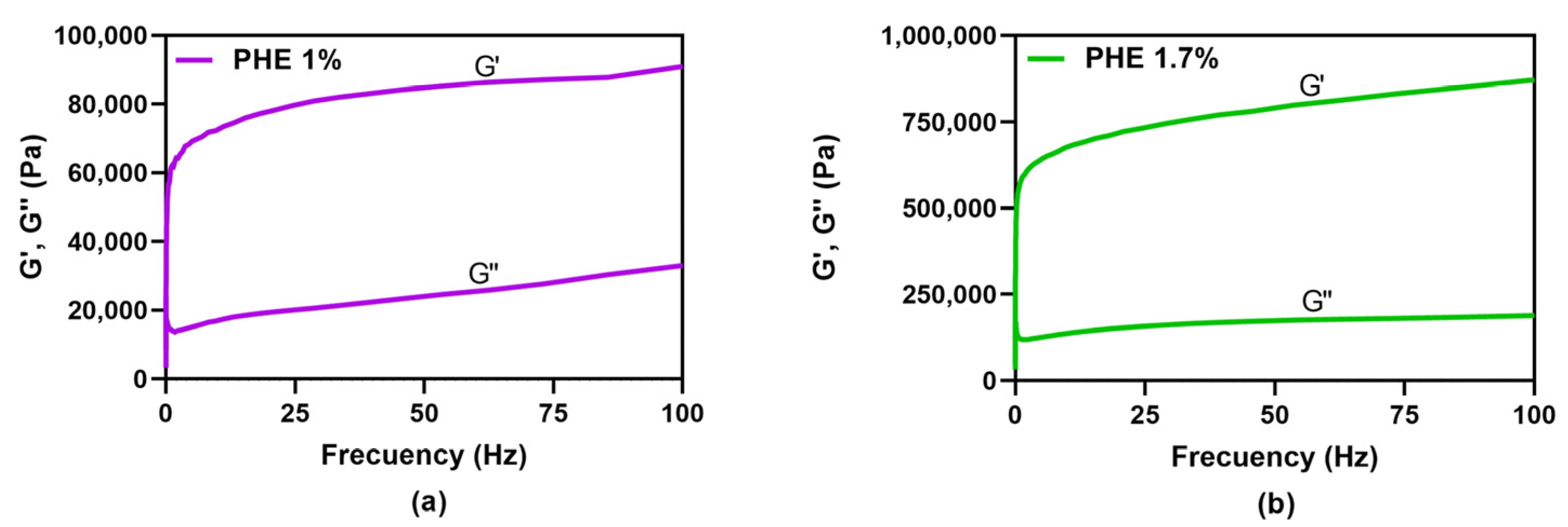
Mechanical spectra for PHE 1.0 or 1.7% m/V obtained by PHE solutions storage at low temperatures were analyzed to characterize gel strength (
Figure 3a and b, respectively). Both storage and loss moduli (G′ and G″, respectively) exhibited an approximately ten-fold increase, and the difference between G′ and G″ became much wider with this raise in PHE concentration. Gel strength increases with PHE concentration.
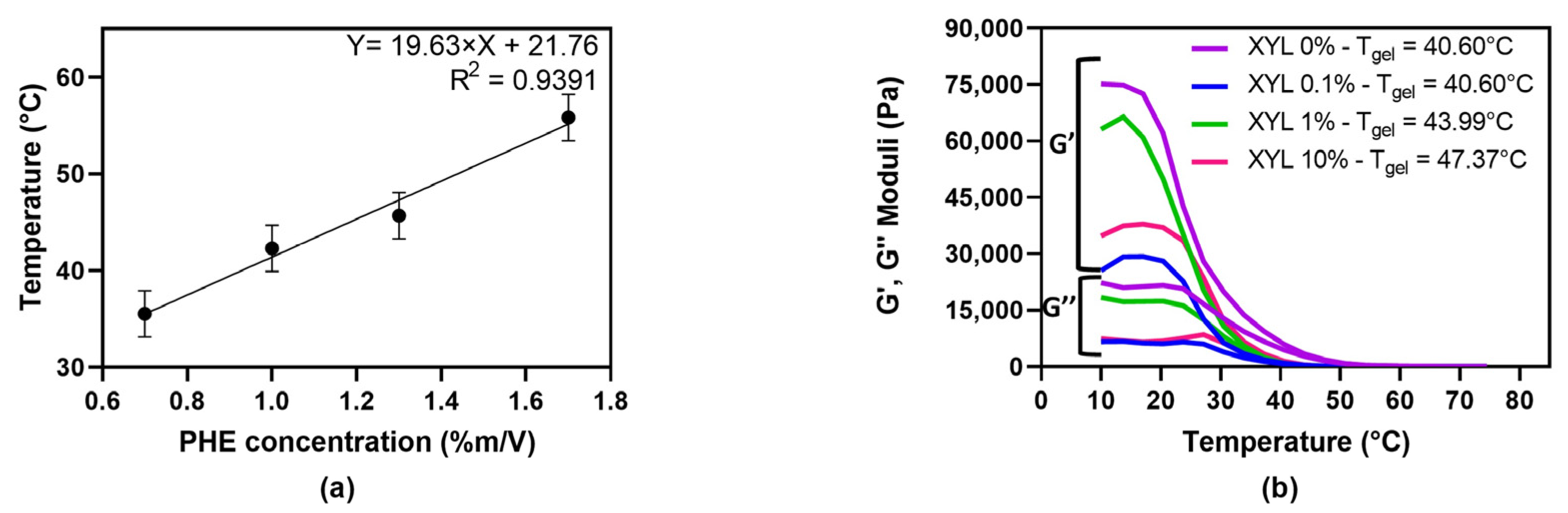
Gelation temperature was also analyzed for PHE gels obtained by low temperature storage. The relationship between PHE concentration and gelation temperature was linear, as shown in
Figure 4a. This observation is in keeping with the mechanical spectra, which showed that gel strength increased with increasing PHE concentration.
Gelation processes have been reported to be affected by the presence of sugars [
20], which are frequent components of foods and other systems. In this work, the influence of different amounts of XYL addition on gelling temperature was analyzed (
Figure 4b). PHE 1.0% m/V gels transition temperature was 40.60 °C. If XYL was added at low concentrations, gelation temperature did not change. However, when XYL and PHE concentrations were similar, gelation temperature increased; this increase was even higher if XYL was added in a higher amount than PHE. These observations indicate that PHE gels strength increase by XYL addition.
The presence of reducing sugars may induce browning by reacting with PHE, via Maillard reaction (MR), which may alter a food’s sensory and nutritional features, either improving or impairing them [
21]. Also, PHE gelation might be affected by the decrease in gelator concentration or by MR products that might interfere in the gel network structure. For this reason, Maillard reaction kinetics were determined by heating PHE or XYL (a reactive five-membered ring monosaccharide) at different concentrations. The development of final products of MR was followed by plotting UV-VIS absorbance at 420 nm as a function of heating time, as shown in
Figure 5.
Figure 5 exhibits the absorbance at 420 nm for different PHE concentrations in excess XYL (
Figure 5a), and for different PHE concentrations at 1.0% m/V XYL (
Figure 5b). Experimental data followed first order kinetics, in accordance with previous PHE model systems studies [
22,
23], and reaction degree increased with both PHE and XYL concentration, especially for a XYL tenfold-increase in concentration. Additionally, for PHE 1.0% m/V in excess XYL (10.0% m/V), there was a longer lag time than those of systems containing similar mass amounts (XYL 1.0% m/V). It must be observed that these heated PHE–XYL systems are characterized by a mild, flowery aroma. Aroma formation in amino acid—sugar systems is dependent on amino acid type, given that it is due to aldehydes produced during Strecker degradation of amino acids, which are intermediate products of the MR [
24]. These compounds have a very low aroma threshold, enhancing food sensorial quality and providing evidence that MR is occurring.
4. Discussion
It has been previously reported that, in aqueous media, PHE self-assembles into fibrils, forming a layered structure stabilized by alternating hydrophobic and hydrophilic non-covalent interactions. Phenylalanine molecules are held together by hydrogen bonds between the carboxylate and amine groups, and interlayer π-π stacking between hydrophobic aromatic rings. Collectively, a strong set of interactions is formed, rendering fibrils [
25,
26]. Phenylalanine is a non-polar amino acid, slightly soluble in water and must be heated for complete dissolution. Adding propylene glycol lowers solvent polarity and enhances PHE–PHE interactions, making it possible to form macroscopic tridimensional networks that do not flow when inverted, also known as gels, at PHE concentrations lower than those previously reported [
27].
Contrary to most gelling food additives, PHE is a LMWG, which forms supramolecular gels. These gels are more easily tunable than biopolymers, given their more dynamic structures. However, because of structure lability, gel characteristics are not reproducible unless preparation is carefully standardized. Thus, solution preparation, gelation methodology, even container volume and shape, are all factors that must be normalized to maintain gel characteristics [
28].
Figure 1 and
Figure 2 show that gels may be formed immediately by PHE solution mechanical stirring, changing gelation formation and characteristics by changing either speeding rate or pH. Mechanically induced gelation process has been previously reported [
18,
29]. Stirring brings the monomers together by convection, quickly generating aggregates from which fibrils are formed. Fibril formation requires high activation energy, so it is promoted by mechanical energy. However, mechanical energy also breaks the formed fibers, causing the viscosity to decrease after reaching a maximum, making the gel reversible. When stirring speed is increased, mechanical energy rises and fibrils are more readily formed. Therefore, viscosity maximum is reached faster. Alternatively, if stirring speed is too low, mechanical energy is not high enough to overcome the activation energy barrier and gels are not formed.
As pH diverges from the PHE isoelectric point, amino acid molecules acquire a net charge. If molecules carry same-sign charges, there is greater repulsion among them, preventing their interaction and thus, gels cannot be formed [
30]. In this way, interaction becomes weaker, and the gel network is destabilized; gel viscosity is lower at pHs farther from the PHE isoelectric point. At extreme pH values, gel is not even formed, and viscosity does not change with stirring. This behavior is illustrated in
Figure 2; at outermost pH values, maximum (gel) viscosity decreases. In fact, at pH 10.48, gel was not formed under experimental conditions.
On the contrary, gels formed upon cooling at rest require more time to develop their tridimensional structure due to fibril formation activation energy. The gels depicted in
Figure 3 and
Figure 4 were prepared by the PHE solution storage at 4 °C for at least 72 h. These gels presented
pseudogel, or weak gel behavior; although, increasing PHE concentration rendered gel structure more rigid, improving gel strength (G′ and G″ became higher and farther apart). Also, gelation temperature increased linearly with PHE concentration. Hydroxylated compounds like XYL may interfere in hydrogen bonding, also changing gelation temperature, although this interaction is not as important as π-π stacking between PHE molecules in gel network.
When the PHE solutions were heated in XYL presence, browning was developed, following first-order kinetics. Browning kinetics were rather slow, which is to be expected, given that the solution pH was too low to enhance carbonyl electrophilicity and amino nucleophilicity, and PHE presented low reactivity [
22,
23], probably due to phenyl group steric hindrance. Browning developed faster as the concentration of PHE or XYL increased, in keeping with first-order kinetics. PHE, being moderately reactive, allows for proper management over MR, which is important in food processing, ensuring appealing flavors, aromas, and colors, thus enhancing consumer experience. Otherwise, it may lead to undesirable effects, such as reduced gelling ability, lower nutritional quality, or potentially harmful compound formation.
5. Conclusions
Characterization of PHE gel formation in water: propylene glycol 50:50 permits their usage as an adequate LMWG alternative to gelling or thickening food agents in specific preparation and storage conditions. Gelation of PHE solutions may be induced mechanically or by storage at a low temperature. In the former case, solution viscosity increases by stirring, reaching a maximum, which may render upscaling more difficult and expensive. However, stirring speed and pH variation allows for fluid viscosity management, while keeping the possibility of reversal to initial conditions. On the other hand, gels formed by cooling at rest exhibit weak gel characteristics, but strength increases with PHE concentration. Additionally, non-enzymatic browning development in PHE–XYL solutions is rather slow and follows first-order kinetics. Also, when gently heated, PHE gels release a floral aroma that could enhance tea-like blends. As such, PHE–water–propylene glycol systems are promising candidates for food products such as desserts or appetizers, offering a unique combination of texture and flavor, and the ability to tailor their properties for specific applications, enhancing versatility across various industries.